Abstract
In this study, activated carbon and piperazine-modified activated carbon adsorbents were prepared and used for CO2 adsorption. The effect of various parameters including adsorbent particle size, adsorbent amount, piperazine weight percent, pressure, and temperature were investigated on the CO2 adsorption capacity. The adsorbents were characterized using nitrogen adsorption/desorption isotherms and FTIR analyses. The results showed that the adsorption capacity decreases with temperature increasing and increases with pressure increasing. In addition, the surface modification of activated carbon improved the CO2 adsorption capacity more than the unmodified adsorbent, and the highest CO2 adsorption was obtained 203.842 mg/g at 25 °C and 8 bar. Additionally, to determine the adsorbent behavior, CO2 adsorption experimental data were fitted by isotherm and kinetic models. CO2 adsorption isotherm modeling was studied up to 8 bar at 25 °C, and kinetic modeling was investigated up to 85 °C at 6 bar. The results show that Hill isotherm model and Elovich kinetic models have a good agreement with the adsorption data. Finally, thermodynamic modeling was carried out for modified and unmodified adsorbents, and enthalpy, entropy, and Gibbs free energy changes of adsorption for piperazine-modified activated carbon at 25 °C and 6 bar obtained 17.078 kJ/mol, − 0.039 kJ/mol.K, and − 5.318 kJ/mol, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing of CO2 production in the environment necessitated more studies and experiments on the removal of this pollutant. Available technologies for CO2 capture are cryogenic distillation, membrane separation, physical and chemical absorption, and adsorption by solid adsorbents (Amiri et al. 2017; Estalkhzir et al. 2018; Ghaemi et al. 2009; Penchah et al. 2021c; Rezakazemi et al. 2017). Adsorption in porous solids such as activated carbon is an attractive alternative technology to overcome the technological and economic constraints of the mentioned technologies (Hauchhum and Mahanta 2014; Najafi et al. 2020; Penchah et al. 2020; Shafeeyan et al. 2015). Generally, adsorption technology is widely considered for gas purification due to its flexibility and efficiency (Penchah et al. 2019, 2021a). Activated carbon is a high microporous material with a large surface area and attractive carbon-based materials to remove gas-phase pollutants (Etemad et al. 2015; Zhua et al. 2014).
In recent years, adsorbent modification has been considered to enhance its essential properties, which include cation or ion exchange within the porous material or the functionalization of pore structure as well as saturation with amines (Najafi et al. 2021; Niknafs et al. 2015; Penchah et al. 2021b; Shafeeyan et al. 2010; Shafeeyan et al. 2015; Sreńscek-Nazzal et al. 2016). To improve the CO2 adsorption capacity, different modifications can be made in the form of physical or chemical processes. Due to corrosion, low stability, and high cost of amine materials, alkaline bases have been used to improve the performance of porous adsorbents (Amiri et al. 2017; Taheri et al. 2019; Yong et al. 2001). One of the possible cases for adsorbing CO2 from the air is using of strong bases such as calcium hydroxide, potassium hydroxide, and sodium hydroxide (Mohammad et al. 2019; Naeem et al. 2016; Shafeeyan et al. 2010).
Tan et al. studied the efficiency of modified activated carbon from coconut shells with NaOH to adsorb carbon dioxide in an adsorption column (Tan et al. 2014). Casco et al. introduced and presented activated carbon from oilfields and modified that with KOH, for adsorbing CO2 at atmospheric pressure (168 mg/g at 298 K) and high pressure (1500 mg/g at 298 K and 4.5 Mpa) (Casco et al. 2014). Deng et al. reported on activated carbon from peanut shells and sunflower seeds to adsorb carbon dioxide. They optimized the activation of activated carbon with a ratio of about 1 KOH/carbon. The CO2 adsorption capacity of the activated carbon of peanut shell and sunflower shell obtained 1.54 mg/g and 1.46 mg/g at 298 K and 0.15 bar, respectively (Deng et al. 2015).
Nazzal et al. investigated the correction of active carbon to improve the CO2 adsorption capacity by activating with KOH, ZnCl2, and K2CO3. The maximum carbon dioxide adsorption was obtained 14.44 mmol/g at 313 K and 40 bar (Sreńscek-Nazzal et al. 2016). In 2001, Ro'denas et al. modified active carbon by activation with NaOH and KOH. The results show that chemical activation with NaOH can be successfully used to produce activated carbon with high surface area and microporous volume; also, pore volume by KOH activation was higher than that with NaOH (Lillo-Rodenas et al. 2001).
