Abstract
The microbial fuel cell (MFC) system is a promising environmental remediation technology due to its simple compact design, low cost, and renewable energy producing. MFCs can convert chemical energy from waste matters to electrical energy, which provides a sustainable and environmentally friendly solution for pollutant degradations. In this review, we attempt to gather research progress of MFC technology in pollutant removal and environmental remediation. The main configurations and pollutant removal mechanism by MFCs are introduced. The research progress of MFC systems in pollutant removal and environmental remediation, including wastewater treatment, soil remediation, natural water and groundwater remediation, sludge and solid waste treatment, and greenhouse gas emission control, as well as the application of MFCs in environmental monitoring have been reviewed. Subsequently, the application of MFCs in environmental monitoring and the combination of MFCs with other technologies are described. Finally, the current limitations and potential future research has been demonstrated in this review.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
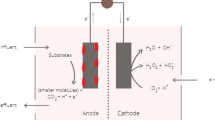
The microbial fuel cell (MFC) system is an emerging environmental treatment technology that integrates microbial and electrochemical processes to generate energy by oxidizing organic matter. MFCs can degrade contaminants under mild and clean reaction conditions and have some the advantages of microbial and electrochemical methods. Compared with other technologies, MFCs consume much less energy and generate electricity while degrading pollutants (Zhang et al. 2016). MFCs can remove a variety of pollutants, including refractory organics, heavy metals, and inorganic non-metals. The main mechanism of pollutant degradations by MFCs are as follows: (1) anodic oxidation—pollutants such as cellulose are degraded as electron donors; (2) anode reduction—pollutants receive electrons at the anode; (3) adsorption—pollutants are adsorbed on the electrode or biofilm; (4) electrodynamics—the electric field generated by MFCs affects the chemical form and distribution of pollutants; (5) cathode alkalization—when the proton is consumed in the cathode, the acidity/alkalinity increases; (6) cathode reduction—NO3−, NO2−, and high-priced metals, etc. obtain electrons from the cathode; and (7) power source—MFCs are used as a power source to drive an electrochemical system to remove pollutants (Wu et al. 2018). At the anode of MFC, many microorganisms can transfer electrons generated by pollutant metabolism to the electrode. There are four possible ways to transfer electrons: (1) metabolic intermediates, (2) redox mediators, (3) conductive nanowire, and (4) periplasmic and membrane-bound cytochromes (He et al. 2017). Some microorganisms can act as electron acceptors and use electrons from electrodes to reduce pollutants, for instance, Methanobacterium palustre was able to accept electrons directly from the electrodes and reduced dehalogenated 2-chlorophenol to pheno (Lovley 2012), and Geobacter metallireducens could also reduce nitrate to nitrite with electrodes acting as the electron donors (Shrestha and Rotaru 2014).
In recent years, various innovative MFCs have been developed as solutions for pollutant removal and environmental restoration, including (1) single-chamber MFC, (2) H-type dual-chamber MFC, (3) cube dual-chamber MFC, (4) SMFC (sediment MFC), and (5) stacking MFC (Fig. 1). In order to systematically introduce MFC technology, the research status of MFCs in environmental restoration was reviewed. This study mainly introduced the latest research of MFCs in pollutant removal and environmental remediation, including wastewater treatment, soil remediation, natural water and groundwater remediation, sludge and solid waste treatment, and greenhouse gas emission control, as well as the application of MFCs in environmental monitoring (Fig. 2).
Microbial fuel cells for wastewater treatment
Domestic sewage
MFCs can be used to treat domestic sewage (Table 1). By reducing the electrode distance, the internal resistance of MFCs can be reduced and the performance of MFCs can be improved. When the electrode distance decreased from 4 to 2 cm, the internal resistance decreased from 161 to 77 Ω, and the maximum power density increased by 68% (Liu et al. 2005). Influent chemical oxygen demand (COD) has little effect on the removal rate of organic matter in domestic sewage but has a greater effect on electricity generation. The influent COD increased from 25 to 300 mg/L; the higher the COD, the better the power generation effect of MFCs. When COD was 300 mg/L, the maximum power density of 25 mW/m2 was obtained (Rodrigo et al. 2007).
There have been attempts at the long-term use and expansion of MFCs for domestic sewage treatment. For example, after stable operation of the system for 60 days, the real domestic sewage flow rate was 600 ± 100 L/day, and the removal rates of COD, biochemical oxygen demand (BOD), and total suspended solids were 86%, 87%, and 95%, respectively. The removal rates of total nitrogen (TN) and total phosphorus (TP) were 84% and 64% (Valladares Linares et al. 2019). A 1000 L modular MFC system was constructed and operated in a municipal sewage treatment plant for more than 1 year to deal with the actual municipal sewage. In the study with low (average of 80 mg/L) and high (average of 250 mg/L) influent COD, the effluent COD was maintained below 50 mg/L and the removal rate was 70–90% (Liang et al. 2018). With the deepening of research, MFCs are expected to be used in actual domestic sewage treatment.
Livestock wastewater
The contents of organic matter and N in livestock wastewater are relatively high, and they produce an odor. Research has shown that the odor of animal wastewater is related to volatile organic acids (Castillo-Gonzalez and Bruns 2005). In the treatment of animal wastewater by MFCs, 99.76% of odor-related chemicals were removed while power was generated (Kim et al. 2008). With the increase of HRT, the removal rates of COD in wine wastewater increased. The COD removal rates at HRT 13, 14, and 20 days were 71, 73, and 83%, respectively (Ma et al. 2016). Pretreatment improved the power generation and comprehensive treatment efficiency of wastewater. After sonicated and autoclaved, the wastewater generated 16% more power (110 ± 4 mW/m2) than before treatment (96 ± 4 mW/m2). The COD removal was increased from 88 to 92% by stirring diluted wastewater (Min et al. 2005).
The feasibility of MFC technology in the long-term and expanded treatment of swine wastewater was evaluated. Two MFCs with a total volume of 115 L were used to treat pig manure wastewater for more than 6 months. Graphite and stainless steel mesh were used as electrodes, respectively, and achieved similar removal rates of COD and N (1.9 ± 0.3 kg/(m3 day) and 0.35 ± 0.02 kg/(m3 day), respectively). However, over time, the graphite electrode was broken and blocked, which reduced the applicability of the electrode. In contrast, stainless steel electrodes have the advantages of high efficiency, low price, and good applicability. This type of electrode material can be used to expand the scale of MFCs (Vilajeliu-Pons et al. 2017).
Industrial wastewater
Industrial wastewater has a complex composition and is difficult to degrade, such as dye wastewater, coal gasification wastewater, vinasse wastewater, and food processing wastewater. MFCs recently become popular in industrial wastewater treatment. Ottoni used a two-chamber MFC to generate electricity and treat vinasse wastewater. The MFC resulted in a maximum current density of 1200 mA/m2 and a power density of 800 mW/m2 within 61 days, while achieving a COD removal rate of 60% (Ottoni et al. 2019). In addition to bioelectrochemical effects, MFCs also are used as a power source to degrade pollutants in wastewater. Using MFCs as a power source, the decolorization rate of dye wastewater reached up to 90.4% after 6 h of aeration. However, when only aeration was conducted without applying voltage, the decolorization rate had little effect (Zhang et al. 2015).
Removal and recovery of metal ions
Unlike organic pollutants, heavy metals are nonbiodegradable and persistent in wastewater. The removal of metals is related to the electrodes, hydraulic retention time (HRT), and electron acceptors. Catalysts improve the performance of MFCs. Compared with the 55.3 ± 1.8% removal rate of Cu(II) by graphene oxide-modified carbon cloth, the removal rate of an electrode modified with reduced graphene oxide increased to 98%. This may have been due to the fact that reduced graphene oxide provides a large adhesion area for bacteria and enhances the electron transfer of cathode biofilms (Wu et al. 2019). In the anode chamber, Pt was recovered from wastewater with a content of less than 16.88 mg/L. The recovery rate of Pt was close to 40%, and the longer the reaction time, the higher the recovery efficiency (Liu et al. 2019d). As an electron acceptor of MFCs, O3 improved the removal rate of metal ions. When O2 was used as an electron acceptor, the removal rates of Cr and Pb were 91.8% and 88.5%, respectively. When O3 was used as an electron acceptor, the removal rates increased to 99.2% and 98.4%, respectively (Gholizadeh et al. 2018).
