Abstract
Environmental pollution caused by inert anthropogenic stressors such as microplastics in aquatic media is constantly increasing. Through the proliferating use of plastic products in daily life, more and more plastic particles enter waters as primary microplastics. Even though large scale plastic items such as plastic bottles and bags represent the highest percentage of plastic waste, their degeneration also generates microparticles and nanoparticles (secondary microplastics). Modern sewage treatment plants require innovative ideas in order to deal with this man-made problem. State-of-the-art technology offers approaches to minimise the amount of microplastics in aquatic systems. These technologies, however, are either insufficient or very costly, as well as time-consuming in both cases. The conceptual idea presented here is to apply innovative inorganic-organic hybrid silica gels which provide a cost-effective and straightforward approach. Currently, the synthesis of preorganised bioinspired compounds is advancing in order to produce functionalised hybrid silica gels in a further step. These gels have the ability to remove stressors such as microplastics from waste water. By means of the sol-gel process, bioinspired silane compounds are currently being permuted to macromolecules and examined with respect to their properties as fixation and filter material in order to remove the hydrophobic anthropogenic stressors sustainably. Here, the reproduction of biological systems plays a significant role. In particular in material sciences, this approach is becoming increasingly important. Among other concepts, new biomimetic molecules form the basis for the investigation of innovative host-guest relationships for anthropogenic stressors in the environment and their implementation in technical processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global plastic production is steadily growing. The greatest demand is for polypropylene (PP), polyethylene (PE) and polyethylene terephthalate (PET) (Fig. 1) (PlasticsEurope 2015). These macromolecules are assigned to the category of thermoplastics. This means that the materials are ductile at a certain temperature range. They are mainly used as packaging, in the automobile industry or in toys (PlasticsEurope 2015).
In addition to the important plastics, there are also water-soluble macromolecules, such as polyvinyl alcohol (PVA) or polyvinyl acetate (PVAc), which are of great commercial interest. They are used, for example, as emulsifiers, stabilisers, protective colloids, complexing agents, cleaning intensifiers, discolouration inhibitors, filming agents or adhesives (Löffler and Morschhäuser 2001; Hartmann et al. 1995). One area of application, which has gained tremendous technical and commercial significance, is the use of hydrophilic polymers to thicken aqueous systems or in the production of so-called hydrogels. In principle, thickening aqueous systems can be achieved by simply adding unlinked natural or synthetic hydrophilic polymers with high molecular weights (Löffler and Morschhäuser 2001). In addition to this, hydrodynamic thickening stable hydrogels can be formed by means of the swelling of networks of relatively long-chained or branched hydrophilic polymers. If the individual polymer chains are linked via covalent bonds, the structures are generally designated as “chemical gels”. If the polymer branches interact with each another via non-covalent cohesion, “physical gels” are formed. Non-covalent cohesive forces are, for instance, crystallisation as well as hydrophobic or electrostatic interactions. However, the application of covalent networked polyacrylic acid as a super absorber in hygiene articles or adhesive plasters has been the greatest commercial significance to date (Brannon-Peppas and Harland 1990). The further development and increase of the variety of products thus also increase the consumption and waste of plastic. Consequently, the stress caused by microplastics is steadily increasing in standing and running waters, as well as in waste water and drinking water. Waste water treatment plants are approaching their limits. Solutions must be found, which can sustainably fix microparticles and are easy as well as cost-effective to use.
Until the end of 2015, more and more plastic particles have been added to the formulas in the cosmetics and personal hygiene industry in order to enhance their cleansing effect. Since December 2015, there has been a general prohibition in the USA against the manufacture of cosmetic products with microplastics. Canada and the Netherlands will follow suit (prospectively in 2016). Nevertheless, there are still ample countries which continue to produce and extensively market cosmetics with microparticle additives. Furthermore, it is suspected that textile fibres from recycled plastics (e.g. PET bottles) generate primary microplastics through friction during washing steps. Washing machines generate more than 1900 microparticles per wash of fleece fabric made of polyester and acrylic textile fibres (Browne et al. 2011). The impact on the environment is serious, since 663 organisms are known to be affected by the negative effects of waste so far. More than half ingest the plastic particles or get caught in them (Essel et al. 2015; Browne et al. 2008). Microplastic particles (<5 mm), as well as larger particles, can lead to physical injuries, such as to influence the digestive tract leading to an inhibition of food uptake (Schrank et al. 2014). In addition, microplastic particles can serve as a means of transport by which harmful substances, such as polychlorinated biphenyl (PCB) or polyaromatic hydrocarbons (PAHs), adsorb on the surface. Subsequently used as nutrients, this results in bioaccumulation and has an effect on the food chain (Köhler et al. 2014; Mintenig et al. 2014; Velzeboer et al. 2014).
