Abstract
Polybrominated diphenyl ethers (PBDEs) are a class of brominated flame retardants (BFRs), present in the environment, animals, and humans. Their levels, distribution, and human exposure have been studied extensively, and over the last decade, various legal measures have been taken to prohibit or minimize their production and use due to the increasing amount of evidence of their harmful effects on human and animal health.Our aim here was to make a comprehensive and up-to-date review of the levels and distribution of PBDEs in the aquatic environment, air, and soil, in indoor dust, and in humans. To fulfill this, we searched through Web of Science for literature data reported in the last five years (2015–2019) on levels of at least six key PBDE congeners in abovementioned matrices. According to our summarized data, significant PBDE mass concentrations/fractions are still being detected in various sample types across the world, which implies that PBDE contamination is an ongoing problem. Secondary sources of PBDEs like contaminated soils and landfills, especially those with electronic and electrical waste (e-waste), represent a particular risk to the future and therefore require a special attention of scientists.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polybrominated diphenyl ethers (PBDEs) are a class of brominated flame retardants (BFRs) that came into use in the 1970s as additives to retard/reduce the combustibility of a variety of textile materials, furniture fillers (polyurethane foam), and electronic equipment (UNEP 2015).
There are 209 theoretically possible PBDE congeners divided into 10 congener groups depending on the number of bromine atoms, but they all share a common structure consisting of a brominated diphenyl ether molecule with two benzene rings connected with an oxygen atom (Fig. 1). For commercial use, they are mixed in various percentages (La Guardia et al. 2006) and marketed in three formulations named after the prevalent congener group in the mixture: (1) the “penta” formulation, (2) the “octa” formulation, and (3) the “deca” formulation. Respectively, they accounted for 11%, 6%, and 83% of the global production in 2001 (La Guardia et al. 2006; US EPA 2010).
In contrast to certain BFRs that are mixed with plastic before polymerization to form covalent bonds, PBDEs are added to polymers without forming chemical bonds with the materials and can therefore easily migrate into the surrounding air, dust, soil, and water during their lifetime (Zhang et al. 2011; UNEP 2015; Anh et al. 2017). They were first measured in the environment in the 1980s (Andersson and Blomkvist 1981), and have penetrated all environmental compartments ever since (US EPA 2010). In 2009, the United Nations Environment Programme’s Stockholm Convention added them to the group of persistent organic pollutants (POPs) because they have an environmental half-life of several years, can travel long distances in the atmosphere, have a tendency to bioaccumulate and biomagnify in the food web, and are toxic to humans and animals (UNEP 2001; De Wit 2002). To reduce these risks, the European Union banned the use of the penta and octa commercial mixtures in 2004 and the use of the decaBDE mixture in electrical and electronic equipment in 2008. The USA followed suit by discontinuing production of the penta and octa mixtures in 2004 (Harrad et al. 2006; US EPA 2010) and of decaBDE in 2013 (US EPA 2014). In 2009, the Stockholm Convention banned the production, use, import, and export of the penta and octa commercial mixtures and in 2017 of decaBDE (Bramwell et al. 2017).
Despite these actions, massive reserves of products containing PBDEs are still in circulation and will continue to release them into the environment for a long time (Abbasi et al. 2015). Part of the problem is the rapidly growing e-waste (Ohajinwa et al. 2019) consisting of discarded electrical and electronic equipment that contains several toxic chemicals, including PBDEs (Robinson 2009; Ilankoon et al. 2018). It is the most prominent in Asian countries that import and recycle e-waste through legal and illegal channels. In these countries, soils at e-waste sites are an important secondary source of pollution (Leung et al. 2007; Tue et al. 2013).
The objective of this review is to summarize the data published in the last five years about the distribution and content of PBDEs in humans and various environmental compartments worldwide. It also addresses concerns about high PBDE levels detected in the areas where e-waste is collected or processed.
Environmental fate and human exposure
Semivolatile pollutants like PBDEs get into the atmosphere as a result of combustion from domestic and industrial sources, emissions from waste incineration or motor vehicles, and (il)legal e-waste landfills (Farrar et al. 2004; Pozo et al. 2016; Degrendele et al. 2018). Their levels and gas/particle partitioning in the atmosphere depend on the physico-chemical properties of a particular PBDE congener, environmental conditions, and the abundance, composition, and size of suspended particles (Besis et al. 2017; Degrendele et al. 2018). Once PBDEs are sorbed onto airborne particles, they reach aquatic and terrestrial environments. Atmospheric transport can take them over long distances, which has been confirmed by the detection of PBDEs in areas as remote as the Arctic and Antarctica (Law et al. 2014; Vecchiato et al. 2015; Khairy et al. 2016; Markham et al. 2018). Being hydrophobic, PBDEs tend to attach to particulate matter and therefore accumulate in sediment and soil, both serving as PBDEs environmental sinks (Law et al. 2006; Anh et al. 2017; Mcgrath et al. 2017; Pei et al. 2018; Tiwari et al. 2018; Ma et al. 2019).