Removal of antibiotics
Due to the hydrophilicity and stable structure of antibiotics, the traditional treatment process cannot effectively remove antibiotics from water. However, biodegradable antibiotics have been proven to be feasible in recent studies, and MFCs are considered a promising method to degrade antibiotic contaminants (Zhang et al. 2019a). There are three main mechanisms of antibiotics removal by MFCs, as follows: (1) antibiotics act as an electron acceptor or C source in the bio-anode; (2) antibiotics are degraded by microorganisms in the cathode or directly reduced by an electrochemical reaction; and (3) antibiotics are degraded by free radicals produced by cathode-modified materials (Yan et al. 2019).
The effect of the initial concentration of antibiotics on its degradation rate in MFCs is interesting. Studies have shown that the higher the concentration of antibiotics (200 ppm), the lower the degradation rate (70%) (Wang et al. 2015b). However, other studies reported that the degradation rate (from 88 to 96%) increased with the increase in antibiotic concentration (from 30 to 50 mg/L) (Wen et al. 2011). In addition, it was reported that the performance of MFCs did not change with the increase in the initial concentration of antibiotics (from 80 to 450 mg/L) (Zhang et al. 2018a), which provided a scientific basis for the treatment of high-concentration antibiotic wastewater by MFCs. This may be related to the different structures and properties of antibiotics and their effects on degrading microorganisms.
Microbial fuel cells for soil remediation
Removal of hydrocarbons
MFCs can greatly promote the removal of refractory organic matter in soil (Table 2). They have the advantages of simple structure, low energy consumption, little damage to soil structure, and little influence on microorganisms (Cao et al. 2015). Difficulties in mass transfer in contaminated soil, including substrate bioavailability and electron transport, limit the biodegradation efficiency of soil MFCs. Biochar has good electrical conductivity and is used as a sustainable electrode material in MFCs. Three types of biochars with a mass ratio of 2% were filled in the soil. After 223 days of operation, the removal rate of TPHs was 17% higher than that without biochar (Li et al. 2019). Soil properties are the determinants of hydrocarbon bioremediation. When the soil water content decreased from 33 to 28%, the internal resistance increased by 46%, thereby resulting in a decrease in the hydrocarbon degradation rate (Wang et al. 2012a).
The pollutant concentration and external resistance also affect the removal efficiency. Some studies have found that with the increase in the hexachlorobenzene (HCB) concentration in soil, the degradation efficiency of pollutants increased, but the removal rate decreased. When the external resistance was 2000, 1000, 510, and 10 Ω, the removal rate of HCB was 57.5%, 61.18%, 62.9%, and 71.15% after 56 days, respectively (Cao et al. 2015). Small external resistance induced a larger current in soil MFCs and the degradation rate of HCB accelerated with the increase in the current. This may have been due to (1) the electrons produced by electrogenic bacteria increasing the metabolic reaction rate of anaerobic bacteria (Li et al. 2010); (2) the electric field changing the permeability of cell membranes, thereby leading to excessive absorption of extracellular substances and changing the metabolism of microorganisms; and (3) the electric field influencing some enzymes in electrogenic bacteria to promote an organic removal reaction (Pitts et al. 2003).
Remediation of heavy metals pollution
Bioremediation of heavy metals is more difficult than that of organic pollutants because the latter can be oxidized to CO2 and water, but heavy metals can only be converted into less toxic forms or immobilized to reduce their bioavailability. Paddy field soil is rich in Fe oxides. After being submerged, Fe(OH)3 acts as an electron acceptor owing to the role of Fe-reducing bacteria, thereby resulting in Fe and As release, an increase in the bioavailability of As, and subsequent accumulation of As in rice, which endangers the environment and human health. MFCs were implanted into paddy soil, and the release of Fe and As into soil pores was significantly reduced by using an anode as an electron acceptor (Gustave et al. 2018).
MFCs can also drive electric remediation of toxic metal-contaminated soil. Under the action of an electric field, metal ions with positive charge migrate from the anode to the cathode. After MFCs treatment, 25% and 18% of Zn and Cd in the soil near the anode were removed, respectively (Chen et al. 2015). Soil pH is one of the key factors affecting the distribution and migration of metals. At low acidity/alkalinity, metals mainly exist in the form of ions in soil pore water, and the migration effect is greater. The increase in acidity/alkalinity leads to heavy metal precipitation and reduces the migration of metals (Al-Hamdan and Reddy 2008). It should be noted that the reduction in water content hinders the migration of heavy metals. With the decrease in soil water content, the internal resistance improves, thereby affecting the power generation of MFCs and hindering the migration of heavy metals (Habibul et al. 2016).
Sediment microbial fuel cells for natural water remediation
Sediment remediation
Degradation of organic matter in sediments
SMFCs consist of an anode embedded in anaerobic sediment and a cathode suspended in an aerobic water column. The use of SMFCs is an effective means to degrade organic pollutants in sediments. However, low conductivity of sediments is an important limiting factor for organic removal. Adding biochar to the sediment improved the conductivity, and the removal rate of TOC was about 4 times higher than that of SMFC without biochar (Chen et al. 2016a). The combination with aquatic plants improved the degradation efficiency of organic matter in sediments. After 367 days of operation, the degradation rates of pyrene and benzopyrene by SMFCs were 55.73 ± 5.65% and 47.20 ± 8.32%, respectively. The combination of calamus and SMFCs resulted in at least a 70% higher degradation rate of pyrene and benzopyrene compared with that of a single SMFC (Yan et al. 2015), which may have been related to the complex aerobic-anaerobic environment of plant roots (Hodge et al. 2009).
Phosphorus immobilization in sediments
SMFCs can affect the form of P in sediments. After 50 days of treatment, metal-bound P (14 to 11%), Ca-bound P (26 to 23%), and refractory P (33 to 28%) increased, which helped to maintain the stability of P in sediments (Martins et al. 2014). The anode of SMFCs is embedded in anaerobic sediments, and the mechanism of controlling P in sediments may be as follows: (1) biodegradation of organic P; (2) acting as an electron acceptor, which prevents the dissolution of Fe, Al, and Ca compounds that adsorb P; and (3) increasing the activity of polyphosphate organisms in sediments (Xu et al. 2018).
Nitrogen removal from sediments
N release from sediments is an important pollution source in the overlying water of aquatic ecosystems. SMFCs are used to reduce the N content in sediments. SMFCs transfer and transform N in sediments through the following mechanisms: (1) NH4+ acts as an electron donor for power generation; (2) NH4+ is synthesized by nitrifying bacteria, which are used by heterotrophic organisms for power generation (He et al. 2009); (3) NH4+ in pore water is released to the overlying water under the action of electromigration; and (4) mineralization of organic N.
Heavy metal removal from sediments
SMFC can fix and remove metal ions in sediments by via electrokinetic processes or changing the redox state of metal ions. Under the action of electric field, the metal ions in the sediment migrate to the overlying water. In Kabutey’s study, it was found that the Cd content in the sediment decreased and the Cd in the overlying water increased (Kabutey et al. 2019). Some microbes can receive electrons from electrodes, and they are used to remedy heavy metals through reduction (Thrash and Coates 2008). For example, Geobacter sulfurreducens can reduce soluble U(VI) into insoluble U(IV) form, which is adsorbed by the electrode surface (Abbas et al. 2017b). Cathodic aeration improved the reduction effect of metal ions in sediment. After 60 days, the aerated SMFC reduced the maximum amount of Cr(VI) to Cr(III) ions (80.70%) and Cu(II) to Cu(I) ions (72.72%) (Abbas et al. 2018). Temperature and pH have important effects on the performance of SMFC. Normally, bacteria obtain the best toxic metal removal effect under neutral pH. At low and high temperature, few toxic metals are removed. Previous research reports pointed out that bacteria can remove toxic metals to the maximum extent at 30 to 45 °C (Abbas et al. 2017a).
