Abstract
In this study, we investigated metal- and metalloid-induced oxidative stress response in two aquatic (cattle egret (Bubulcus ibis) (n = 10), pond heron (Ardeola grayii) (n = 10)), as well as two terrestrial (spotted owlet (Athene brama) (n = 6) and bank myna (Acridotheres ginginianus) (n = 16)) bird species collected from the outskirts of Lahore city, Pakistan. For this purpose, glutathione (tGSH) and lipid peroxidation (thiobarbituric acid-reactive substances (TBARS)) levels and activities of antioxidant enzymes (superoxide dismutase (SOD); catalase (CAT)) were analyzed as biomarkers of oxidative stress against metal (Pb, Cd, Cu, Zn) and metalloid (As) concentrations in kidney liver and blood of birds. Our results depicted significant correlation for Pb, Cd, and As with oxidative stress biomarkers in birds. The levels of heavy metals and As and their corresponding effects on oxidative stress biomarkers were comparably higher in aquatic species (p ≤ 0.01) except for Pb and Zn. In comparison of species, SOD and tGSH activities were higher in bank myna and cattle egret, while CAT activity and TBARS concentrations were higher in pond heron and cattle egret, respectively. We deduced that tissues with higher accumulation of metal(loid)s such as liver and kidney were under a great risk to oxidative damage. The overall order of metal accumulation and subsequent oxidative damage among families followed the pattern as Strigidae ≥Ardieda ≥ Sturnidae with their respective trophic levels. Globally, metal- and As-induced oxidative stress is least emphasized in multiple tissues of birds that is needed to be addressed with focus on case-control studies using dose-response approach.

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High levels of toxic trace metals and metalloids in the environment and their subsequent health effects have become a cause of great concerns at local, regional, and global scale (Wu et al. 2016). Small quantity of trace metals, e.g., Pb, Cd, and Cr and essential elements such as Cu and Zn if present in high concentration in tissue are of major concern because of their serious health effects in living organisms (Wen et al. 2016; Abbasi et al. 2015a). These metals are mainly released in the environment from mining, smelting, electroplating, and preparation of paint, pigments, batteries, photovoltaic cells, synthetic plastic, insecticides, and leather product. Besides metals, some metalloid such as As mainly originated from rocks, volcanoes, and ores, as well as from different industrial and agricultural sources and are of serious concern because of their adverse health effects (Yang et al. 2018; Lucia et al. 2010). Once released in the environment, metal(loid)s resist photolytic, chemical, and biological degradation hence persist in the environment for a longer period of time (Ali and Khan 2018). Although not biomagnified as persistent organic pollutants, metals and As show increasing bioaccumulation trend through the food chain. Because of potential trophic transfer, species staying at higher trophic levels are more vulnerable due to their toxicity (Green et al. 2010; Cai et al. 2009). Moreover, distribution pattern, behavior, and toxicity mechanism of metal(loid)s differ between terrestrial and aquatic habitat. When compared with terrestrial environment, metals and As are readily bioavailable in aquatic habitat (Ali and Khan 2019; Szynkowska et al. 2018; Burger and Eichhorst 2005). In modern ecotoxicological studies, best indicator species are used to assess the environmental health and associated risks. Birds from terrestrial and aquatic food chains are among the best indicator for metal(loid) toxicity because they stay higher at the food chain, having longer life span and wide range of dietary sources hence increasing their exposure (Abbasi et al. 2015a).
In past literature, it has been established that exposure to elevated levels of metal(loid)s are associated with various neurological, physiological, reproductive, developmental, and behavioral impairments in birds (Schmude et al. 2018; Espín et al. 2014a, b). In few of recent studies on birds, it has been reported that high metal(loid) concentrations are responsible for oxidative damage (Espín et al. 2014a, b, 2016a, b; Koivula et al. 2011; Martinez-Haro et al. 2011). In living organisms, reactive oxygen species (ROS) and free radicals are generated as a result of aerobic metabolism. To encounter the adverse effects of these, antioxidants defense mechanism is co-evolved with aerobic metabolism (Monaghan et al. 2009). The antioxidant defense system is composed of different enzymatic system such as, superoxide dismutase (SOD) and catalase (CAT), as well as non-enzymatic like glutathione (GSH) antioxidants which are released to regulate the level of preoxidants (Koivula and Eeva 2010; Monaghan et al. 2009). Glutathione is a major antioxidant, which has an ability to bind directly with ROS and convert them in less-harmful substances (Koivula and Eeva 2010). In normal conditions, glutathione is present in its reduced form (GSH), but when ROS levels are high, another enzyme glutathione peroxidase (GPx) converts GSH into its oxidized form (glutathione disulfide (GSSG)) which facilitates the conversion of ROS into their less-reactive form. Some other important enzymes that are involve in breakdown and conversion of (ROS) into less-reactive species are SOD, CAT, and glutathione S-transferase (GST) which catalyze ROS into their less-reactive forms (Espín et al. 2014a). However, in response to various environmental stressors such as increased exposure to metal(loid)s, antioxidants may fail to overcome the effects of ROS which are responsible to damage the protein, lipids, and DNA in cells and causes serious health effects (Sánchez-Virosta et al. 2019). This condition is termed as oxidative stress. Few of the previous studies has reported the antioxidant activities and lipid peroxidation as useful biomarkers to measure oxidative stress in living organisms in relation to metal concentrations (Costantini and Verhulst 2009; Berglund et al. 2007). However, there are very few studies available on oxidative damage caused by As and heavy metals in birds using internal tissues (Espín et al. 2014a; Koivula et al. 2011; Martinez-Haro et al. 2011; Berglund et al. 2007; Custer et al. 2006; Hoffman et al. 1998, 2011). Moreover, the accumulation, detoxification, and elimination mechanism of metal(loid)s vary among species as well as among various tissues within an individuals and so does oxidative stress (Koivula and Eeva 2010; Lucia et al. 2010). Hence, it is also of great importance to compare metal(loid)-induced oxidative stress among various tissues of birds from the same species. Further, there is a need to investigate the differences in metal(loid)-induced oxidative stress among various species, habitat, and taxonomic and trophic groups. Although there is a dearth of literature on this important concern of metal(loid) toxicity, this aspect is particularly neglected in South Asian region. Seeing this important research gap in ecotoxicological studies, current study was designed to elucidate the metal (Pb, Cd, Cr, and Cu) and metalloid (As) levels and their potential effects on oxidative stress biomarkers in four selected species of birds from Pakistan. Further, we have also investigated the interspecific differences as well as metal(loid)-induced oxidative stress differences between terrestrial and aquatic food chains. The selected bird species were native and relatively abundant in the study area. Their position at the upper level of the food chain makes them a suitable indicator of environmental contamination. Further, their responses can provide information regarding environmental changes occurring at lower trophic levels.