Huang et al. worked on CO2 capture using metal-organic framework NH2-MIL-101 that was grafted with amine. NH2-MIL-101 is a standard metal-organic framework adsorbent with a chromium basis that is synthesized and modified with tetraethylenepentamine (TEPA) by wet impregnation method. As a result, NH2-MIL-101 is modified with 50 %wt. So this amine-grafted adsorbent with a high CO2 capture capacity is found as an efficient material for CO2 adsorption (Huang et al. 2016).
Pino et al. modified activated carbon with two amines, polyethyleneimine (PEI) and tetraethylenepentamine (TEPA), using wet impregnate method. The effects of amine amount (from 10 to70 %wt.), the concentration of CO2 in the feed, temperature and gas hourly space velocity were investigated on the CO2 adsorption capacity. The best sorption capacity was 6.90 mmol/g, when the adsorbent was modified with 70 wt.% of supported TEPA at 70 °C and the stream contained 80 vol.% of CO2 (Pino et al. 2016).
Gholami et al. carried out a series of experiments in order to find the optimum condition for grafting 3-[2-(2-aminoethylamino) ethylamino] propyltrimethoxysilane (TRI) on bi-modal porous structure MCM-41(BPS-MCM-41). The optimum conditions for the modified adsorbent was obtained 85 °C, 0.3 cc/g, and 1.5 cc/g for temperature, water to support ratio, and aminosilane to support ratio, respectively. The BPS-MCM-41 grafted with amine achieves 2.31 mmol/g adsorption capacity under this condition (Gholami et al. 2015).
Bezerra et al. (2014) studied the CO2 adsorption on Zeolite 13X as solid adsorbent which was functionalized with amine groups. They found that more loading of amine to the adsorbent caused more decrease in micropore volume and adsorbent was pore-blocking with monoethanol amine (MEA). According to this study, one part of loaded MEA is bonded to the zeolite structure covalently, and excess MEA is adsorbed physically that can be desorbed by raising the temperature more than MEA boiling point (Bezerra et al. 2014).
Chiang et al. investigate the material properties of activated carbon fibers (ACFs) modified with potassium hydroxide (KOH) or tetraethylenepentamine (TEPA) to determine the CO2 adsorption capacities of adsorbents. The results showed that the KOH-modified ACFs exhibited a great deal of pore volume and a specific surface area of 1565 m2/g. Also, the CO2 uptakes at 1 atm and 25 °C on KOH-activated ACFs reached 2.74 mmol/g (Chiang et al. 2019).
Acevedo et al. prepared activated carbon from lignocellulosic material, African palm shells, by chemical impregnation of the precursor with solutions of cupric nitrate at five different concentrations. The results showed that the BET surface areas varied between 473 and 1361 m2/g and micropore volume between 0.18 and 0.51 cm3/g and the CO2 adsorption capacity was obtained between 103 and 217 mg/g (Acevedo et al. 2020).
Giraldo et al. studied on CO2 adsorption by chemically modified activated carbon with nitric acid and ammonium aqueous. The results show that the activated carbons had a BET surface area between 634 and 865 m2/g, with a micropore volume between 0.25 and 0.34 cm3/g. CO2 adsorption capacity at 273 K and 1 bar was achieved between 205 and 333 mg/g (Giraldo et al. 2020).
Considering the previous researches, CO2 adsorption, because of its advantages, was an appropriate method to omit CO2 as pollutant. In this study, activated carbon because of its high adsorption capacity and low price was chosen as the base adsorbent for modifying. Improvement of the activated carbon for CO2 adsorption was carried out by piperazine that is an organic compound and consists of a six-membered ring containing two nitrogen atoms. Also, unmodified and modified adsorbent characterizations were carried out by FTIR and BET analyses. The effect of various parameters such as adsorbent particle size, adsorbent amount, piperazine amount, piperazine weight percent, pressure, and temperature were investigated on the CO2 adsorption capacity. Finally, isotherm, kinetic, and thermodynamic modeling were accomplished using the CO2 adsorption system.