Remediation of overlying water
Phosphorus removal from overlying water
At present, the main method of removing P from water is the adsorption and precipitation of soluble P by minerals (such as Fe hydroxide) at the sediment-water interface (Yang et al. 2016). SMFCs form internal ion currents in water and sediment systems, such as H + and PO43−, which increase the P flux from the overlying water to sediment, and are expected to be used for eutrophication control. In one study, SMFCs reduced the TP content in the overlying water from 0.1 to 0.01 mg/L. This may have been due to the electromigration of phosphate (Wolf et al. 2002), in which PO43− flowed downward to the interface between the water and sediment to adsorb or deposit on the surface of some metal (Al, Ca, and Fe) salts in the sediment. With the increase in initial PO43− concentration, the migration from overlying water to sediment improved, even though the removal rate decreased (Xu et al. 2018).
Nitrogen removal from overlying water
In SMFCs, NO2−-N and/or NO3−-N is used as the electron acceptor of the cathode, thereby reducing the contents of NO2−-N and NO3−-N in the overlying water (Virdis et al. 2008). Acidity/alkalinity, electrode distance, and external resistance are the key factors affecting the removal of TN in overlying water. The removal rate of TN improves with the increase in acidity/alkalinity and electrode distance, and decreases with the increase in external resistance (Sajana et al. 2014). Excessive O2 in the overlying water resulted in a significant decrease in N removal efficiency, but the N removal rate improved with the increase in the organic matter concentration in the sediments (Zhang and Angelidaki 2012a).
Removal of heavy metals from overlying water
In the SMFC system, electrons generated by degradation of organic substances in the sediment are transmitted to the cathode through wires, and the metal ions can become terminal electron acceptors. Li’s research pointed out that when Cu(II) in the overlying water was less than 3 mg/L, it was found that Cu(II) was reduced to metallic copper at the cathode and the power generation of SMFC was improved (Li et al. 2017). The mechanism by which SMFC removes metals from overlying water is not only bioreduction but also biosorption and bioaccumulation. In Wu’s study, Hg(II) and Ag(I) in overlying water reached removal rates of 25% and 35%, respectively, under open-circuit conditions, which was mainly due to the effects of biosorption and bioaccumulation (Wu et al. 2017).
Microbial fuel cells for groundwater remediation
Refractory organic compounds
Refractory organic compounds in groundwater include aromatic compounds and chlorinated hydrocarbons, whose existence is mainly attributed to human pollution. Because of the lack of electron acceptors, these substances exist in the environment for a long time. Studies have shown that benzene is anaerobically degraded in the anode chamber of MFCs, which provides the possibility for MFCs to remove organic pollutants from groundwater (Rakoczy et al. 2013). The composite anode improved the removal rate of toluene. The performance of the composite anode made of conductive coke and conductive carbon black was better than that of a single anode, and the removal rate (88.2%) of toluene was the highest at 1:3. This may have been due to the large porosity of coke and the high specific surface area of carbon black, which were beneficial for microorganisms to adhere to the surface of the anode (Liu et al. 2019c). Stacked MFCs improve the removal rate of organic matter. When three MFCs were connected in a series, the removal rate of benzene was 19.6% higher than that of a single MFC. The removal rate by parallel MFCs was better than that of a series of MFCs. The performance of three parallel MFCs (removal rate of 92.7%) was much higher than that of a series of MFCs (removal rate of 75.5%) (Chang et al. 2017).
Metal contaminants
The objective of groundwater treatment with metal contaminants is to change the metal ions to a state of low toxicity and/or solubility, and then remove them by precipitation or adsorption. For example, Geobacillus can reduce soluble U(VI) to relatively insoluble U(IV) by using organic compounds as electron donors, thus effectively removing U from contaminated groundwater (Gregory and Lovley 2005). In microbial metabolism, As(III) can be converted into less toxic As(V) by using electrodes as direct electron acceptors (Pous et al. 2015). Hao applied bioelectricity generated by MFC directly to a bioelectrical reactor to promote microbial reduction of V(V) in groundwater. V(V) acted as an electron acceptor, and V(V) with high toxicity was reduced to V(IV), and the removal rate of V(V) reached 93.6%. When the output voltage of MFC improved from 200 to 700 mV, the removal efficiency of V(V) increased with the improved of the voltage (Hao et al. 2015). The electrochemical system based on MFC power supply provides an efficient and economical method for controlling metal pollution in groundwater. Under the 600 mV provided by MFCs, the oxidation rate of Tl(I) reached nearly 80.5% within 4 h of operation, and Tl(I) was gradually oxidized into Tl(III) that was easy to precipitate, which was more conducive to the removal from groundwater (Tian et al. 2017).
Nonmetallic inorganic pollutants
In recent years, MFC system has made remarkable achievements in the removal of nitrate from groundwater. With acetate as the electron donor of the anode chamber, groundwater was injected into the cathode chamber of MFCs, and 64% of NO3−-N was removed (Pous et al. 2013). Four different volumes of sand/water (0, 10, 50, and 100%) were added into two chambers of the MFCs to simulate the working condition of the denitrification bioreactor in an aquifer. The denitrification time was 15, 25, 24, and 20 days, respectively. The results showed that the reduction efficiency of NO3−-N was related to the sand/water ratio, and the biological community was slightly different under different sand/water ratios, which might have been related to the conditions of the liquid phase circulation in the cathode chamber (Van Khanh et al. 2016).
Nitrate loading, COD, and HRT all affect the removal of nitrate. With the increase in nitrate loading, the concentration of NO3−-N in effluent increases. Contrary to the effect of nitrate loading, the NO3−-N content in wastewater is reduced with the increase in COD concentration. When the HRT is longer, the bacteria have enough time to denitrify. With the gradual shortening of the HRT, the nitrate removal rate in groundwater decreases owing to the lack of denitrification time (Liu et al. 2016).
Microbial fuel cells for sludge and solid waste treatment
Sludge treatment
Sludge treatment and disposal have become difficult and expensive problems. MFC provides a promising method for sludge treatment. MFC improves the degradation efficiency of organic matter in sludge, mainly because the electric field generated by MFC has an impact on microbial community. On the one hand, electric field can change the permeability of cell membrane, leading to excessive absorption of extracellular material, and then change the metabolism of microorganisms. On the other hand, the electric field acts on some enzymes in the bacteria to promote the removal of organic compounds (Xin et al. 2019). The degradation of 2,4-dichlorophenoxyacetic acid were studied by electro biological coupling system, and it was found that the electron transfer was controlled by the surface diffusion, while the electric field can provide more electrons and accelerate the electron transfer rate, which is conducive to the degradation of pollutants by microorganisms (Zhang et al. 2013).
Electrode distance, water content, and temperature all affect the degradation of organic matter in dewatered sludge. Adding the distance between the electrodes leads to a longer time for protons to transfer from the anode to the cathode, which improves the internal resistance of MFCs and reduces the degradation rate of organic matter. The internal resistance can be reduced by decreasing the electrode spacing. However, in single-chamber MFCs, electrode distances that are too short allow O2 from the cathode to more easily diffuse to the anode, which is detrimental to the performance of MFCs. Low water content of dewatered sludge reduces the microbial activity and proton transfer rate (Wang et al. 2015a). Ideal humidity allows bacteria to more easily consume organic matter and is conducive to the hydrolysis of organic compounds (Liang et al. 2003). The increase in temperature contributes to the diffusion of organic matter from dewatered sludge to electrogenic bacteria, and also promotes the growth rate of bacteria and the rate of biochemical reactions in cells (Larrosa-Guerrero et al. 2010). However, excessive temperature damages the protein and nucleic acid in cells, thereby resulting in cell death (Liu et al. 2005).
Solid waste treatment
Low-value organic matter in municipal solid waste is a potential source of electronic donors for MFCs. Different combinations of solid wastes have an effect on organic matter removal and power generation. The highest COD removal rate (78%) and the highest Coulomb efficiency (24%) were observed when food wastes, cardboard wastes, and garden wastes were mixed at a ratio of 1:1:1. This may have been related to the influence of different types and proportions of solid wastes on the diversity of microorganisms in the anode (Pendyala et al. 2016). The research showed that there was a linear relationship between temperature and the maximum power generation of MFCs, which may have been related to the decrease in internal resistance with the increase in temperature. The effect of temperature on the removal of organic matter is relatively small. With the temperature increased from 20 to 35 °C, the removal efficiency of COD increased slightly from 45.7 to 52.8%, respectively (Karluvah et al. 2015). As the temperature decreased from 30 to 20 °C, the removal rate of COD only decreased from 87 to 85%, respectively (Feng et al. 2008).