Material and methods
Study area and sampling
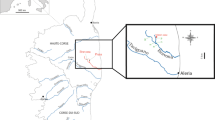
Sampling was conducted from September 2016 to April 2017 in two different regions viz. Manga mandi (31° 18′ 5″ N and 74° 3′ 43″ E) and Muridke (31° 48′ 9.00″ N and 74° 15′ 42.01″ E) (Fig. 1) located in the outskirts of Lahore which is the second largest metropolis of Pakistan. Manga mandi and Muridke are about 45 and 29 km from Lahore, respectively. These are typical agro-industrial areas of Lahore having chemicals, metallurgy, plastics, smelting, and small electroplating units around it (Khan et al. 2016). Further, these areas receive agricultural, industrial, and urban run-off in large quantity from Lahore City (Ahmad and Bhattacharya 2018; Abbas et al. 2007). A few studies reported high levels of metal(loid)s in soil, water, and food stuff from the study sites (Randhawa et al. 2014; Waseem et al. 2014). We collected two terrestrial (spotted owlet (Athene brama F; n = 6), bank myna (Acridotheres ginginianus L; n = 16)) and two aquatic (pond heron (Ardeola grayii F. n = 10), cattle egret (Bubulcus ibis L; n = 10)) bird species from the study area. The samples for this study were collected from the birds captured by WWF Pakistan in the framework of some other studies. Blood samples (3–4 ml) were collected in heparinized tubes containing anti-coagulating reagent from brachial vein of birds using 23G needles and stored at − 80 °C. After blood collection, birds were euthanized and internal tissues (liver and kidney) were collected in acetone cleaned zipped bags and subsequently stored in Environmental Biology Laboratory, Quaid-i-Azam University Islamabad at − 80 °C prior to analysis. The handling of birds samples was approved from bioethical committee of Quaid-i-Azam University Islamabad, Pakistan.
Map showing sampling sites from different locations of Lahore, Pakistan. The detail of birds collected at different sampling sites are given in corresponding Table S1
Arsenic and metal analysis
The glassware (Pyrex, Germany) used for analysis were first soaked overnight in 10% HNO3 bath followed by washing and rinsing with distilled water (Gochfeld and Michael 2000). Weighed samples (0.5 g) of all the tissues were firstly homogenized (0.1 M KCL, 50 mM potassium phosphate, pH 7.4 with 20% glycerol) using a Teflon homogenizer. These homogenized samples were then digested on a hotplate at 120 °C for about 20 min with 2 ml of 70% (V/V) nitric acid (HNO3) and 1 ml of (30% H2O2) for quantifications of Cd, Pb, Cu, Zn, and As (Abbasi et al. 2015a, b). Similarly, weighed blood samples (1 ml) were digested on hotplate with 2.5 ml mixture of sulfuric acid (95% H2SO4), perchloric acid (70% HClO4), and nitric acid (70% HNO3) (1:8:8) at 160 °C for 15–20 min (Dolan et al. 2017). The digested samples were filtered using Whatman filter paper No. 42 (Kavon, USA), and the volumes of the samples were raised up to 25 ml with Milli-Q water prior to their analysis. Analytical blanks were run after every five samples in the same way to ensure analytical precision. Pesticide-grade solvent obtained from Merck, Germany were used throughout the experiment. The certified reference material DOLT-3 (dogfish liver, National Research Council of Canada, 11 Ottawa, Canada) was used to evaluate the precision of the method. The concentrations of metals (Pb, Cd, Cu, Zn) were determined by using Voltammeter VA-797, (Computrace, 797) whereas As was analyzed by ICP-MS (Agilent, 7500) and values were expressed in micrograms per gram ww. For each element, the limit of detection was calculated by repeated blank analysis three times as used for sample analysis, determining the standard deviation (SD) and multiplying by three times. The LOD were 0.007 μg l−1 for As, 0.021 μg l−1 for Pb, 0.0009 μg L−1 for Cd, 0.09 μg l−1 for Cu, and 0.3 μg L−1 for Zn.
Biochemical analysis
Activity of oxidative stress biomarkers (CAT, SOD, tGSH, and thiobarbituric acid-reactive substances (TBARS)) were analyzed in the liver, kidney, and blood of all the bird species. For analysis, homogenates of tissues were prepared using 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer containing 0.1 mg phenylmethylsulphonyl fluoride (PMSF) in homogenizer. Whole blood samples were taken in Eppendorf tubes, homogenized, and subsequently diluted in 0.9% NaCl. The TBARS levels were used as proxy to evaluate lipid peroxidation following the procedure given by Alonso-Alvarez et al. (2008) using 0.1 ml of sample homogenate, 0.1 ml of a freshly prepared 1 mM FeSO4 solution, 0.1 ml of 150 Mm tris-HCl, 0.1 ml of 1.5 mM ascorbic acid, 0.6 ml of water, 1 ml of 0.37% of TBA, and 1 ml of 10% TCA, and absorbance was noted at 532 nm by spectrophotometer. The levels of lipid peroxidation were expressed as micromoles per gram. The activity of SOD enzyme was analyzed using the method early followed by Beyer and Fridovich (1987). For SOD, the absorbance was measured at 560 nm using a spectrophotometer. The activity of SOD was expressed as units per gram of protein. Similarly, level of tGSH was measured by following the method Reglero et al. (2009) using GSH standard. The level of tGSH in tissues was determined by mixing an aliquot of 0.1 ml from homogenized tissue, 1 ml of sodium phosphate buffer (0.4 M) (pH = 7.5) and 0.5 ml of 5,5′dithiobis (2-nitro benzoic acid (DTNB)). Mixture was stirred vigorously and placed at room temperature for 15 min until yellow color appear and absorbance was noted at 512 nm. The activity of tGSH was expressed in micromoles per gram. The activity of CAT was measured using method earlier described by Aebi (1984) based on the decomposition of hydrogen peroxide (H2O2) in molecular oxygen and water by this enzyme. The rate of enzymatic decomposition of H2O2 was determined by change in absorbance at 240 nm with a spectrophotometer (UV-1603, Shimadzu). The activity of CAT was expressed as micromoles H2O2 consumed per minute per milligram of protein. The activities of enzymes were expressed in relation to protein in homogenates and blood samples using Bradford’s (1976) method where bovine serum albumin (BSA) was used as standard. The absorbance was noted at 595 nm. The concentrations of samples were determined using BSA standard curve (S.I. Fig. 3).