Materials and methods
Materials
Activated carbon was chosen as adsorbent and was prepared from BASF Company of Germany. Piperazine with the IUPAC name of hexahydropiprazine as the amine that consists of a six-membered ring containing two nitrogen atoms and methanol with the 99.99% purity as solvent was used and prepared from Merck. Furthermore, CO2 with purity of 99.99% was bought from Sablan Gas Co., Iran.
Adsorbent modification
For the modification of the activated carbon with piperazine, at first, the adsorbent was washed by nitric acid to create C-O-O-H group on activated carbon surface to link with piperazine under reflux condition with concentrated nitric acid for 24 h. The adsorbent was washed with ionized distilled water until its pH reached to 5, and then, it was dried under 100°C for 24 h in the oven. 1 %wt piperazine in different amount of methanol (5, 10, 20, and 60 ml) was used for modifying of 1 g of activated carbon. Each sample was stirred for 6 h at temperature of 45 °C. After that, it was dried at temperature of 75 °C for 24 h in oven. The adsorption experiment was done for each sample. Finally, the modified sample with 5 ml of piperazine solution in methanol had the best result. It was assumed that in other loading of piperazine in methanol, this amount of solution was the optimum amount. So, 5 ml of varied weight percentage of piperazine in methanol solvent was prepared, and the effect of weight percentage of piperazine (1–4%) was investigated. The adsorbent that was modified with 5 ml of solution with 2 %wt of piperazine in methanol had the maximum adsorption capacity. So, investigating the effect of the temperature and the pressure on the adsorption capacity was continued with modified adsorbent with 5 ml of 2 %wt of piperazine in methanol.
Adsorbent characterization
Surface structure, morphology, and porosity properties of adsorbent were accomplished by nitrogen adsorption and desorption at 77 K at a pressure of 106.65 kPa (Micromeritics, Model ASAP 2020, USA). FTIR spectroscopy analysis was performed using a spectrometer (Perkin Elmer, Model 2000 FTIR, USA) to identify surface functional groups of modified and unmodified activated carbon.
Adsorption setup
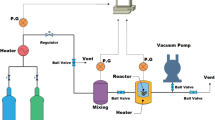
An adsorption batch system was designed for CO2 adsorption capacity measurement (Fig. 1). As it is shown, the setup included a stainless style batch vessel (with the height of 9 cm, internal radius of 3 cm, and internal volume of 255 cm3), CO2 gas cylinder, pressure adjusting valves, electrical heater, pressure and temperature transmitter, and a computer. The vessel was able to control the main parameters that are effective in CO2 adsorption like temperature, pressure, and the amount of adsorbent. At the first of each experiment, gas temperature was set, and then, the sample of adsorbent was put in the vessel. After that, related valves and pressure gauge set the initial pressure of CO2 in the vessel. After setting the pressure and reaching the temperature to the set point amount, the experiment started and in the computer; temperature and pressure were saved versus the time. The amount of adsorption capacity was calculated using the real gas law by measuring the loss of pressure due to CO2 adsorption (Eqs. 1 and 2).
where i and f subscripts refer to initial and final conditions. P, T, V, Z, Mw, R, w, B, and m are pressure, temperature, reactor volume, compressibility factor, gas molecular weight, universal gas constant, mass of adsorbent, virial second coefficient, and mass of adsorbed gas, respectively.
Result and discussion
Characterization
The FTIR spectra of activated carbon and piperazine-modified activated carbon before and after CO2 adsorption are presented in Figs. 2 and 3. According to Fig. 2, in the range of 1300–1400 cm−1, the vibration of C-O bond and, at the point of 1600 cm−1, the vibration of C=O (aromatics) bond were noticeable and observed. In the range of 1700–1800 cm−1, the vibration of C=O (carboxylic acid) bond was evident. As it is shown in Fig. 2, the FTIR spectra are developed after adsorption (Li et al. 2016). According to Fig. 3, in the range of 1260–1340 cm−1, the vibration of C-N bond and, in the range of 1430–1650 cm−1, the vibration of C-C bond are shown. Also the vibration of C-H and N-H bonds is visible in the range of 2750–3080 cm−1 and 3220–3500 cm−1, respectively. These vibrations prove the modification of activated carbon surface by piperazine (Li et al. 2016).