Landfilling is the main form of solid waste management. Landfill leachate is the liquid emitted from landfill systems. The organic load of leachate is relatively high and difficult to treat. MFCs were used to treat landfill leachate without additional energy input. After 52 days of operation, the removal rates of BOD, TOC, and NH4+-N reached 74%, 27%, and 25%, respectively (Damiano et al. 2014).
Microbial fuel cell control of gas emissions
Paddy fields and wetlands are the main sources of CH4 emissions. MFCs are used to control CH4 emissions because the electrogenic bacteria on the anode compete with methanogens for organic substrates, thereby reducing CH4 production (Arends et al. 2014). The application of MFCs reduced the emissions by 17.9–36.9% for CH4, 7.2–38.7% for N2O, and 5.9–32.4% for CO2 from constructed wetlands (CWs). With the increase in external resistance (over 500 Ω), the CH4 and N2O emissions of the CW-MFC increased significantly, while the CO2 emissions decreased. With the increase in organic load, the emissions of CO2 and CH4 increased and the emissions of N2O decreased, which may have been related to the effect of organic load on microbial nitrification and denitrification (Wang et al. 2019).
Biochar has the characteristics of good stability, high porosity, and large surface area. There are very different research results on the effect of biochar on CH4 emissions in paddy fields, as follows: (1) incorporating biochar into paddy fields reduced the CH4 emissions in the rice growth cycle; (2) adding biochar had no effect on CH4 emissions (Xie et al. 2013); and (3) adding biochar increased CH4 emissions in paddy fields (Zhang et al. 2012). However, biochar as the anode of MFCs seems to reduce CH4 emissions from rice paddies. Studies have shown that the CH4 emissions of biochar anodes are 39% lower than those of carbon felt anodes (Khudzari et al. 2019).
Microbial fuel cells for environmental monitoring
In recent years, with the gradual development of MFCs in chemistry, electrochemistry, and microorganisms, they have been widely used in the field of environmental monitoring. MFCs are used as energy supply devices to provide the required power for remote sensors, and are also used as self-powered biosensors to detect environmental pollutants (Huang et al. 2011).
Because of the sustainability of power generation, MFCs are used as a long-term alternative power supply for remote monitoring sensors in remote areas. As a power supply for sensors, MFCs need sufficient power output and stable potential. The use of supercapacitors and DC/DC converters contribute to solve these two problems (Donovan et al. 2011). Through a power management system, MFCs operate sensors and integrate a telemetry system to transmit remote signals wirelessly (Kim et al. 2007). Under natural conditions, there are several factors that affect the power and potential output of MFCs, such as temperature, acidity/basicity (Raghavulu et al. 2009), the presence of toxic substances and inhibitors. With the increase in temperature, the resistivity of the solution decreases and microbial metabolism and membrane permeability are enhanced, which improves the power output of MFCs. However, some microorganisms achieve optimal performance at low temperatures, which contributes to the application of MFC-driven sensors in cold regions (Vazquez-Larios et al. 2010). The acidity/alkalinity affects the activity of microorganisms. Most MFCs operate best under neutral conditions (Oliveira et al. 2013).
When MFCs are used as self-powered sensors for in situ online environmental monitoring, they can monitor a variety of pollutants, including BOD, heavy metals (Hg, Pb, Cr), bentazon, formaldehyde, DO, and p-nitrophenol (Table 3). MFCs use electroactive microorganisms as probes, and the existence or change in the target analyte level affects the electron transfer process of microorganisms, thereby generating electrical signals. By focusing on the changes in battery output under different environmental conditions, the purpose of pollutant monitoring can be achieved (Abrevaya et al. 2015). MFC sensors provide a potential alternative method for monitoring pollutants, but also face several challenges. These include limited selectivity, low recognition limit, and high potential contamination of other strains. Further research is required to determine how to eliminate the above challenges and improve the application ability of MFC-based biosensors (ElMekawy et al. 2018).
Integration of microbial fuel cells with other technologies
Integration with anaerobic digestion
Anaerobic digestion technology has been widely used in organic waste treatment and energy recovery. Anaerobic digestion is suitable for the treatment of high contents of organic matter above 30 °C, but at low concentrations of substrate and low temperatures, the effect is not adequate. MFCs have good performance under low COD and low temperature (10–20 °C), which allows the integration of MFCs and anaerobic digestion to have greater pollutant removal efficiency (Pham et al. 2006).
An upflow anaerobic sludge bed reactor-microbial fuel cell-biological aerated filter (UASB-MFC-BAF) system was developed to treat high-concentration molasses wastewater. The removal rates of COD, sulfate, and chroma were 53.2%, 52.7%, and 41.1%, respectively. In this system, the up-flow anaerobic sludge bed (UASB) reactor was mainly responsible for removing COD and reducing sulfate, while the MFC unit produced electricity by oxidizing sulfide to elemental S (Zhang et al. 2009). Embedding MFCs in an anaerobic-anoxic-oxic (A2/O) process is conducive to solving the problem of substrate competition and high energy consumption in the A2/O process. An anaerobic tank was used as the anode chamber of the MFC, while an anoxic cell was used as the cathode chamber. The MFC-A2/O reactor generated electricity continuously. Compared with the control, the removal efficiencies of COD, TN, and TP increased by 15.9%, 9.3%, and 1.4%, respectively (Xie et al. 2014). An anaerobic fluidized bed reactor (AFB) was used as the anode chamber of the MFC (Fig. 3). A combined system of AFB and MFC was designed. After stable operation, the COD removal rate of alcohol wastewater was 80–90%. Moreover, a longer HRT resulted in a higher COD removal rate (Huang et al. 2011).
Integration with ecological treatment technologies
The rhizosphere of plants can release O2 and organic matter. The large surface area of plant roots and the complex aerobic-anaerobic environment are conducive to the growth and attachment of microorganisms (Hodge et al. 2009). A plant-SMFC system was constructed by combining Acorus calamus and a SMFC. Within 367 days, the degradation rate of pyrene and benzopyrene in shallow lake sediments was at least 70% higher than that of a single SMFC (Yan et al. 2015). Plants also absorbed heavy metals. A Plant-SMFC including Pennisetum removed 35% of Cr from soil, of which 95% was absorbed by plants; the contents of Cr in roots and stems were the highest at 44.7% and 37.9%, respectively (Guan et al. 2019a). PMFCs are widely used in wastewater treatment (Fig. 3). A duckweed-MFC reactor achieved a 71% removal rate of B from wastewater. The COD, NH4+-N, and PO43− removal rates were 84%, 81%, and 76%, respectively (Turker 2018).
The constructed wetland (CW) system is a wastewater treatment technology that effectively removes many pollutants. MFCs can utilize the natural redox gradient in CWs. Combining MFCs with CWs improves the efficiency of wastewater treatment and obtains additional energy output (Hartl et al. 2019). Temperature is the key factor for pollutant removal. The COD of CW-MFC effluent in summer was lower than that in winter because the microbial activity was higher in summer. Up-flow microbial fuel cell-coupled constructed wetland (UCW-MFC) was used to treat high-concentration pharmaceutical wastewater. It was found that closed-circuit operation was more effective than open-circuit operation. Compared with those in open-circuit mode, the removal rates of ibuprofen and bisphenol A increased by 9.3% and 18%, respectively, and when the HRT was decreased from 16 to 4 h, the removal rates decreased by 14.6% and 23.7%, respectively (Liu et al. 2019b). DO in the cathode area is of great significance to the removal of organic matter and N in CW-MFCs. With the increase in DO concentration from 1.0 to 2.0 mg/L, the average removal rate of COD increased from 76.54 to 87.00%, respectively. However, when the DO concentration was 1.5 mg/L, the average removal rate of TN was the highest (84.40%), which was 13.75%, 2.62%, 6.32%, and 9.68% higher than that of 1.0, 2.0, 2.5, and 3.0 mg/L, respectively (Liu et al. 2019a).