Statistical computation
All the statistical computations were carried out using SPSS (IBM Statistics 20). For all models, significant values were set at 5% (α = 0.05) to reject null hypothesis. A first normality of data was evaluated using Kolmogorov–Smirnov test (KS test), and skewness was found within range. Descriptive statistics were calculated for all variables. Analysis of variance (ANOVA) was applied to determine the variations of metal(loid) levels and biomarker response among species, tissues, and taxonomic and trophic groups. Multiple comparison test (Tukey test) was used to investigate differences among variables when null hypothesis in ANOVA was rejected. Simple Pearson correlation and regression were performed to evaluate associations of metal(loid)s with oxidative stress biomarkers activities. Significant associations of metal(loid)s on biomarker activities were shown by regression graphs using Minitab 18 (trial version). Levels of metal(loid) and biomarker response in all species are illustrated through bar graphs.
Results and discussion
Concentration profile of arsenic and metals
The holistic summary of metal (Pb, Cd, Zn, Cu) and metalloid (As) concentration as well as activities and levels of corresponding oxidative stress biomarkers in different matrices (liver, kidney, and blood) of birds is summarized in Table 1 and S.I. Figs. 1 and 2. The accumulation and subsequent distribution pattern for both metals and As differ among tissue and in general are either comparable or fairly higher than previous reported studies from Pakistan (Abbasi et al. 2015a, b; Mustafa et al. 2015). These high concentrations of metal(loids)s in different tissues of birds are the reflection of our polluted sampling sites receiving bulk of urban and industrial waste of Lahore City. In general, we observed the highest concentration trend of Zn followed by Pb, As, Cu, and Cd in studied tissues of birds (Table 1). Similarly, liver followed by kidney were generally found as the major tissues for metal(loid) bioaccumulation as they are involved in detoxification and excretion of harmful substances in the body (Abbasi et al. 2017; Dolan et al. 2017; Lucia et al. 2008; Nam et al. 2005). We compared our results with globally reported studies on similar or related species and tissues to evaluate the analogy and differences by converting literature values to approximate wet weight equivalent (Kim and Koo 2007) wherever required. In the current study, highest concentration (mean (min-max) μg g−1 ww) were recorded for Pb (17.74 (13.21–23.3)) and As (12.58 (9.98–19.14)) in the kidney of pond heron, Cd (1.37 (0.93–1.65)) in the kidney of cattle egret, and Zn (202.71 (166.65–237.95)) and Cu (29.86 (14.23–43.12) in the liver of pond heron and cattle egret, respectively. On the contrary, the lowest values of all the metals were recorded in the blood of these species (Table 1). Considerably high concentration in internal tissues reflects constant historical exposure of the species where lower concentrations in the blood showed least exposure through diet. The highest concentrations of metals and As recorded in this study is either comparable or fairly higher in the same tissues of these or closely related species studied elsewhere (Teraoka et al. 2018; Zarrintab and Mirzaei 2018; Ishii et al. 2017; Mikoni et al. 2017; Finger et al. 2016; Nikolic et al. 2016; Binkowski et al. 2013; Kim and Oh 2013; Jayakumar and Muralidharan 2011; Lucia et al. 2010; Nam and Lee 2009; Perez-Lopez et al. 2008; Kim et al. 2008; Kim and Koo 2007; Deng et al. 2007; Dauwe et al. 2005; Kalisińska et al. 2004; Kavun 2004; Beyer et al. 2004; Golden et al. 2003). To summarize this section, considerably high exposure of metals and As in different tissues of birds was recorded in the vicinity of Lahore, indicating heavy pollution load coming from various industrial and urban sources. We suspect that still the use of metals and metalloids in different industries and their subsequent discharge through untreated effluent as well as improper waste disposal from urban sources are posing serious health concerns to human and wildlife.
Metal- and As-induced oxidative stress response
In this study, we investigated the relationship between the activity of oxidative stress biomarkers such as SOD, CAT, and tGSH, as well as TBARS with metal(loid) concentrations to study the potential oxidative damage caused through these contaminants. As a result, correlation (S.I. Tables 3, 4, and 5) and regression analysis (S.I. Tables 3 and 4) showed a significant association for metals and As with oxidative stress biomarkers. Among metals, Cd was strongly an associated activity of CAT in the kidney (R2 = 0.72, p < 0.00) and SOD activity in the liver (R2 = 0.60, p < 0.00) of cattle egret, respectively. In addition, Cd was found to mediate TBARS levels in liver (R2 = 0.71, p < 0.00) of cattle egret and blood (R2 = 0.64, p < 0.05) of spotted owlet. Similarly, As was associated with activity of CAT in the kidney (R2 = 0.53, p < 0.05) of a spotted owlet. High concentration of Pb was found to affect the activities of SOD in the blood (R2 = 0.58, p < 0.01) of pond heron and CAT activity in the liver (R2 = 0.52, p < 0.05) of cattle egret (S.I. Table 3; Fig. 2). The enhanced activities of SOD and CAT can be interpreted as an antioxidant defense mechanism of bird species against high levels of metal(loid) exposure. The lipid peroxidation may reflect that oxidative stress has surpassed the defense mechanism leading to lipid damage. Conclusively, the significant associations of As and metals on oxidative stress biomarkers reflect the internal mechanism of species which triggers to upregulate these antioxidants to overcome oxidative stress. Our findings are parallel to few previous studies where metals have been found to influence the activity of oxidative stress biomarkers (Espín et al. 2014a, b, 2016a, b).