BET analysis (Fig. 4) was used to determine the surface area and porosity properties of activated carbon and modified activated carbon by N2 adsorption-desorption at 77 K. Surface area, total volume, pore volume, and mean pore diameter are reported in Table 1. According to adsorption-desorption curve of activated carbon and adapting it with IUPAC isotherms, this type of curve is isotherm type III, and this type of isotherm shows that the adsorption of the first layer was lower than other layers. Considering the mean pore diameter equal to 10.49 nm, it was found that this adsorbent is mesoporous. BJH curve shows that the highest amount of adsorption is in the range of mesoporous pores (Ruthven and Reyes 2007). Adsorption-desorption curve of modified activated carbon is like of activated carbon. Considering the mean pore diameter equal to 2.49 and 2.42 nm for activated carbon and modified activated carbon with piperazine, respectively, both adsorbents are mesoporous. Figure 5 represents that for both activated carbon and its modified sample, the most adsorption has taken place in mesoporous region and also between particles with 0–20 nm radius.
Effect of parameters on co2 adsorption capacity
In order to investigate the adsorbent particle size in the CO2 adsorption capacity, different adsorbent sizes are divided by mesh, and adsorption is tested as shown in Fig. 6. According to this figure, it is shown that increasing the particle size decreases the adsorption capacity.
In order to evaluate the effect of adsorbent amount on CO2 adsorption capacity, different amounts of adsorbent are tested as shown in Fig. 7. In the experiments which were done with different amounts of adsorbent, it was found that by increasing the amount of adsorbent, the adsorption capacity decreases.
In order to modify the activated carbon by piperazine, the effect of the amount of piperazine in methanol (modifying solution) was investigated. Hence, various amounts of piperazine-methanol solution (5-10-20-60-100 ml) were considered for modifying of 1 g of activated carbon adsorbent at 25 °C and 6 bar (Fig. 8). The results show that by increasing the amount of piperazine in methanol, the adsorption capacity decreases. So in the following experiments, 5 ml of piperazine-methanol solution was used for modifying of 1 g of activated carbon adsorbent (Norouzbahari et al. 2015).
In order to evaluate the weight percent of piperazine in the modifying solution on adsorption capacity, 1 g of activated carbon was modified with 5 ml of 1, 2, and 4 wt.% of piperazine. CO2 adsorption capacity is plotted in Fig. 9, and as a result, it is shown that optimum CO2 adsorption capacity was obtained using the adsorbent sample which was modified with 2 %wt of piperazine in methanol.
For investigating the effect of pressure on CO2 adsorption capacity, the adsorption experiments were done with 1 g of piperazine-modified activated carbon in various pressure (2–8 bar) at temperature of 25 °C. As it is shown in Fig. 10, by increasing the pressure, the CO2 adsorption capacity increases.
For investigating the effect of temperature on adsorption capacity, the adsorption experiments were done at pressure of 6 bar and temperature range of 25 up to 85 °C. According to, Fig. 11, CO2 adsorption capacity decreases by increasing the temperature.
Figures 12 and 13 show the comparison of CO2 adsorption capacity between activated carbon and piperazine-modified activated carbon at various pressure and temperature. According to these figures, piperazine-modified activated carbon has a higher adsorption capacity than unmodified activated carbon.
Adsorption isotherm
In order to study the amount of adsorbed material on the adsorbent surface and its relation to the gas pressure or the dissolved concentration at a constant temperature, conventional equations for the adsorption isotherms are applied. The isotherm shows the CO2 adsorption in the adsorbent samples as a pressure function. In physical adsorption, molecules adsorbed to all parts of the adsorbent surface are limited to the number which are compressed in each molecular layer. Langmuir and Freundlich isotherm models are related to adsorption in low temperature and are appropriate for physical adsorption (Langmuir 1916). The Langmuir isotherm model is the best description for physical adsorption because it is limited to one layer on the surface of adsorbent, while Freundlich model indicates a physisorption process. The value of nF < 1 in Freundlich model indicates that CO2 adsorption is a chemical process, while the value of nF > 1 is the indicator of a physical process (Ko and Schmidt 1974).
Dubinin-Radushkevich isotherm model give useful information about energy parameters. In these models, ɛ and β are adsorption energy and the heat of adsorption, respectively. If 9<ɛ<16 kj/mol, the adsorption is Schmidtchemisorption; otherwise if ɛ<8 kj/mol, the adsorption is physisorption (Ko and Schmidt 1974).