Algae can absorb CO2 and release O2 through photosynthesis. Algae were introduced into the cathode of a MFC to form a photosynthetic algae microbial fuel cell (Fig. 3). Meanwhile, the power generation and wastewater treatment capacity of MFCs were improved. By fixing Chlorella in the cathode, a new type of microbial C capture battery was constructed, which achieved zero CO2 emissions and an 84.8% COD removal rate in wastewater treatment (Lakaniemi et al. 2012). A MFC with a Chlorella-modified cathode was constructed to treat livestock wastewater. Compared with those of a MFC without Chlorella modification, the removal rates of NH4+-N, TN, and TOC in the anode chamber were increased by 3.0%, 25.4%, and 7.7%, respectively. The removal rate of NH4+-N in the cathode chamber (68.7%) was also much higher than that in the MFC (47.5%). This indicated that algae could improve the performance of MFCs in wastewater treatment (Zhang et al. 2019b). Light affects the performance of algal MFCs. Under high light intensity, photosynthetic activity in the cathode improved, the MFC output voltage increased, and the consumption rate of organic matter in the anode chamber increased (Gouveia et al. 2014).
Integration with membrane bioreactor
A membrane bioreactor (MBR) is a wastewater treatment technology that combines the activated sludge process and membrane filtration technology (Meng et al. 2017) and achieves high pollutant removal efficiency and complete biomass retention. However, during the operation of MBRs, suspended particles (microorganisms and cell debris), colloids, solutes, and sludge flocs are deposited on the membrane surface and/or in the membrane pore, thereby resulting in the blockage of membrane pores and a decrease in membrane flux (Meng et al. 2009). Studies have shown that additional weak current fields effectively control membrane fouling (Akamatsu et al. 2010), which provides a possibility for the combination of MFCs and MBRs (Fig. 3). The electric field generated by MFC can change the characteristics of sludge, reduce core foulants, and improve the activity of microorganisms. It can also change the composition of bacteria species and increase the particle size of flocs. It can improve the performance of sludge flocs by accelerating the growth of filamentous bacteria, thus slowing down membrane pollution and prolonging the filtration period. The main fouling on the membrane surface consists of negatively charged sludge flocs, microorganisms, and their metabolites. Under the bioelectric field, the fouling moves away from the membrane surface and reduces the fouling adhesion rate. Therefore, MFC-MBR coupling system can effectively control membrane fouling (Liu et al. 2018).
Influent load is a key factor affecting the control of membrane fouling in MBRs by MFCs. At the low load stage, MFCs play a negative role, while at the high load stage, the electric field generated by MFCs contributes to reduce membrane fouling (Wang et al. 2018). The initial concentration of mixed liquid suspended solids (MLSS) has a serious impact on the power generation and membrane fouling of MFC-MBR systems. With the increase in the MLSS concentration, the rate of membrane fouling improves. With the decrease in the MLSS concentration and the increase in DO, power generation increases. The combined MFC-MBR system not only reduces the MBR membrane fouling but also improves the wastewater treatment effect (Su et al. 2013). The MBR aeration tank was directly used as the MFC cathode chamber, which was conducive to the better utilization of O2 and the improvement in effluent quality. At the same time, it was expected that the energy consumption of the MBR process would be offset by MFC power generation to achieve more sustainable wastewater treatment (Wang et al. 2012b).
Integration with Fenton reaction
The Fenton reaction was first discovered by Fenton in 1894. Hydroxyl radicals produced by the reaction of H2O2 with Fe2+ are highly oxidative and have good removal effect on refractory organic pollutants in wastewater. In the past few years, Fenton reaction has been used in wastewater treatment. However, the traditional Fenton process is still costly, energy-intensive, and requires additional unstable reagents (Li et al. 2018a).
The protons and electrons generated by the MFC anode chamber enter the cathode chamber through the membrane and external circuit, and then they can react with O2 to form H2O2 in the cathode chamber, which makes it possible to combine MFC with Fenton reaction. MFC and Fenton reaction constitute a bio-electro-Fenton system, which has the following advantages: (1) no need for external electric energy input; (2) refractory organic pollutants can be removed in the cathode chamber; (3) biodegradable organic matter can be removed in the anode chamber. Fe2+ needed for Fenton reaction can be supplied by composite cathode, which can reduce the excessive addition of metal and the content of impurities in water (Kahoush et al. 2018). Some methods are needed to make more iron loaded on the cathode surface to improve the sustained release capacity of iron ions. For example, after oxidizing carbon felt with nitric acid, the highest iron load reached 11.02% (Ling et al. 2016). MFC can also be used to power the electro-Fenton system, which will save energy consumption and operating costs. However, the low-voltage output of MFC is a key limitation of large-scale operation.
Current limitations and future outlook
There are still many challenges to overcome before MFCs can be applied to the real environment. There are few studies on the long-term operation of MFCs. The execution time of MFCs for pollutants removal is usually short. Improving long-term operation stability is the key to the full field application of MFCs. In addition, most of the current research on MFCs is at the laboratory scale, and the actual application needs to be expanded. One way to achieve this is to enlarge the size of a single MFC, which will lead to the improvement in reactor internal resistance; another method is to use a stacking system to increase the total reactor volume, which will lead to complex interactions between stacking units. It is easy to cause additional losses owing to voltage reversal. Cost issues also need to be considered; for example, Pt or graphene-modified electrodes improve the performance of MFCs, but their prices are relatively high. In dual-chamber MFCs, the use of ion-exchange membranes increases the cost of MFCs. Cost reduction is an important condition for the practical application of MFCs.
In order to promote the sustainable development of MFCs in environmental remediation and pollutant treatment, future research should focus on the following issues: (1) electrochemically active microorganisms play an important role in MFCs. Therefore, it is necessary to further understand the metabolic mechanism and electron transfer mechanism in order to improve the capacity of MFCs for pollutant treatment and power generation. (2) In order to improve the long-term operation performance and reduce the cost of MFCs, it is necessary to study materials and develop sustainable, low-cost, and high-performance electrodes, catalysts, and separator materials. (3) Combining MFCs with other technologies can improve the application of pollutant treatment. Combination technologies will compensate for the shortcomings of single technologies, and have broad application prospects. More efforts should be made to improve the performance of composite technologies. (4) The research on energy harvesting system needs to be further strengthened, which is the key to the practical application of MFC. The system needs to convert the weak energy storage generated by MFC into intermittent driving load and have appropriate stable output.
Conclusion
This review summarized the application of MFC technology to wastewater treatment, soil remediation, natural water and groundwater remediation, sludge treatment, gas emission control, and environmental monitoring. Clearly, research on MFCs in the field of pollution control and environmental remediation is progressing, but there are still some challenges, including less long-term research, high cost, and small scale. It should be noted that although these problems exist, MFCs still provide a promising method for pollutant treatment and environmental remediation.