Regenerative filter systems or innovative precipitation basins (separation by differences in density) are required in order to remove such hazardous microparticles from the waste water. Existing filter systems, such as the membrane filter procedure, are associated with additional energy and investment costs and are not recyclable (Moore 2008; Leslie et al. 2013; Backer et al. 2014). Due to this inefficient process, additional research is required in order to provide materials and processes which are simple, inexpensive and environmentally friendly, and which sustainably clean the waters in order to keep the risk to man, animal and nature as low as possible.
The state of the art
Despite the fast-paced development in the area of microplastics according to van Cauwenberghe et al. 2015, approximately 80 publications concerning microplastics in sediments have been published in the last 5 years, but no standardised method for the sampling and extraction of microplastics has been established. Classic water treatment by means of activated charcoal is not possible due to the particle size of microplastics. Additionally, activated charcoal bears the risk that the adsorbed materials can be washed out again (desorption) and that the processing of the charcoal is disproportionately expensive, especially in the case of plastics. Furthermore, this method is not suitable for an extensive application over a wide area. Another problem is the removal of the polluted activated carbon. This must be removed by means of cloth filters, so that the pollutants do not reach back again into the environment.
The density difference of the individual particles can be used to isolate microplastics. The density of a liquid, for example, can be increased by adding mineral salt. By means of a concentrated sodium chloride (NaCl) solution, it is possible to achieve a density of 1.2 g/cm3 (Thompson et al. 2004). Particles with a lower density will float to the surface and can be easily extracted. This method is inapplicable for plastics with a higher density distribution such as polyvinyl chloride (PVC; 1.14–1.56 g/cm3) or PET (1.32–1.41 g/cm3). These materials account for 17.3 % of the total demand for plastics (PlasticsEurope 2015), which cannot be isolated using the aforementioned method. Since they were not removed from the waste water, sediments in the waters have been massively contaminated. Therefore, researchers have been working on investigating mineral solutions with a higher density than a NaCl solution. As a result, ZnCl2 (1.5–1.7 g/cm3) (Imhof et al. 2012) or NaI (3.3 M; 1.6–1.8 g/cm3) (Dekiff et al. 2014; Claessens et al. 2013) have been determined. An additional method for the separation of microplastics is the principle of elutriation. In this method, a stream of gas or liquid is introduced to separate lighter from heavier particles. This technique is primarily used in marine biology and is known as “Barnett’s fluidised sand bath” (Southwood and Henderson 2000). The sampling, extraction, detection and purification of microplastics are gaining more and more global research interest, which confirms the risk and the need to act (Fries et al. 2013; Nuelle et al. 2014; Harrison et al. 2012). The term microparticle includes all plastic types. Therefore, it can be assumed that the contamination consists of a variety of plastics with different physicochemical properties (Table 1).
A concept for the application of bioinspired compounds containing silicium for the removal of hydrophobic, anthropogenic stressors from the water
Bioinspired molecules or biomaterials are deliberately engineered materials, which, alone or as part of a complex system, steer the course of certain processes by controlling the interactions with components of living systems. Interfaces between biomaterials and for instance cells/tissue play a special role in the research and application of biomaterials. Also, the utilisation of the effects induced by biomaterials on waste water treatment and the purification of drinking water is moving into the focus of scientists (Tu et al. 2015). Furthermore, there are many materials in nature, which far exceed the possible applications of synthesised materials thanks to their properties optimised over millions of years. In order to ensure the optimal implementation of bioinspired materials, it is essential to understand the underlying functionality of nature, to implement it accordingly and to emphasise environmental compatibility and degradability especially in the development of new strategies for the conservation of water quality. Bioinspired molecules have a combination of organic and inorganic molecular building blocks, which has several advantages. The organic unit is used as a spacer, texturing agent and additionally reactive site and the inorganic unit interacts as a crosslinker (Pfeifer et al. 2013; Hoffmann et al. 2006; Vallé et al. 2006). Moreau et al. 2005 also show that functionalised hybrid materials consisting of organic and inorganic elements have special characteristics, such as preorganization. In addition to the properties of so-called host-guest systems, as they are already known as crown ethers, zeolites or calixarene, our concept was formed.