Regression layout between concentrations of metal(loid)s (As, Pb, Cd, Cu) and related biomarker activities in blood and different tissues (liver, kidney) of bank myna, spotted owlet, pond heron and cattle egret. Only significant regression was plotted. Regression is significant (p < 0.05) and (p = 0.01)
To further explain the metal(loid)-mediated oxidative stress, correlation analysis also showed significant associations of As and metals with biomarkers of oxidative stress. We found that SOD activity was negatively influenced by Cd concentration (r = − 0.64, p = < 0.04) in the liver of pond heron (S.I. Table 4) which is coupled with an earlier study (Kamiński et al. 2009) on white stork (Ciconia ciconia) from Poland. In our study, Pb was found to be negatively correlated with SOD activity (r = −0.74, p = <0.01) in blood of pond heron (S.I. Table 4) as previously found (Espín et al. 2014a, b) in Eurasian eagle owl (Bubo bubo) from a mining site in Spain. This is because SOD needs Cu for its proper functioning, while Pb may reduce Cu levels in the body hence decreasing the activity of SOD (Gurer and Ercal 2000). Among all the studied metals, Cd showed strong positive correlation with CAT activities. Our findings are in accordance with a few previous studies (Kant et al. 2017; Bharavi et al. 2010). It is attributed to the fact that CAT use H2O2 as substrate and Cd exposure have been shown to increase the inorganic H2O2 levels by Fenton type reaction (Bharavi et al. 2010; Liochev and Fridovich 1999) resulting in high oxidative stress (Watjen and Beyersmann 2004). The normal rate of H2O2 production is mainly balanced by GPx, but as H2O2 production increases, CAT becomes more important in oxidative effects (Halliwell and Gutteridge 1999) preventing oxidative stress and breakdown of H2O2 (Ahmad 1995). In the spotted owlet, Cd in liver was associated with TBARS (r = 0.82, p = 0.04) (S.I. Table 4). Several studies have reported similar relationship of As, Pb, and Cd with TBARS in different bird species (Espín et al. 2014a, b). Similarly, tGSH activity was also found to be significantly associated with As, Cd, Zn, Cu, and Pb in different tissues (S.I. Tables 4 and 5). Earlier, the study conducted on starlings also showed that long-term exposure to Cd resulted in increased activity of tGSH levels (Congiu et al. 2000). The correlation and regression analysis suggest that a high level of metal(loid)s strongly influenced the activities of antioxidants and TBARS in birds. In general, our results also suggested that SOD and CAT are more sensitive toward metal(loid) concentration while As has the potential to induce lipid peroxidation in birds (S.I. Tables 2, 3, 4, and 5). Further, certain oxidative stress biomarkers are influenced by the activity of each other (S.I Table 6) as they may balance each other during oxidative stress. The differential response in tissues and species is because of several intra- and interspecific variables (Espín et al. 2016a, b). The species-specific differences in birds suggest that antioxidant defense system can vary widely depending upon species because the mechanism to rectify the contaminants may vary among species, tissues, and taxonomic and trophic groups, as well as certain other confounding variables (Abbasi et al. 2017; Espín et al. 2014a, b, 2016a, b) which are discussed in the following section.
Interspecific variations
In this study, we aimed to analyze the differences of metal and As levels on oxidative stress responses among species, tissues, habitats, as well as taxonomic and trophic levels. The interspecific variation among metals and As and subsequent implications on antioxidant defense were analyzed with ANOVA (Tables 2, 3, and 4) and subsequent Tukey HSD test. The levels of heavy metals and As as well as the activities of oxidative stress biomarkers generally differ significantly (< 0.05) for the tested variables such as species, tissues, habitat, and trophic and taxonomic affiliations. Hence, the results showed a strong influence of these aforementioned variables in the overall metal and metalloid burden in birds.
Species-specific variations
In the present study, concentrations of metals and As as well as activities/level of oxidative stress biomarkers differ significantly (p < 0.05) except TBARS levels in blood of different species (Table 2). Our findings of interspecies differences are in accordance to previous findings (Abbasi et al. 2015a, b; Kim and Koo 2007; Dauwe et al. 2005). In general, we found spotted owlet as most contaminated species with relatively higher accumulation of metal(loid)s in different tissues followed by cattle egret, pond heron, and bank myna (Table 2). This differential accumulation of metal(loid)s could be partially attributed to the dietary habit of the species (Aloupi et al. 2017; Sinka-Karimi et al. 2015; Abigail et al. 2013). Spotted owlet being specific carnivorous accumulate higher concentration of metal(oid)s. The diet of cattle egret and gray heron mainly comprised of small fishes and invertebrates hence reflect similar levels of metal(loid) accumulation in their tissues. On the other hand, bank myna feeds mainly on fruits such as berries as well as small insect hence accumulate least concentrations of metal(loid)s. Further, this differential accumulation of species could be attributed to the differences in levels of pollution from where these species are collected. Besides these, there are several confounding factors such as age, gender, and reproductive status which may influence the bioaccumulation of metal(loid)s in birds (Espín et al. 2014a, b). Overall, species differences for metal- and As-induced oxidative stress were in the sequence of bank myna > cattle egret > spotted owlet > pond heron (Table 2; S.I. Fig. 1). This trend of metal- and As-induced oxidative stress biomarkers can be explained by several confounding variables such as age, gender, trophic level, habitat differences, metabolic rates, storage, and excretion of contaminants, species-specific resistance to metal(loid)s, basal antioxidant capacity, exposure, dietary habits, molting period, variation in nutritional needs during breeding, life span, migration of different bird species, as well as variable environmental conditions (Aloupi et al. 2017; Sinka-Karimi et al. 2015). Hence, we think that several confounding variables may synergistically influence the level of metal(loid)s and corresponding oxidative stress responses in different species of birds.
Tissue-specific variations
The metal and As distribution in internal tissues of birds is regulated by their uptake, storage, and excretion mechanism (Aloupi et al. 2017). The organotropism of metals and As can be determined by the level of exposure, speciation of each element, reactions with other toxins, and inherent physiological mechanism of the species (Aloupi et al. 2017; Gochfeld and Burger 1987). In general, we found a trend of metal(loid) accumulation as liver > kidney > blood (Table 3; Fig. S.I. 2). High levels of metal accumulation in liver is well established in the literature which is because of major role of liver in the detoxification as well as storage of metals (Lucia et al. 2010). The metallothioneins (MTs) of liver play an important part in this as they are known to strongly bind metallic bivalent cations (Pb2+, Cd2+, Zn2+, Cu2+, etc.) hence to regulate the homeostasis, storage, and detoxification of non-essential and regulation of additional essential metals (Nam et al. 2005). Comparison between tissues for metals and As and oxidative stress biomarkers (TBARS, CAT, GSH, SOD) reflected a significant (p < 0.001) difference among tissues except for TBARS and tGSH in spotted owlet (Table 3). Relatively higher levels of Pb, Cd, and As were found in kidneys of birds while essential elements (Zn and Cu) were higher in liver of birds (Fig. S.I. 2). Activities of SOD were higher in kidneys of bank myna. While CAT activities were higher in liver of pond heron, and lipid peroxidation damage was highest in kidneys of cattle egret. The tGSH activities were higher in cattle egret liver followed by spotted owlet kidneys. High tGSH activities in liver is attributed to the fact that liver is the main site for synthesis of GSH and is also responsible for detoxification of xenobiotic hence high rate of antioxidant levels is derived in the liver (Dauwe et al. 2005). On the other hand, blood reflects lowest concentrations of all the metals and As as well as oxidative stress biomarkers (Abbasi et al. 2017). Blood reflects dietary exposure which was found relatively lower in this study. Our results suggest that tissues involved in storage, transportation, and excretion of elements such as liver and kidney are under a high risk to oxidative damage in birds.