References
Abbas SZ, Rafatullah M, Ismail N, Nastro RA (2017a) Enhanced bioremediation of toxic metals and harvesting electricity through sediment microbial fuel cell. Int J Energy Res 41:2345–2355
Abbas SZ, Rafatullah M, Ismail N, Syakir MI (2017b) A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: mechanisms and future prospective. Int J Energy Res 41:1242–1264
Abbas SZ, Rafatullah M, Ismail N, Shakoori FR (2018) Electrochemistry and microbiology of microbial fuel cells treating marine sediments polluted with heavy metals. RSC Adv 8:18800–18813
Abrevaya XC, Sacco NJ, Bonetto MC, Hilding-Ohlsson A, Corton E (2015) Analytical applications of microbial fuel cells. Part I: biochemical oxygen demand. Biosens Bioelectron 63:580–590
Akamatsu K, Lu W, Sugawara T, Nakao S-I (2010) Development of a novel fouling suppression system in membrane bioreactors using an intermittent electric field. Water Res 44:825–830
Al-Hamdan AZ, Reddy KR (2008) Transient behavior of heavy metals in soils during electrokinetic remediation. Chemosphere 71:860–871
Arends JBA, Speeckaert J, Blondeel E, De Vrieze J, Boeckx P, Verstraete W, Rabaey K, Boon N (2014) Greenhouse gas emissions from rice microcosms amended with a plant microbial fuel cell. Appl Microbiol Biotechnol 98:3205–3217
Ayyaru S, Dharmalingam S (2014) Enhanced response of microbial fuel cell using sulfonated poly ether ether ketone membrane as a biochemical oxygen demand sensor. Anal Chim Acta 818:15–22
Cao X, Song H-L, Yu C-Y, Li X-N (2015) Simultaneous degradation of toxic refractory organic pesticide and bioelectricity generation using a soil microbial fuel cell. Bioresour Technol 189:87–93
Castillo-Gonzalez HA, Bruns MA (2005) Dissimilatory iron reduction and odor indicator abatement by biofilm communities in swine manure microcosms. Appl Environ Microbiol 71:4972–4978
Chang S-H, Wu C-H, Wang R-C, Lin C-W (2017) Electricity production and benzene removal from groundwater using low-cost mini tubular microbial fuel cells in a monitoring well. J Environ Manag 193:551–557
Chen Z, Zhu B-K, Jia W-F, Liang J-H, Sun G-X (2015) Can electrokinetic removal of metals from contaminated paddy soils be powered by microbial fuel cells? Environ Technol Innov 3:63–67
Chen S, Tang J, Fu L, Yuan Y, Zhou S (2016a) Biochar improves sediment microbial fuel cell performance in low conductivity freshwater sediment. J Soils Sediments 16:2326–2334
Chen Z, Niu Y, Zhao S, Khan A, Ling Z, Chen Y, Liu P, Li X (2016b) A novel biosensor for p-nitrophenol based on an aerobic anode microbial fuel cell. Biosens Bioelectron 85:860–868
Cheng S, Liu H, Logan BE (2006) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40:2426–2432
Damiano L, Jambeck JR, Ringelberg DB (2014) Municipal solid waste landfill leachate treatment and electricity production using microbial fuel cells. Appl Biochem Biotechnol 173:472–485
Dominguez-Garay A, Esteve-Nunez A (2018) Designing strategies for operating microbial electrochemical systems to clean up polluted soils under non-flooded conditions. Bioelectrochemistry 124:142–148
Dong Y, Qu Y, He W, Du Y, Liu J, Han X, Feng Y (2015) A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour Technol 195:66–72
Donovan C, Dewan A, Peng H, Heo D, Beyenal H (2011) Power management system for a 2.5W remote sensor powered by a sediment microbial fuel cell. J Power Sources 196:1171–1177
ElMekawy A, Hegab HM, Pant D, Saint CP (2018) Bio-analytical applications of microbial fuel cell-based biosensors for onsite water quality monitoring. J Appl Microbiol 124:302–313
Ewing T, Phuc Thi H, Beyenal H (2017) Evaluation of long-term performance of sediment microbial fuel cells and the role of natural resources. Appl Energy 192:490–497
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880
Gholizadeh A, Salmani MH, Ebrahimi AA, Hosseini SS, Ehrampoush MH, Miri M, Nikoonahad A, Pasalari H (2018) Improved power density and Cr/Pb removal using ozone in a microbial desalination cell. Environ Chem Lett 16:1477–1485
Gouveia L, Neves C, Sebastiao D, Nobre BP, Matos CT (2014) Effect of light on the production of bioelectricity and added-value microalgae biomass in a photosynthetic alga microbial fuel cell. Bioresour Technol 154:171–177
Gregory KB, Lovley DR (2005) Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ Sci Technol 39:8943–8947
Guan C-Y, Hu A, Yu C-P (2019a) Stratified chemical and microbial characteristics between anode and cathode after long-term operation of plant microbial fuel cells for remediation of metal contaminated soils. Sci Total Environ 670:585–594
Guan C-Y, Tseng Y-H, Tsang DCW, Hu A, Yu C-P (2019b) Wetland plant microbial fuel cells for remediation of hexavalent chromium contaminated soils and electricity production. J Hazard Mater 365:137–145
Gustave W, Yuan Z-F, Sekar R, Chang H-C, Zhang J, Wells M, Ren Y-X, Chen Z (2018) Arsenic mitigation in paddy soils by using microbial fuel cells. Environ Pollut 238:647–655
Habibul N, Hu Y, Sheng G-P (2016) Microbial fuel cell driving electrokinetic remediation of toxic metal contaminated soils. J Hazard Mater 318:9–14
Hao L, Zhang B, Tian C, Liu Y, Shi C, Cheng M, Feng C (2015) Enhanced microbial reduction of vanadium (V) in groundwater with bioelectricity from microbial fuel cells. J Power Sources 287:43–49
Hartl M, Bedoya-Rios DF, Fernandez-Gatell M, Rousseau DPL, Du Laing G, Garfi M, Puigagut J (2019) Contaminants removal and bacterial activity enhancement along the flow path of constructed wetland microbial fuel cells. Sci Total Environ 652:1195–1208
Hassan H, Jin B, Dai S, Ma T, Saint C (2016) Chemical impact of catholytes on Bacillus subtilis-catalysed microbial fuel cell performance for degrading 2,4-dichlorophenol. Chem Eng J 301:103–114
He Z, Kan J, Wang Y, Huang Y, Mansfeld F, Nealson KH (2009) Electricity production coupled to ammonium in a microbial fuel cell. Environ Sci Technol 43:3391–3397
He L, Du P, Chen Y, Lu H, Cheng X, Chang B, Wang Z (2017) Advances in microbial fuel cells for wastewater treatment. Renew Sustain Energy Rev 71:388–403
Hodge A, Berta G, Doussan C, Merchan F, Crespi M (2009) Plant root growth, architecture and function. Plant Soil 321:153–187
Huang J, Yang P, Guo Y, Zhang K (2011) Electricity generation during wastewater treatment: an approach using an AFB-MFC for alcohol distillery wastewater. Desalination 276:373–378
Jiang Y, Liang P, Liu P, Wang D, Miao B, Huang X (2017) A novel microbial fuel cell sensor with biocathode sensing element. Biosens Bioelectron 94:344–350
Kabutey FT, Antwi P, Ding J, Q-l Z, Quashie FK (2019) Enhanced bioremediation of heavy metals and bioelectricity generation in a macrophyte-integrated cathode sediment microbial fuel cell (mSMFC). Environ Sci Pollut Res 26:26829–26843
Kahoush M, Behary N, Cayla A, Nierstrasz V (2018) Bio-Fenton and bio-electro-Fenton as sustainable methods for degrading organic pollutants in wastewater. Process Biochem 64:237–247
Karluvah A, Koroglu EO, Manav N, Cetinkaya AY, Ozkaya B (2015) Electricity generation from organic fraction of municipal solid wastes in tubular microbial fuel cell. Sep Purif Technol 156:502–511
Khan N, Khan MD, Ansari MY, Ahmad A, Khan MZ (2019) Bio-electrodegradation of 2,4,6-Trichlorophenol by mixed microbial culture in dual chambered microbial fuel cells. J Biosci Bioeng 127:353–359
Khudzari JM, Gariepy Y, Kurian J, Tartakovsky B, Raghavan GSV (2019) Effects of biochar anodes in rice plant microbial fuel cells on the production of bioelectricity, biomass, and methane. Biochem Eng J 141:190–199
Kim M, Hyun MS, Gadd GM, Kim HJ (2007) A novel biomonitoring system using microbial fuel cells. J Environ Monit 9:1323–1328
Kim JR, Dec J, Bruns MA, Logan BE (2008) Removal of odors from swine wastewater by using microbial fuel cells. Appl Environ Microbiol 74:2540–2543