According to the results which are obtained from CO2 adsorption isotherm on activated carbon (Fig. 14) and piperazine-modified activated carbon (Fig. 15), it is obvious that Hill model has the best correlation coefficient, so this model has the best conformity with experimental data (Table 2). As a result, CO2 adsorption using activated carbon and modified activated carbon is physical and limited to one layer. Also the maximum amount of CO2 adsorption using activated carbon and modified activated carbon according to Langmuir model can be 414.74 mg/g and 609.92 mg/g, respectively. Considering the high Dubinin-Radushkevich correlation coefficient, it is possible to confirm that CO2 adsorption by activated carbon and modified activated carbon is a physical process according to its high average energy equal to 0.640 kJ and 0.668 kJ, respectively.
Adsorption kinetic
Kinetic modeling can be used to determine the equilibrium and kinetic constants. Predicting the adsorption rate is the most important factor in the design of process systems and control the time required for the adsorption process. Due to the complexity of predicting kinetic parameters, a conventional approach involves matching the experimental data to a set of fixed models and choosing the best model (Foo and Hameed 2010; Ho 2004). Experimental data and kinetic models of CO2 adsorption using activated carbon and piperazine-modified activated carbon are presented in Figs. 16 and 17, respectively, and kinetic model parameters are listed in Table 3. According to the results, the Elovich kinetic model has the best agreement with the experimental data.
Absorbent thermodynamic
It is necessary to consider both energy and entropy factors to evaluate the feasibility and spontaneity of a process. Each of the thermodynamic parameters has its significance, and their values and symbols can be help to understand how the adsorption process is performed (Ghaemi et al. 2009; Niknafs et al. 2015). According to Fig. 18, the values of ΔH° and ΔS° are obtained from the slope (ΔH°/R) and the intercept (ΔS°/R) of the line from the plot of ln (kd) versus 1/T. This figure shows that modified activated carbon has better CO2 adsorption capacity than activated carbon. Figure 18 expresses that by increasing the temperature, CO2 adsorption capacity decreases. So, activated carbon has better CO2 adsorption capacity in low temperature (Zhang et al. 2014). ΔH, ΔS, and ΔG of activated carbon and modified activated carbon are listed in Table 4. It is considered that the positive value of ΔS and negative value of ΔG show that the adsorption process is spontaneous.
Conclusion
In this study, CO2 adsorption was investigated, and the effect of several effective parameters such as the amount and particle size of adsorbent, temperature, and pressure for adsorption with activated carbon in addition to the amount of modifier solution and the weight percentage of piperazine in methanol for adsorption with modified activated carbon was done. The results show that increasing the amount of adsorbent decreases the CO2 adsorption capacity; also increasing particle size leads to decrease CO2 adsorption capacity. By increasing temperature and pressure, CO2 adsorption capacity decreases and increases respectively. Modification of activated carbon surface shows an enhancement in CO2 adsorption capacity for both parameters of temperature and pressure more than unmodified activated carbon. Experimental data show that the optimum amount of piperazine in methanol for modification of 1 g of activated carbon is 5 ml, and increasing the amount of solution for this amount of activated carbon decreases the CO2 adsorption capacity. By investigating different weight percentage of piperazine in methanol, it is found that the optimum weight percentage is 2 %wt.
For investigating adsorption isotherm, Langmuir, Freundlich, Dubinin-Radushkevich, and Hill models and, for investigating adsorption kinetic, first-order, second-order, Elovich, and rate controlling models were used. The results show that the experimental adsorption data by activated carbon and modified activated carbon meets Hill isotherm model. Kinetic modeling shows that Elovich model has a good agreement with the experimental data for activated carbon and piperazine-modified activated carbon and the controlling step is diffusion in boundary layer. Thermodynamic studies of CO2 adsorption with modified activated carbon show that, ΔH, ΔS, and ΔG are 17.078 kJ/mol, − 0.039 kJ/mol.K, and − 5.318 kJ/mol at 25 °C, respectively. In addition, for both CO2 adsorption processes, by increasing the temperature, CO2 adsorption capacity decreases, and both adsorbents have maximum adsorption capacity in low temperature.
Data availability
All data generated or analyzed during this study are included in the manuscript.