Habitat-specific variations
Bioaccumulation of metals and As varied between aquatic and terrestrial habitat because of different pathways in both the ecosystems. In this study, concentrations of all the metals and As differ significantly (p < 0.05) between aquatic and terrestrial species except Pb and Zn (Table 4). Similarly, the activities of SOD, CAT, and tGSH and level of TBARS also differ significantly (p < 0.05) between aquatic and terrestrial habitats (Table 4). The Zn is an essential element regulated by birds effectively even in case of high exposure. The mute swans (Cygnus olor) has been found to accumulate three times the concentration of hepatic Zn as compared with tundra swans (Cygnus columbianus) when fed with the same diet (Day et al. 2003). Hence, it is established that not only the exposure level but also the detoxification and excretion mechanism of species may induce changes in different habitats. Interestingly, we have found that concentrations of Cu, As, and Cd were fairly higher in aquatic species whereas Pb and Zn were slightly higher in terrestrial species. The elevated concentration of As in aquatic species is attributed to the established groundwater contamination of As from geogenic sources of the study area (Ali and Khan 2018). On the other hand, Pb and Cd are mainly discharged through industrial effluent, deposited in sediment, and become bioavailable for species which subsequently reaches higher trophic levels through food chain (Abbasi et al. 2015b; Lucia et al. 2010). Similarly, other metals such as Zn and Cu are also originated through anthropogenic as well as natural sources and reaches higher trophic level through dietary intake (Ali and Khan 2018; Wen et al. 2016; Franson et al. 2012). From the above discussion, it is clear that metals and As are readily available in aquatic habitat and pose more health risk when compared with terrestrial habitat. However, it largely depends upon the nature of pollutant, soil, sediment, water, and certain metrological factor which are responsible for transfer of pollutant from their sources of release to the aquatic habitat (Kim and Oh 2013). We concluded that differences of metals and As between terrestrial and aquatic habitat is mainly attributed to the magnitude of discharge from sources, pathways taken to reach living organisms, nature, and bioavailability of the pollutant in the environment. Further, aquatic species are more prone to bioaccumulate metals and As in their tissues which may cause some oxidative damage in birds inhabiting aquatic environment.
Taxonomic and trophic level-based variations
It is established that the level of contaminants and biomarker activities in birds are strongly influenced by factors such as trophic level and taxonomic affiliation (Abbasi et al. 2015a, b, 2017). To evaluate the potential effects of diet type on metal levels, the examined birds were divided into three groups viz. carnivorous, piscivorous, and omnivorous (Karmiris et al. 2010) and three families. Cattle egret and pond heron are categorized as piscivorous feeding on frogs, insects, tadpoles, and mice (Abigail et al. 2013) belong to Ardiedae family. Bank myna was categorized as grainivorous feeding on grains, insects, fruits, and crop pest (Bose and Das 2012) and represent the Strunidae family (Gopi and Pandav 2007). Spotted owlet was classified as carnivorous feeding on lizard, moths, carcasses of birds, etc. (Jadhav and Parasharya 2003) and belongs to the Strigidae family. Interestingly, all three families of birds considered for this study exhibits different feeding habits hence categorized under different trophic level. In this study, all the metal(loid)s except Pb nd Zn differed significantly (p < 0.05) among three families/trophic levels (Table 4). In fact, ingestion through diet is the major route of metal intake in birds which is responsible for differential accumulation of metals and As in different trophic levels (Abbasi et al. 2015b). Species having different dietary habits and flexibilities bioaccumulate metals and As differently in their tissues which may trigger oxidative stress. Although metals and As cannot be biomagnified as persistent organic pollutants, however, the increasing bioaccumulation trend through the food chain is observed which could majorly be explained through relatively greater dietary intake of the species residing high at the trophic level (Pérez-López et al. 2008). Further, the levels of metals and As also depend on the intake, distribution, storage, detoxification, and excretion mechanism exhibited by the species (Zolfaghari et al. 2009). Besides these, differences in bioaccumulation of metals and As as well as those of oxidative stress biomarkers could be attributed to the differences defense mechanism of each species controlled through inherited genetic makeup (Abbasi et al. 2015a; Zolfaghari et al. 2009). Species may respond differently under stress conditions because of different genetic and exposure history. Hence, it is concluded that trophic level and taxonomic affiliation of species are among the most important variables in determining the levels of metals and As in birds and subsequent possible oxidative stress responses.
Conclusion
Metals and As are found in elevated concentrations in tissues of birds which reflect still the use of these contaminants in developing parts of the world. In general, aquatic species showed more affinity for metal and As accumulation in their tissues consequently increased oxidative damage when compared with terrestrial species. Among tissues, the liver and kidney were found as major recipients of metal(loid) burden because of their physiological role in the body. Activities of SOD and CAT were found to be sensitive in terrestrial environment so is the tGSH and TBARS in aquatic environment. Variables such as species, tissues, habitat, and taxonomic and trophic levels significantly influence the concentration levels of metals and As and corresponding oxidative stress responses. However, consideration of variables such as age, gender, and reproductive status of birds from wider geographical scale would best predict the results. In the future, dose-response-based studies are recommended to evaluate the threshold level of metals and As in tissues which can trigger oxidative stress in birds.