Lakaniemi A-M, Tuovinen OH, Puhakka JA (2012) Production of electricity and butanol from microalgal biomass in microbial fuel cells. Bioenergy Res 5:481–491
Larrosa-Guerrero A, Scott K, Head IM, Mateo F, Ginesta A, Godinez C (2010) Effect of temperature on the performance of microbial fuel cells. Fuel 89:3985–3994
Leiva E, Leiva-Aravena E, Rodriguez C, Serrano J, Vargas I (2018) Arsenic removal mediated by acidic pH neutralization and iron precipitation in microbial fuel cells. Sci Total Environ 645:471–481
Li J, Liu G, Zhang R, Luo Y, Zhang C, Li M (2010) Electricity generation by two types of microbial fuel cells using nitrobenzene as the anodic or cathodic reactants. Bioresour Technol 101:4013–4020
Li X, Mu S, Ren Y, Wang X (2017) Behavior of copper in membrane-less sediment microbial fuel cell. J Renew Sustain Energy 9
Li X, Chen S, Angelidaki I, Zhang Y (2018a) Bio-electro-Fenton processes for wastewater treatment: advances and prospects. Chem Eng J 354:492–506
Li X, Zhao Q, Wang X, Li Y, Zhou Q (2018b) Surfactants selectively reallocated the bacterial distribution in soil bioelectrochemical remediation of petroleum hydrocarbons. J Hazard Mater 344:23–32
Li X, Li Y, Zhang X, Zhao X, Sun Y, Weng L, Li Y (2019) Long-term effect of biochar amendment on the biodegradation of petroleum hydrocarbons in soil microbial fuel cells. Sci Total Environ 651:796–806
Liang C, Das KC, McClendon RW (2003) The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour Technol 86:131–137
Liang P, Duan R, Jiang Y, Zhang X, Qiu Y, Huang X (2018) One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Res 141:1–8
Ling T, Huang B, Zhao M, Yan Q, Shen W (2016) Repeated oxidative degradation of methyl orange through bio-electro-Fenton in bioelectrochemical system (BES). Bioresour Technol 203:89–95
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046
Liu H, Cheng SA, Logan BE (2005) Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ Sci Technol 39:5488–5493
Liu Y, Zhang B, Tian C, Feng C, Wang Z, Cheng M, Hu W (2016) Optimization of enhanced bioelectrical reactor with electricity from microbial fuel cells for groundwater nitrate removal. Environ Technol 37:1008–1017
Liu W, Jia H, Wang J, Zhang H, Xin C, Zhang Y (2018) Microbial fuel cell and membrane bioreactor coupling system: recent trends. Environ Sci Pollut Res 25:23631–23644
Liu F, Wang L, Zuo K, Luo S, Zhang X, Liang P, Huang X (2019a) A novel operational strategy to enhance wastewater treatment with dual-anode assembled microbial desalination cell. Bioelectrochemistry 126:99–104
Liu Q, Zhou B, Zhang S, Xu D, Pan R, Xia S (2019b) Embedding microbial fuel cells into the vertical flow constructed wetland enhanced denitrogenation and water purification. Pol J Environ Stud 28:1799–1804
Liu S-H, Lai Y-C, Lin C-W (2019c) Enhancement of power generation by microbial fuel cells in treating toluene-contaminated groundwater: developments of composite anodes with various compositions. Appl Energy 233:922–929
Liu Y, Song P, Gai R, Yan C, Jiao Y, Yin D, Cai L, Zhang L (2019d) Recovering platinum from wastewater by charring biofilm of microbial fuel cells (MFCs). J Saudi Chem Soc 23:338–345
Lovley DR (2012) Electromicrobiology. In: Gottesman S, Harwood CS, Schneewind O (eds) Annual review of microbiology, Vol 66. Annual Review of Microbiology, pp 391–409
Ma D, Jiang Z-H, Lay C-H, Zhou D (2016) Electricity generation from swine wastewater in microbial fuel cell: hydraulic reaction time effect. Int J Hydrog Energy 41:21820–21826
Mani P, Kumar VTF, Keshavarz T, Chandra TS, Kyazze G (2018) The role of natural laccase redox mediators in simultaneous dye decolorization and power production in microbial fuel cells. Energies 11
Martins G, Peixoto L, Teodorescu S, Parpot P, Nogueira R, Brito AG (2014) Impact of an external electron acceptor on phosphorus mobility between water and sediments. Bioresour Technol 151:419–423
Meng F, Chae S-R, Drews A, Kraume M, Shin H-S, Yang F (2009) Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res 43:1489–1512
Meng F, Zhang S, Oh Y, Zhou Z, Shin H-S, Chae S-R (2017) Fouling in membrane bioreactors: an updated review. Water Res 114:151–180
Min B, Kim JR, Oh SE, Regan JM, Logan BE (2005) Electricity generation from swine wastewater using microbial fuel cells. Water Res 39:4961–4968
Oliveira VB, Simoes M, Melo LF, Pinto AMFR (2013) Overview on the developments of microbial fuel cells. Biochem Eng J 73:53–64
Ottoni CA, Simoes MF, Santos JG, Peixoto L, Martins CR, Silva BP, Neto AO, Brito AG, Maiorano AE (2019) Application of microbial fuel cell technology for vinasse treatment and bioelectricity generation. Biotechnol Lett 41:107–114
Pendyala B, Chaganti SR, Lalman JA, Heath DD (2016) Optimizing the performance of microbial fuel cells fed a combination of different synthetic organic fractions in municipal solid waste. Waste Manag 49:73–82
Pham TH, Rabaey K, Aelterman P, Clauwaert P, De Schamphelaire L, Boon N, Verstraete W (2006) Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng Life Sci 6:285–292
Pitts KE, Dobbin PS, Reyes-Ramirez F, Thomson AJ, Richardson DJ, Seward HE (2003) Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA. J Biol Chem 278:27758–27765
Pous N, Puig S, Coma M, Balaguer MD, Colprim J (2013) Bioremediation of nitrate-polluted groundwater in a microbial fuel cell. J Chem Technol Biotechnol 88:1690–1696
Pous N, Casentini B, Rossetti S, Fazi S, Puig S, Aulenta F (2015) Anaerobic arsenite oxidation with an electrode serving as the sole electron acceptor: a novel approach to the bioremediation of arsenic-polluted groundwater. J Hazard Mater 283:617–622
Raghavulu SV, Mohan SV, Reddy MV, Mohanakrishna G, Sarma PN (2009) Behavior of single chambered mediatorless microbial fuel cell (MFC) at acidophilic, neutral and alkaline microenvironments during chemical wastewater treatment. Int J Hydrog Energy 34:7547–7554
Rakoczy J, Feisthauer S, Wasmund K, Bombach P, Neu TR, Vogt C, Richnow HH (2013) Benzene and sulfide removal from groundwater treated in a microbial fuel cell. Biotechnol Bioeng 110:3104–3113
Rodrigo MA, Canizares P, Lobato J, Paz R, Saez C, Linares JJ (2007) Production of electricity from the treatment of urban waste water using a microbial fuel cell. J Power Sources 169:198–204
Sajana TK, Ghangrekar MM, Mitra A (2014) Effect of operating parameters on the performance of sediment microbial fuel cell treating aquaculture water. Aquac Eng 61:17–26
Shrestha PM, Rotaru A-E (2014) Plugging in or going wireless: strategies for interspecies electron transfer. Front Microbiol 5
Song T-S, Zhang J, Hou S, Wang H, Zhang D, Li S, Xie J (2018) In situ electrokinetic remediation of toxic metal-contaminated soil driven by solid phase microbial fuel cells with a wheat straw addition. J Chem Technol Biotechnol 93:2860–2867
Song X, Yang W, Lin Z, Huang L, Quan X (2019) A loop of catholyte effluent feeding to bioanodes for complete recovery of Sn, Fe, and Cu with simultaneous treatment of the co-present organics in microbial fuel cells. Sci Total Environ 651:1698–1708
Stein NE, Hamelers HMV, van Straten G, Keesman KJ (2012) On-line detection of toxic components using a microbial fuel cell-based biosensor. J Process Control 22:1755–1761
Su X, Tian Y, Sun Z, Lu Y, Li Z (2013) Performance of a combined system of microbial fuel cell and membrane bioreactor: wastewater treatment, sludge reduction, energy recovery and membrane fouling. Biosens Bioelectron 49:92–98
Thrash JC, Coates JD (2008) Review: Direct and indirect electrical stimulation of microbial metabolism. Environ Sci Technol 42:3921–3931
Tian C, Zhang B, Borthwick AGL, Li Y, Liu W (2017) Electrochemical oxidation of thallium (I) in groundwater by employing single-chamber microbial fuel cells as renewable power sources. Int J Hydrog Energy 42:29454–29462
Turker OC (2018) Simultaneous boron (B) removal and electricity generation from domestic wastewater using duckweed-based wastewater treatment reactors coupled with microbial fuel cell. J Environ Manag 228:20–31