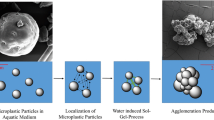
The concept for the removal of hydrophobic, anthropogenic stressors (cf. PE, PP and PET) consists of three synthesis steps. These steps include the synthesis of the inclusion unit (IU), the bioinspired component of the entire molecule. The second step is the synthesis of the capture unit (CU). The CU is characterised by a preorganisation and the ability to bond with the material to be included via the interactions of the introduced functional groups. The CU and the IU are then combined in the last synthesis step to create the inclusion compound (IC). Among further functions, alkoxysilyl serves to stretch the desired three-dimensional network. Through the organisation structure of the CU, structured hybrid silica gels will be obtained resulting from the sol-gel process (Fig. 2). In the next step, the concept provides for the utilisation of the bioinsipiration and the ability of the CU to interact so that the inclusion of the inert compounds results. Subsequently, a simple separation is conducted by which the hydrophobic stressors trapped in the new hybrid silica gel are easy to isolate (due to the increase in volume, which is considerably larger in comparison to granulated activated carbon) with a separation process, such as by means of a cost-effective sand trap. Finally, there is the recycling concept, in which the CU can generate additional energy by means of thermal utilisation for instance.
The drivers for the complexation of hydrophobic “guests” in the aqueous phase through bioinspired molecules are, first and foremost, the hydrophobic and van der Waals interactions. The hydrophobic interaction has enthalpic as well as entropic causes. Through the capture of a suitable guest molecule in the hydrophobic cavity of the alkoxysilyl functionalised biomolecule, embedded water molecules are displaced. Through their release, the water molecules obtain greater mobility (entropy gain) and are able to form new hydrogen bonds with adjacent water molecules (gain of cohesion energy, loss of enthalpy). The van der Waals forces, which also act as a driver for the complexation, only have a very short range so that, as a rule, the more stable inclusion compounds are, the better the cavity is filled by the guest molecules (Tu et al. 2015). In addition, the biofunctionalised unit has a plurality of variable functions, resulting in intrinsic and extrinsic host-guest interactions. Therefore, it is possible to respond to different types of plastic strain and is highly flexible in the waste water treatment of various streams.
Outlook
In a multistage synthesis, di- and trialkoxysilyl functionalised preorganised precursors as CU with alkoxysilyl functionalised bioinspired compounds (as IU) are systematically formed to macromolecules (IC) (Schuhen 2015) and examined with respect to their ability to form inclusion compounds with plastic particles. Additionally, the functionalities of prescursors (IU) will be varied to enlarge the potential due to the reduction of, for example, heavy metals or pesticides. Based on the results, a study about the effectiveness of the CU in the aquatic environment will be carried out.