References
Abbas ST, Sarfraz M, Mehdi SM, Hassan G (2007) Trace elements accumulation in soil and rice plants irrigated with the contaminated water. Soil Tillage Res 94(2):503–509. https://doi.org/10.1016/j.still.2006.10.004
Abbasi NA, Jaspers VLB, Chaudhry MJI, Ali S, Malik RN (2015a) Influence of taxa, trophic level, and location on bioaccumulation of toxic metals in bird’s feathers: a preliminary biomonitoring study using multiple bird species from Pakistan. Chemosphere 120:527–537. https://doi.org/10.1016/j.chemosphere.2014.08.054
Abbasi NA, Khan MU, Jaspers VLB, Chaudhry MJI, Malik RN (2015b) Spatial and interspecific variation of accumulated trace metals between remote and urbane dwelling birds of Pakistan. Ecotoxicol Environ Saf 113:279–286. https://doi.org/10.1016/j.ecoenv.2014.11.034
Abbasi NA, Arukwe A, Jaspers VLB, Eulaers I, Mennillo E, Ibor OR, Frantz A, Covaci A, Malik RN (2017) Oxidative stress responses in relationship to persistent organic pollutant levels in feathers and blood of two predatory bird species from Pakistan. Sci Total Environ 580:26–33. https://doi.org/10.1016/j.scitotenv.2016.11.197
Abigail K, Rosina K, Daniel AK, Holbech LH (2013) Foraging activities, success and efficiency of cattle egrets (Bubulcus ibis) in three habitat types in the Greater Accra region of Ghana. J Biol Food Sci Res 2(4):45–50
Aebi H (1984) [13] Catalase in vitro. In: Methods in enzymology, vol 105. Academic Press, pp 121–126
Ahmad S (1995) Antioxidant mechanism of enzymes and proteins. In: Ahmad S (ed) Oxidative stress and antioxidant defences in biology. Chapman & Hall, New York, pp 238–272. https://doi.org/10.1007/978-1-4615-9689-9_7
Ahmad A, Bhattacharya P (2018) Arsenic contamination of groundwater in Indus River Basin of Pakistan. In: Groundwater of South Asia. Springer, Singapore, pp 393–403. https://doi.org/10.1007/978-981-10-3889-1_24
Ali H, Khan E (2018) Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—concepts and implications for wildlife and human health. Hum Ecol Risk Assess Int J 25(6):1353–1376. https://doi.org/10.1080/10807039.2018.1469398
Alonso-Alvarez C, Pérez-Rodríguez L, Mateo R, Chastel O, Vinuela J (2008) The oxidation handicap hypothesis and the carotenoid allocation trade-off. J Evol Biol 21(6):1789–1797. https://doi.org/10.1111/j.1420-9101.2008.01591.x
Aloupi M, Karagianni A, Kazantzidis S, Akriotis T (2017) Heavy metals in liver and brain of waterfowl from the Evros Delta, Greece. Arch Environ Contam Toxicol 72(2):215–234. https://doi.org/10.1007/s00244-016-0349-6
Berglund ÅM, Sturve J, Förlin L, Nyholm N (2007) Oxidative stress in pied flycatcher (Ficedula hypoleuca) nestlings from metal contaminated environments in northern Sweden. Environ Res 105(3):330–339
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161(2):559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Beyer W, Dalgarn J, Dudding S, French J, Mateo R, Miesner J, Spann J (2004) Zinc and lead poisoning in wild birds in the Tri-State Mining District (Oklahoma, Kansas, and Missouri). Arch Environ Contam Toxicol 48(1):108–117. https://doi.org/10.1007/s00244-004-0010-7
Bharavi K, Reddy AG, Rao G, Reddy AR, Rao SR (2010) Reversal of cadmium-induced oxidative stress in chicken by herbal adaptogens Withania somnifera and Ocimum sanctum. Toxicol Int 17(2):59. https://doi.org/10.4103/0971-6580.72671
Binkowski ŁJ, Sawicka-Kapusta K, Szarek J, Strzyżewska E, Felsmann M (2013) Histopathology of liver and kidneys of wild living mallards Anas platyrhynchos and coots Fulica atra with considerable concentrations of lead and cadmium. Sci Total Environ 450:326–333. https://doi.org/10.1016/j.scitotenv.2013.02.002
Bose SN, Das VK (2012) Distribution, habit and reproductive activity of Bank myna, Acridotheres ginginianus (Latham) in relation to natural photoperiod. J Appl Biosci 38(1):108–112
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Burger J, Eichhorst B (2005) Heavy metals and selenium in grebe eggs from Agassiz National Wildlife Refuge in northern Minnesota. Environ Monit Assess 107:285–295
Cai Q, Long ML, Zhu M, Zhou QZ, Zhang L, Liu J (2009) Food chain transfer of cadmium and lead to cattle in a lead-zinc smelter in Guizhou, China. Environ Pollut 157:3078e3082
Congiu L, Chicca M, Pilastro A, Turchetto M, Tallandini L (2000) Effects of chronic dietary cadmium on hepatic glutathione levels and glutathione peroxidase activity in starlings (Sturnus vulgaris). Arch Environ Contam Toxicol 38(3):357–361. https://doi.org/10.1007/s002449910047
Costantini D, Verhulst S (2009) Does high antioxidant capacity indicate low oxidative stress? Funct Ecol 23(3):506–509. https://doi.org/10.1111/j.1365-2435.2009.01546.x
Custer CM, Custer TW, Warburton D, Hoffman DJ, Bickham JW, Matson CW (2006) Trace element concentrations and bioindicator responses in tree swallows from northwestern Minnesota. Environ Monit Assess 118(1–3):247–266. https://doi.org/10.1007/s10661-006-1499-1
Dauwe T, Janssens E, Bervoets L, Blust R, Eens M (2005) Heavy-metal concentrations in female laying great tits (Parus major) and their clutches. Arch Environ Contam Toxicol 49(2):249–256. https://doi.org/10.1007/s00244-003-0209-z
Day DD, Beyer WN, Hoffman DJ, Morton A, Sileo L, Audet DJ, Ottinger MA (2003) Toxicity of lead-contaminated sediment to mute swans. Arch Environ Contam Toxicol 44:510–522. https://doi.org/10.1007/s00244-002-1140-4
Deng H, Zhang Z, Chang C, Wang Y (2007) Trace metal concentration in great tit (Parus major) and greenfinch (Carduelis sinica) at the Western Mountains of Beijing, China. Environ Pollut 148(2):620–626. https://doi.org/10.1016/j.envpol.2006.11.012
Dolan KJ, Ciesielski TM, Lierhagen S, Eulaers I, Nygård T, Johnsen TV, Ortiz-Santaliestra ME (2017) Trace element concentrations in feathers and blood of northern goshawk (Accipiter gentilis) nestlings from Norway and Spain. Ecotoxicol Environ Saf 144:564–571. https://doi.org/10.1016/j.ecoenv.2017.06.062
Espín S, Martínez-López E, Jiménez P, María-Mojica P, García-Fernández AJ (2014a) Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture (Gyps fulvus). Environ Res 129:59–68. https://doi.org/10.1016/j.envres.2013.11.008