Valladares Linares R, Dominguez-Maldonado J, Rodriguez-Leal E, Patron G, Castillo-Hernandez A, Miranda A, Diaz Romero D, Moreno-Cervera R, Camara-chale G, Borroto CG, Alzate-Gaviria L (2019) Scale up of microbial fuel cell stack system for residential wastewater treatment in continuous mode operation. Water 11
Van Khanh N, Park Y, Yu J, Lee T (2016) Bioelectrochemical denitrification on biocathode buried in simulated aquifer saturated with nitrate-contaminated groundwater. Environ Sci Pollut Res 23:15443–15451
Vazquez-Larios AL, Rios-Leal E, Solorza-Feria O, Poggi-Varaldo HM (2010) Effect of the temperature on two types microbial fuel cells performance. J Biotechnol 150:S145–S146
Vilajeliu-Pons A, Puig S, Salcedo-Davila I, Balaguer MD, Colprim J (2017) Long-term assessment of six-stacked scaled-up MFCs treating swine manure with different electrode materials. Environ Sci-Water Res Technol 3:947–959
Virdis B, Rabaey K, Yuan Z, Keller J (2008) Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res 42:3013–3024
Wang X, Cai Z, Zhou Q, Zhang Z, Chen C (2012a) Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnol Bioeng 109:426–433
Wang Y-P, Liu X-W, Li W-W, Li F, Wang Y-K, Sheng G-P, Zeng RJ, Yu H-Q (2012b) A microbial fuel cell-membrane bioreactor integrated system for cost-effective wastewater treatment. Appl Energy 98:230–235
Wang C-T, Lee Y-C, Liao F-Y (2015a) Effect of composting parameters on the power performance of solid microbial fuel cells. Sustainability 7:12634–12643
Wang L, Wu Y, Zheng Y, Liu L, Zhao F (2015b) Efficient degradation of sulfamethoxazole and the response of microbial communities in microbial fuel cells. RSC Adv 5:56430–56437
Wang G-H, Cheng C-Y, Liu M-H, Chen T-Y, Hsieh M-C, Chung Y-C (2016) Utility of Ochrobactrum anthropi YC152 in a microbial fuel cell as an early warning device for hexavalent chromium determination. Sensors 16
Wang Y, Jia H, Wang J, Cheng B, Yang G, Gao F (2018) Impacts of energy distribution and electric field on membrane fouling control in microbial fuel cell-membrane bioreactor (MFC-MBR) coupling system. Bioresour Technol 269:339–345
Wang X, Tian Y, Liu H, Zhao X, Peng S (2019) The influence of incorporating microbial fuel cells on greenhouse gas emissions from constructed wetlands. Sci Total Environ 656:270–279
Wen Q, Kong F, Zheng H, Yin J, Cao D, Ren Y, Wang G (2011) Simultaneous processes of electricity generation and ceftriaxone sodium degradation in an air-cathode single chamber microbial fuel cell. J Power Sources 196:2567–2572
Wolf C, Fischer R, Koster R, Weidler P (2002) Investigation on the behaviour of iron manganese, and phosphate at the sediment/water interface influenced by an electric field. Acta Hydrochim Hydrobiol 30:75–86
Wu MS, Xu X, Zhao Q, Wang ZY (2017) Simultaneous removal of heavy metals and biodegradation of organic matter with sediment microbial fuel cells. RSC Adv 7:53433–53438
Wu Y, Jing X, Gao C, Huang Q, Cai P (2018) Recent advances in microbial electrochemical system for soil bioremediation. Chemosphere 211:156–163
Wu Y, Wang L, Jin M, Kong F, Qi H, Nan J (2019) Reduced graphene oxide and biofilms as cathode catalysts to enhance energy and metal recovery in microbial fuel cell. Bioresour Technol 283:129–137
Xie Z, Xu Y, Liu G, Liu Q, Zhu J, Tu C, Amonette JE, Cadisch G, Yong JWH, Hu S (2013) Impact of biochar application on nitrogen nutrition of rice, greenhouse-gas emissions and soil organic carbon dynamics in two paddy soils of China. Plant Soil 370:527–540
Xie B, Dong W, Liu B, Liu H (2014) Enhancement of pollutants removal from real sewage by embedding microbial fuel cell in anaerobic-anoxic-oxic wastewater treatment process. J Chem Technol Biotechnol 89:448–454
Xin X, Chen B-Y, Hong J (2019) Unraveling interactive characteristics of microbial community associated with bioelectric energy production in sludge fermentation fluid-fed microbial fuel cells. Bioresour Technol:289
Xu P, Xiao E, Xu D, Li J, Zhang Y, Dai Z, Zhou Q, Wu Z (2018) Enhanced phosphorus reduction in simulated eutrophic water: a comparative study of submerged macrophytes, sediment microbial fuel cells, and their combination. Environ Technol 39:1144–1157
Xu H, Quan X, Chen L (2019) A novel combination of bioelectrochemical system with peroxymonosulfate oxidation for enhanced azo dye degradation and MnFe2O4 catalyst regeneration. Chemosphere 217:800–807
Yan Z, Jiang H, Cai H, Zhou Y, Krumholz LR (2015) Complex interactions between the macrophyte Acorus calamus and microbial fuel cells during pyrene and benzo a pyrene degradation in sediments. Sci Rep 5
Yan W, Xiao Y, Yan W, Ding R, Wang S, Zhao F (2019) The effect of bioelectrochemical systems on antibiotics removal and antibiotic resistance genes: a review. Chem Eng J 358:1421–1437
Yang Q, Zhao H, Zhao N, Ni J, Gu X (2016) Enhanced phosphorus flux from overlying water to sediment in a bioelectrochemical system. Bioresour Technol 216:182–187
Zhang Y, Angelidaki I (2012a) Bioelectrode-based approach for enhancing nitrate and nitrite removal and electricity generation from eutrophic lakes. Water Res 46:6445–6453
Zhang Y, Angelidaki I (2012b) A simple and rapid method for monitoring dissolved oxygen in water with a submersible microbial fuel cell (SBMFC). Biosens Bioelectron 38:189–194
Zhang B, Zhao H, Zhou S, Shi C, Wang C, Ni J (2009) A novel UASB-MFC-BAF integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour Technol 100:5687–5693
Zhang A, Bian R, Pan G, Cui L, Hussain Q, Li L, Zheng J, Zheng J, Zhang X, Han X, Yu X (2012) Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: a field study of 2 consecutive rice growing cycles. Field Crop Res 127:153–160
Zhang J, Cao Z, Zhang H, Zhao L, Sun X, Mei F (2013) Degradation characteristics of 2, 4-dichlorophenoxyacetic acid in electro-biological system. J Hazard Mater 262:137–142
Zhang B, Wang Z, Zhou X, Shi C, Guo H, Feng C (2015) Electrochemical decolorization of methyl orange powered by bioelectricity from single-chamber microbial fuel cells. Bioresour Technol 181:360–362
Zhang Q, Hu J, Lee D-J (2016) Microbial fuel cells as pollutant treatment units: research updates. Bioresour Technol 217:121–128
Zhang Q, Zhang Y, Li D (2017) Cometabolic degradation of chloramphenicol via a meta-cleavage pathway in a microbial fuel cell and its microbial community. Bioresour Technol 229:104–110
Zhang E, Yu Q, Zhai W, Wang F, Scott K (2018a) High tolerance of and removal of cefazolin sodium in single-chamber microbial fuel cells operation. Bioresour Technol 249:76–81
Zhang X, Zhang D, Huang Y, Zhang K, Lu P (2018b) Simultaneous removal of organic matter and iron from hydraulic fracturing flowback water through sulfur cycling in a microbial fuel cell. Water Res 147:461–471
Zhang S, Song H-L, Cao X, Li H, Guo J, Yang X-L, Singh RP, Liu S (2019a) Inhibition of methanogens decreased sulfadiazine removal and increased antibiotic resistance gene development in microbial fuel cells. Bioresour Technol 281:188–194
Zhang Y, Zhao Y, Zhou M (2019b) A photosynthetic algal microbial fuel cell for treating swine wastewater. Environ Sci Pollut Res 26:6182–6190
Zhong D, Liu Y, Liao X, Zhong N, Xu Y (2018) Facile preparation of binder-free NiO/MnO2-carbon felt anode to enhance electricity generation and dye wastewater degradation performances of microbial fuel cell. Int J Hydrog Energy 43:23014–23026
Funding
This project was supported by the Special S&T Project on the Treatment and Control of Water Pollution (2014ZX07203-009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Q., Jiao, S., Ma, M. et al. Microbial fuel cell system: a promising technology for pollutant removal and environmental remediation. Environ Sci Pollut Res 27, 6749–6764 (2020). https://doi.org/10.1007/s11356-020-07745-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07745-0