References
Backer H, Frias M, Nicolas F, HELCOM (2014) Baltic sea sewage port reception facilities
Brannon-Peppas L, Harland RS (1990) Absorbent polymer technology. Studies in polymer science, vol 8. Elsevier; Distributors for the United States and Canada, Elsevier Science Pub, Amsterdam, New York, New York, NY, U.S.A
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42(13):5026–5031. doi:10.1021/es800249a
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol 45(21):9175–9179. doi:10.1021/es201811s
Claessens M, van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of microplastics in sediments and field collected organisms. Mar Pollut Bull 70(1–2):227–233. doi:10.1016/j.marpolbul.2013.03.009
Dekiff JH, Remy D, Klasmeier J, Fries E (2014) Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ Pollut 186:248–256. doi:10.1016/j.envpol.2013.11.019
Essel R, Engel L, Carus M, Ahrens RH (2015) Quellen für Mikroplastik mit Relevanz für den Meeresschutz in Deutschland
Fries E, Dekiff JH, Willmeyer J, Nuelle M, Ebert M, Remy D (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci: Processes Impacts 15(10):1949. doi:10.1039/c3em00214d
GDV, Gesamtverband der Deutschen Versicherungswirtschaft e.V. (2000) Kunststoffe
Harrison JP, Ojeda JJ, Romero-González ME (2012) The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci Total Environ 416:455–463. doi:10.1016/j.scitotenv.2011.11.078
Hartmann H, Görtz H, Grzesitza J, BASF AG (1995) Synthetische wasserlösliche Polymere-stand und ausblick. Angew Makromol Chem 123(124):1–44
Hoffmann F, Cornelius M, Morell J, Fröba M (2006) Silica-based mesoporous organic–inorganic hybrid materials. Angew Chem Int Ed 45(20):3216–3251. doi:10.1002/anie.200503075
Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C (2012) A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol Oceanogr Methods 524–537
Köhler A, Moos N, Fritsch J, Löder M (2014) Aufnahme und Wirkung von Mikroplastik in Muscheln
Leslie HA, van Velzen, M. J. M., Vethaak AD (2013) Microplastic survey of the Dutch environment
Löffler MD, Morschhäuser RD (2001) Wasserlösliche Polymere und ihre Verwendung in kosmetischen und pharmazeutischen Mitteln. http://www.google.de/patents/EP1069142A1?cl=de
Mintenig S, Int-Veen I, Löder M, Gerdts G (2014) Mikroplastik in ausgewählten Kläranlagen des Oldenburgisch- Ostfriesischen Wasserverbandes (OOWV) in Niedersachsen
Moore CJ (2008) Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ Res 108(2):131–139. doi:10.1016/j.envres.2008.07.025
Moreau JJE, Pichon, BP, Wong Chi Man M (2005) Lamellar phenylene-bridged hybrid silicones. Composite Interfaces 11(8–9):609–616
Nuelle M, Dekiff JH, Remy D, Fries E (2014) A new analytical approach for monitoring microplastics in marine sediments. Environ Pollut 184:161–169. doi:10.1016/j.envpol.2013.07.027
Pfeifer S, Schwarzer A, Schmidt D, Brendler E, Veith M, Kroke E (2013) Precursors for pyromellit-bridged silica sol–gel hybrid materials. New J Chem 37(1):169–180. doi:10.1039/C2NJ40538E
PlasticsEurope (2015) Plastics—the facts
Schrank I, Imhof HK, Laforsch C (2014) Auswirkungen von Mikroplastik auf limnische Invertebraten. StatuskolloquiumBayerisches Landesamt für Umwelt
Schuhen, K (2015) Polykondensiertes Hybridkieselsäurematerial zur Fixierung anthropogener Verunreinigungen aus einem aquatischen Umfeld, Patent pending
Southwood TRE, Henderson PA (2000) Ecological methods, 3rd edn
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John A (2004) Lost at sea: where is all the plastic? Science 304(5672):838. doi:10.1126/science.1094559
Tu Y, Peng F, Adawy A, Men Y, Abdelmohsen LKEA, Wilson DA (2015) Mimicking the cell: bio-inspired functions of supramolecular assemblies. Chem Rev. doi:10.1021/acs.chemrev.5b00344
Vallé K, Belleville P, Pereira F, Sanchez C (2006) Hierarchically structured transparent hybrid membranes by in situ growth of mesostructured organosilica in host polymer. Nat Mater 5(2):107–111. doi:10.1038/nmat1570
van Cauwenberghe L, Devriese L, Galgani F, Robbens J, Janssen CR (2015) Microplastics in sediments: a review of techniques, occurrence and effects. Mar Environ Res 111:5–17. doi:10.1016/j.marenvres.2015.06.007
Velzeboer I, Kwadijk CJAF, Koelmans AA (2014) Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ Sci Technol 48(9):4869–4876. doi:10.1021/es405721v
Acknowledgments
The project is financed by the Bundesministerium für Wirtschaft und Energie (BMWi, Germany) due to a decision of the German Bundestag (Central Innovation Program for SME). The authors also acknowledge technical and research support acknowledge of the SAS Hagmann GmbH, Horb am Neckar, Germany (www.sashagmann.de) and abcr GmbH, Karlsruhe, Germany (www.abcr.de).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Herbort, A.F., Schuhen, K. A concept for the removal of microplastics from the marine environment with innovative host-guest relationships. Environ Sci Pollut Res 24, 11061–11065 (2017). https://doi.org/10.1007/s11356-016-7216-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7216-x