Espín S, Martínez-López E, León-Ortega M, Martínez JE, García-Fernández AJ (2014b) Oxidative stress biomarkers in Eurasian eagle owls (Bubo bubo) in three different scenarios of heavy metal exposure. Environ Res 131:134–144. https://doi.org/10.1016/j.envres.2014.03.015
Espín S, García-Fernández AJ, Herzke D, Shore RF, van Hattum B, Martínez-López E, Gómez-Ramírez P (2016a) Tracking pan-continental trends in environmental contamination using sentinel raptors—what types of samples should we use? Ecotoxicology 25(4):777–801. https://doi.org/10.1007/s10646-016-1636-8
Espín S, Martínez-López E, Jiménez P, María-Mojica P, García-Fernández AJ (2016b) Interspecific differences in the antioxidant capacity of two Laridae species exposed to metals. Environ Res 147:115–124. https://doi.org/10.1016/j.envres.2016.01.029
Finger A, Lavers JL, Orbell JD, Dann P, Nugegoda D, Scarpaci C (2016) Seasonal variation and annual trends of metals and metalloids in the blood of the little penguin (Eudyptula minor). Mar Pollut Bull 110(1):261–273. https://doi.org/10.1016/j.marpolbul.2016.06.055
Franson JC, Lahner LL, Meteyer CU, Rattner BA (2012) Copper pellets simulating oral exposure to copper ammunition: absence of toxicity in American kestrels (Falco sparverius). Arch Environ Contam Toxicol 62(1):145–153. https://doi.org/10.1007/s00244-011-9671-1
Gochfeld M, Burger J (1987) Factors affecting tissue distribution of heavy metals. Biol Trace Elem Res 12(1):389–399. https://doi.org/10.1007/BF02796695
Gochfeld JB, Michael (2000) Effects of lead on birds (Laridae): a review of laboratory and field studies. J Toxicol Environ Health B Crit Rev 3(2):59–78. https://doi.org/10.1080/109374000281096
Golden N, Rattner B, McGowan P, Parsons K, Ottinger M (2003) Concentrations of metals in feathers and blood of nestling black-crowned night-herons (Nycticorax nycticorax) in Chesapeake and Delaware bays. Bull Environ Contam Toxicol 70(2):0385–0393. 10.1007%2Fs00128-002-0203-6
Gopi GV, Pandav B (2007) Avifauna of Bhitarkanika mangroves, India. Zoos’ Print J 22(10):2839–2847
Green ID, Diaz A, Tibbett M (2010) Factors affecting the concentration in seven spotted ladybirds (Coccinella septempunctata L.) of Cd and Zn transferred through the food chain. Environ Pollut 158:135e141
Gurer H, Ercal N (2000) Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med 29(10):927–945. https://doi.org/10.1016/S0891-5849(00)00413-5
Halliwell B, Gutteridge JM (1999) Free radicals in biology and medicine. Clarendon Press, Oxford
Hoffman DJ, Ohlendorf HM, Marn CM, Pendleton GW (1998) Association of mercury and selenium with altered glutathione metabolism and oxidative stress in diving ducks from the San Francisco Bay region, USA. Environ Toxicol Chem 17(2):167–172. https://doi.org/10.1002/etc.5620170205
Hoffman DJ, Eagles-Smith CA, Ackerman JT, Adelsbach TL, Stebbins KR (2011) Oxidative stress response of Forster’s terns (Sterna forsteri) and Caspian terns (Hydroprogne caspia) to mercury and selenium bioaccumulation in liver, kidney, and brain. Environ Toxicol Chem 30(4):920–929. https://doi.org/10.1002/etc.459
Ishii C, Ikenaka Y, Nakayama SM, Mizukawa H, Yohannes YB, Watanuki Y, Ishizuka M (2017) Contamination status and accumulation characteristics of heavy metals and arsenic in five seabird species from the central Bering Sea. J Vet Med Sci 79(4):807–814
Jadhav A, Parasharya BM (2003) Some observations on the nesting behaviour and food of the spotted owlet Athene brama. Zoos’ Print J 18(8):1163–1165
Jayakumar R, Muralidharan S (2011) Metal contamination in select species of birds in nilgiris district, Tamil Nadu, India. Bull Environ Contam Toxicol 87(2):166–170. https://doi.org/10.1007/s00128-011-0323-y
Kalisińska E, Salicki W, Mysłek P, Kavetska KM, Jackowski A (2004) Using the mallard to biomonitor heavy metal contamination of wetlands in North-Western Poland. Sci Total Environ 320(2):145–161. https://doi.org/10.1016/j.scitotenv.2003.08.014
Kamiński P, Kurhalyuk N, Jerzak L, Kasprzak M, Tkachenko H, Klawe JJ, Wiśniewska E (2009) Ecophysiological determinations of antioxidant enzymes and lipoperoxidation in the blood of white stork Ciconia ciconia from Poland. Environ Res 109(1):29–39. https://doi.org/10.1016/j.envres.2008.07.013
Kant V, Mehta M, Varshneya C, Chauhan S (2017) Induction of oxidative stress by subacute oral exposure of cadmium sulphate in adult poultry. Braz J Vet Pathol 4(2):117–121
Karmiris I, Kazantzidis S, Papachristou TG (2010) Variation in diet composition of wintering waterfowl among Greek wetlands. Avocetta 34(1):21–28
Kavun VY (2004) Heavy metals in organs and tissues of the European black vulture (Aegypius monachus): dependence on living conditions. Russ J Ecol 35(1):51–54
Khan MU, Shahbaz N, Waheed S, Mahmood A, Shinwari ZK, Malik RN (2016) Comparative health risk surveillance of heavy metals via dietary foodstuff consumption in different land-use types of Pakistan. Hum Ecol Risk Assess Int J 22(1):168–186
Kim J, Koo TH (2007) Heavy metal concentrations in diet and livers of black-crowned night heron Nycticorax nycticorax and Grey heron Ardea cinerea chicks from Pyeongtaek, Korea. Ecotoxicology 16(5):411–416. https://doi.org/10.1007/s10646-007-0143-3
Kim J, Oh JM (2013) Assessment of trace metals in four bird species from Korea. Environ Monit Assess 185(8):6847–6854. https://doi.org/10.1007/s10661-013-3069-7
Kim J, Lee H, Koo T-H (2008) Heavy-metal concentrations in three owl species from Korea. Ecotoxicology 17:21–28
Koivula MJ, Eeva T (2010) Metal-related oxidative stress in birds. Environ Pollut 158(7):2359–2370. https://doi.org/10.1016/j.envpol.2010.03.013
Koivula MJ, Kanerva M, Salminen J-P, Nikinmaa M, Eeva T (2011) Metal pollution indirectly increases oxidative stress in great tit (Parus major) nestlings. Environ Res 111(3):362–370. https://doi.org/10.1016/j.envres.2011.01.005
Liochev SI, Fridovich I (1999) Superoxide and iron: partners in crime. IUBMB Life 48(2):157–161. https://doi.org/10.1080/713803492
Lucia M, André JM, Bernadet MD, Gontier K, Guy G, Davail S (2008) Concentrations of metals (zinc, copper, cadmium, and mercury) in three domestic ducks in France: Pekin, Muscovy, and Mule ducks. J Agric Food Chem 56:281–288