References
Acevedo S, Giraldo L, Moreno-Piraján JC (2020) Adsorption of CO2 on activated carbons prepared by chemical activation with cupric nitrate. ACS Omega 5:10423–10432
Amiri M, Shahhosseini S, Ghaemi A (2017) Optimization of CO2 capture process from simulated flue gas by dry regenerable alkali metal carbonate-based adsorbent using response surface methodology (RSM). Energy Fuel 31:5286–5296
Bezerra DP, da Silva FWM, de Moura PAS, Sousa AGS, Vieira RS, Rodriguez-Castellon E, Azevedo DCS (2014) CO2 adsorption in amine-grafted zeolite 13X. Appl Surf Sci 314:314–321
Casco ME, Martínez-Escandell M, Jn S-A, Rodríguez-Reinoso F (2014) Effect of the porous structure in carbon materials for CO2 gerccapture at atmospheric and high-pressure. Carbon 67:230–235
Chiang Y-C, Yeh C-Y, Weng C-H (2019) Carbon dioxide adsorption on porous and functionalized activated carbon fibers. Appl Sci 9:1977–1991
Deng S, Hu B, Chen T, Wang B, Huang J, Wang Y, Yu G (2015) Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption 21:125–133
Estalkhzir FM, Penchah HR, Gilani HG, Mikayilzadeh H (2018) Experimental data of citric acid extraction from aqueous solution with 1 - decanol by using liquid − liquid equilibrium. J Chem Eng Data 63:3227–3236
Etemad E, Ghaemi A, Shirvani M (2015) Rigorous correlation for CO2 mass transfer flux in reactive absorption processes. Int J Greenh Gas Cont 42:288–295
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Ghaemi A, Shahhosseini S, Maragheh MG (2009) Nonequilibrium dynamic modeling of carbon dioxide absorption by partially carbonate d ammonia solutions. Chem Eng J 149:110–117
Gholami M, Talaie MR, Aghamiri SF (2015) CO2 adsorption on amine functionalized MCM-41: effect of bi-modal porous structure. J Taiwan Inst Chem Eng:1–5
Giraldo L, Vargas DP, Moreno-Piraján JC (2020) Study of CO2 adsorption on chemically modified activated carbon with nitric acid and ammonium aqueous. Front Chem 8:543452
Hauchhum L, Mahanta P (2014) Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int J Energy Environ Eng 5:349–356
Y-S HO (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59:171–177
Huang X, Lu J, Wang W, Wei X, Ding J (2016) Experimental and computational investigation of CO2 capture on amine grafted metal-organic framework NH2-MIL-101. Appl Surf Sci 371:307–313
Ko SM, Schmidt LD (1974) Adsorption and solution of H2 and N2 by ta and Nb. Surf Sci 42:508–524
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I Solids J Am Chem Soc 38:2221–2295
Li Z, Zhong D-L, Lu Y-Y, Wang J-L, Qing S-L, Yan J (2016) Enhanced separation of carbon dioxide from a CO2 + CH4 gas mixture using a hybrid adsorption-hydrate formation process in the presence of coal particles. J Nat Gas Sci Eng 35:1472–1479
Lillo-Rodenas MA, Lozano-Castello D, Cazorla-Amoros D, Linares-Solano A (2001) Preparation of activated carbons from Spanish anthracite II. Activation by NaOH Carbon 39:751–759
Mohammad NK, Ghaemi A, Tahvildari K (2019) Hydroxide modified activated alumina as an adsorbent for CO2 adsorption: experimental and modeling. Int J Greenh Gas Control 88:24–37
Naeem S, Ghaemi A, Shahhosseini S (2016) Experimental investigation of CO2 capture using sodium hydroxide particles in a fluidized bed. Korean J of Chem Eng 33:1278–1285
Najafi P, Penchah HR, Ghaemi A (2020) Improving CO2 /N2 and CO2/H2 selectivity of hypercrosslinked carbazole-based polymeric adsorbent for environmental protection. J Chem Pet Eng 54:311–321
Najafi P, Penchah HR, Ghaemi A (2021) Synthesis and characterization of benzyl chloride-based hypercrosslinked polymers and its amine-modification as an adsorbent for CO2 capture. Environ Technol Innov 23:101746
Niknafs H, Ghaemi A, Shahhosseini S (2015) Dynamic heat and mass transfer modeling and control in carbon dioxide reactive absorption process. Heat Mass Transf 51:1131–1140