Lucia M, André J-M, Gontier K, Diot N, Veiga J, Davail S (2010) Trace element concentrations (mercury, cadmium, copper, zinc, lead, aluminium, nickel, arsenic, and selenium) in some aquatic birds of the Southwest Atlantic Coast of France. Arch Environ Contam Toxicol 58(3):844–853. https://doi.org/10.1007/s00244-009-9393-9
Martinez-Haro M, Green AJ, Mateo R (2011) Effects of lead exposure on oxidative stress biomarkers and plasma biochemistry in waterbirds in the field. Environ Res 111(4):530–538. https://doi.org/10.1016/j.envres.2011.02.012
Mikoni NA, Poppenga R, Ackerman JT, Foley J, Hazlehurst J, Purdin G, Tell LA (2017) Trace element contamination in feather and tissue samples from Anna’s hummingbirds. Ecol Indic 80:96–105. https://doi.org/10.1016/j.ecolind.2017.04.053
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12(1):75–92
Mustafa I, Ghani A, Arif N, Asif S, Khan MR, Waqas A, Ahmed H (2015) Comparative metal profiles in different organs of house sparrow (Passer domesticus) and black kite (Milvus migrans) in Sargodha District, Punjab, Pakistan. Pak J Zool 47(4)
Nam DH, Lee D-P (2009) Abnormal lead exposure in globally threatened cinereous vultures (Aegypius monachus) wintering in South Korea. Ecotoxicology 18(2):225–229. https://doi.org/10.1007/s10646-008-0275-0
Nam DH, Anan Y, Ikemoto T, Tanabe S (2005) Multielemental accumulation and its intracellular distribution in tissues of some aquatic birds. Mar Pollut Bull 50:1347–1362. https://doi.org/10.1016/j.marpolbul.2005.05.004
Nikolic D, Djinovic-Stojanovic J, Jankovic S, Stefanovic S, Petrovic Z, Grujic S, Lausevic M (2016) Cadmium in pheasant tissues as a bioindicator of environmental pollution in 23 Serbian districts. Sci J “Meat Technol” 57(2):115–119
Pérez-López M, de Mendoza MH, Beceiro AL, Rodríguez FS (2008) Heavy metal (Cd, Pb, Zn) and metalloid (As) content in raptor species from Galicia (NW Spain). Ecotoxicol Environ Saf 70(1):154–162. https://doi.org/10.1016/j.ecoenv.2007.04.016
Randhawa MA, Ahmad G, Anjum FM, Asghar A, Sajid MW (2014) Heavy metal contents and their daily intake in vegetables under peri-urban farming system of Multan, Pakistan. Pak J Agric Sci 51(4)
Reglero MM, Taggart MA, Monsalve-Gonzalez L, Mateo R (2009) Heavy metal exposure in large game from a lead mining area: effects on oxidative stress and fatty acid composition in liver. Environ Pollut 157(4):1388–1395. https://doi.org/10.1016/j.envpol.2008.11.036
Sánchez-Virosta P, Espín S, Ruiz S, Stauffer J, Kanerva M, García-Fernández AJ, Eeva T (2019) Effects of calcium supplementation on oxidative status and oxidative damage in great tit nestlings inhabiting a metal-polluted area. Environ Res 171:484–492
Schmude E, Ertl HM, Taylor RJ, Mora MA (2018) Using feathers to evaluate adverse effects of metals on northern bobwhites (Colinus virginianus) in Texas. Arch Environ Contam Toxicol 75(1):87–95. https://doi.org/10.1007/s00244-018-0520-3
Sinka-Karimi MH, Pourkhabbaz AR, Hassanpour M, Levengood JM (2015) Study on metal concentrations in tissues of mallard and pochard from two major wintering sites in Southeastern Caspian Sea, Iran. Bull Environ Contam Toxicol 95(3):292–297. https://doi.org/10.1007/s00128-015-1591-8
Szynkowska MI, Pawlaczyk A, & Maćkiewicz E (2018) Bioaccumulation and Biomagnification of Trace Elements in the Environment. In: Chojnacka, K., & Saeid, A. (Eds.). Recent Advances in Trace Elements. John Wiley and Sons, Oxford UK. pp. 251–273. https://doi.org/10.1002/9781119133780
Teraoka H, Miyagi H, Haraguchi Y, Takase K, Kitazawa T, Noda J (2018) Contamination status of seven elements in hooded cranes wintering in south-West Kyushu, Japan: comparison with red-crowned cranes in Hokkaido, Japan. Arch Environ Contam Toxicol:1–9. https://doi.org/10.1007/s00244-018-0541-y
Waseem A, Arshad J, Iqbal F, Sajjad A, Mehmood Z, Murtaza G (2014) Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. Biomed Res Int 2014:1–29. https://doi.org/10.1155/2014/813206
Watjen W, Beyersmann D (2004) Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals 17(1):65–78
Wen J, Yi Y, Zeng G (2016) Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J Environ Manag 178:63–69. https://doi.org/10.1016/j.jenvman.2016.04.046
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 23(9):8244–8259. https://doi.org/10.1007/s11356-016-6333-x
Yang F, Xie S, Liu J, Wei C, Zhang H, Chen T, Zhang J (2018) Arsenic concentrations and speciation in wild birds from an abandoned realgar mine in China. Chemosphere 193:777–784
Zarrintab M, Mirzaei R (2018) Tissue distribution and oral exposure risk assessment of heavy metals in an urban bird: magpie from Central Iran. Environ Sci Pollut Res:1–10. https://doi.org/10.1007/s11356-018-1642-x
Zolfaghari G, Esmaili-Sari A, Ghasempouri SM, Baydokhti RR, Kiabi BH (2009) A multispecies-monitoring study about bioaccumulation of mercury in Iranian birds (Khuzestan to Persian Gulf): effect of taxonomic affiliation and trophic level. Environ Res 109(7):830–836. https://doi.org/10.1016/j.envres.2009.07.001
Acknowledgments
The authors are highly thankful to WWF Pakistan for providing support during field sampling of this project.
Funding
This research was conducted under university research funds from the Quaid-i-Azam University, Islamabad, Pakistan. Research was done when the first author was an MPhil Scholar under the supervision of Dr. Riffat Naseem Malik in the Environmental Laboratory, Quaid-i-Azam University, Islamabad.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• First ever report on metal(loid)-induced oxidative stress response in birds of Pakistan.
• Evaluated differences between habitat, species, tissues, and taxonomic and trophic groups.
• Elevated levels of metals and As were found in different tissues of studied birds.
• Found significant correlations between metal(loid)s and oxidative stress biomarkers.
• Highlighted the need of dose-response-based studies to best predict the results in future studies.
Electronic supplementary material
ESM 1
(DOC 406 kb)
Rights and permissions
About this article
Cite this article
Kanwal, S., Abbasi, N.A., Chaudhry, M.J.I. et al. Oxidative stress risk assessment through heavy metal and arsenic exposure in terrestrial and aquatic bird species of Pakistan. Environ Sci Pollut Res 27, 12293–12307 (2020). https://doi.org/10.1007/s11356-020-07649-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07649-z