Norouzbahari S, Shahhosseini S, Ghaemi A (2015) CO2 chemical absorption into aqueous solutions of piperazine: modeling of kinetics and mass transfer rate. J Nat Gas Sci Eng 26:1059–1067
Penchah HR, Ghaemi A, Gilani HG (2019) Benzene-based hypercrosslinked polymer with enhanced adsorption capacity for CO2 capture. Energy Fuel 33:12578–12586
Penchah HR, Gilani HG, Ghaemi A (2020) CO2, N2, and H2 adsorption by hyper-cross-linked polymers and their selectivity evaluation by gas−solid equilibrium. J Chem Eng Data 65:4905–4913
Penchah HR, Ghaemi A, Gilani HG (2021a) Efficiency increase in hypercrosslinked polymer based on polystyrene in CO2 adsorption process. Polym Bull
Penchah HR, Ghaemi A, Godarziani H (2021b) Eco-friendly CO2 adsorbent by impregnation of diethanolamine in nanoclay montmorillonite. Environ Sci Pollut Res
Penchah HR, Najafi P, Ghaemi A, Gilani HG (2021c) Characterization of hypercrosslinked polymer adsorbent based on carbazole to achieve higher CO2 capture. Environ Prog Sustain Energy
Pino L, Italiano C, Vita A, Fabiano C, Recupero V (2016) Sorbents with high efficiency for CO2 capture based on amines-supported carbo for biogas upgrading. J Environ Sci 48:138–150
Rezakazemi M, Heydari I, Zhang Z (2017) Hybrid systems: combining membrane and absorption technologies leads to more efficient acid gases (CO2 and H2S) removal from natural gas. J CO2 Util 18:362–369
Ruthven DM, Reyes SC (2007) Adsorptive separation of light olefins from paraffins. Microporous Mesoporous Mater 104:59–66
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrolysis 89:143–151
Shafeeyan MS, Houshmand A, Arami-Niya A, Razaghizadeh H, Daud WMAW (2015) Modification of activated carbon using nitration followed by reduction for carbon dioxide capture. Bull Kor Chem Soc 36:533–538
Sreńscek-Nazzal J, Narkiewicz U, Morawski AW, Wróbel R, Gęsikiewicz-Puchalska A, Michalkiewicz B (2016) Modification of commercial activated carbons for CO2 adsorption. Acta Phys Pol A 129:394–401
Taheri FS, Ghaemi A, Maleki A, Shahhosseini S (2019) High CO2 adsorption on amine-functionalized improved mesoporous silica nanotube as an eco-friendly nanocomposite. Energy Fuel 33:5384–5397
Tan YL, Islam MA, Asif M, Hameed BH (2014) Adsorption of carbo n dioxide by sodium hydroxide-modified granular coconut shell activated carbon in a fi xed bed. Energy 77:926–931
Yong Z, Mata VG, Rodrigues AE (2001) Adsorption of carbon dioxide on chemically modified high surface area carbon-based adsorbents at high temperature. Adsorption 7:41–50
Zhang J, Burke N, Zhang S, Liu K, Pervukhina M (2014) Thermodynamic analysis of molecular simulations of CO2 and CH4 adsorption in FAU zeolites. Chem Eng Sci 113:54–61
Zhua X-L, Wanga P-Y, Peng C, Yang J, Yan X-B (2014) Activated carbon produced from paulownia sawdust for high-performance CO2 sorbents. Chin Chem Lett 25:929–932
Funding
All authors are grateful to their representative universities/institutes for providing experimental facilities and financial support.
Author information
Authors and Affiliations
Contributions
Hamid Ramezanipour Penchah: Supervision, formal analysis and investigation, methodology, software, validation, visualization, and writing review and editing. Ahad Ghaemi: Supervision and writing review and editing, methodology, software, visualization, writing original draft preparation. Fatemeh Jafari: Conceptualization, data curation, formal analysis and investigation, methodology, software, validation, visualization, writing original draft preparation, and writing review and editing.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramezanipour Penchah, H., Ghaemi, A. & Jafari, F. Piperazine-modified activated carbon as a novel adsorbent for CO2 capture: modeling and characterization. Environ Sci Pollut Res 29, 5134–5143 (2022). https://doi.org/10.1007/s11356-021-16040-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16040-5