Aquatic environments are exposed to PBDEs not only through atmospheric deposition but also through effluent and sewage sludge from wastewater treatment plants and landfill leaches (Aigars et al. 2017; Tombesi et al. 2017; Liu et al. 2018; Pei et al. 2018). With their low vapor pressure, very low water solubility, and high octanol/water partition coefficient (log Kow), PBDEs in aquatic environments adsorb onto the organic fraction of sediments, suspended particulate matter, or enter aquatic organisms. There, they bioaccumulate in lipid-rich tissues of organisms and biomagnify along food chains (Webster et al. 2010; Govaerts et al. 2018). This is why aquatic organisms tend to be highly burdened with PBDEs.
Terrestrial animals, in turn, are much less exposed to bioavailable PBDEs, and—to our knowledge—only three studies have reported PBDE levels in them over the last five years: two in carnivore species from the USA (Boyles and Nielsen 2017; Boyles et al. 2017) and one in two herbivore and one omnivore species from Latvia (Zacs et al. 2018).
Humans are exposed to PBDEs through diet, inhalation, accidental ingestion of dust, and dermal contact. According to the European Food Safety Authority (EFSA 2011), the main source of exposure would be food of animal origin with higher fat content (fish, meat, and dairy products), in which PBDEs tend to accumulate due to their lipophilicity. One of the first reports that found a strong association between the consumption of contaminated fish and elevated PBDE levels in human serum comes from Sweden. Median PBDE level in the serum of Swedes who did not consume fish was 0.4 ng/g lipid weight (lw) (< 0.1–2.5; 10–90 percentile) compared to 2.2 ng/g lw (0.96–5.7; 10–90 percentile) in consumers who ate between 12 and 20 meals of fatty Baltic Sea fish per month (Sjödin et al. 2000). Another recent study from the USA also suggests that people who eat over 10 servings of seafood per week have a significantly higher ∑PBDE in serum than those who eat less than 1 serving per week (Kuo et al. 2019).
Martellini et al. (2016) reported PBDEs in various foodstuffs commonly consumed in Italy (meat, eggs, milk, cheese, fish, fish oil, and mussels). The highest mass fraction of total PBDEs was measured in dairy products (18,537 pg/g ww), meat (12,672 pg/g ww), and eggs (9729 pg/g ww). PentaBDEs were dominant in fish oil, while BDE 209 dominated in other food groups. The authors also showed that exposure to PBDEs through food varied considerably with region and personal food habits.
Lorber (2008) pointed out much higher PBDE levels found in a US population than the rest of the world, even though PBDE levels in US foodstuffs were not. These findings pointed to dust inhalation and/or ingestion as one of the most significant routes of human exposure to PBDEs, especially considering the dominantly indoor lifestyle (house, car, school/kindergarten, office/working place). That PBDE exposure through dust inhalation/ingestion is even greater than from food has been confirmed by several reports (Schecter et al. 2006; Wu et al. 2007; Stapleton et al. 2008; Lorber 2008; Fraser et al. 2009; Johnson-Restrepo and Kannan 2009; Wei et al. 2009). Indoor environments are contaminated with PBDEs from flame retardants in a wide range of consumer products that remain in use for a long time, such as polyurethane foam in furniture and automobile seats, textiles, and electrical and electronic equipment. From these products, PBDEs are released into the ambient air through volatilization, mechanical abrasion, and/or sorption to dust particles. Higher PBDE concentrations in dust from the USA than from Europe and Asia seems to directly correlate with the historical usage of PBDEs (Whitehead et al. 2011). Similarly, higher PBDE human body burdens found in California (Zota et al. 2008; Rose et al. 2010) and the UK (Bramwell et al. 2017) than in the rest of the USA and Europe seem to be related to very strict fire protection regulations in California and the UK.
Regardless of the exposure routes, the most vulnerable group to PBDE exposure are children. Numerous studies found higher PBDE levels in children’s blood than in blood of their mothers (US EPA 2010; Lunder et al. 2010; Shin et al. 2016; Terry et al. 2017), parents (Fischer et al. 2006; Wu et al. 2015), or generally of the adult population (Toms et al. 2009; Rose et al. 2010).
Similar to other lipophilic contaminants, PBDEs enter the organism as early as in the prenatal period, as evidenced by their findings in the umbilical cord blood (Herbstman et al. 2008; Zota et al. 2018), placenta (Dassanayake et al. 2009), fetal blood (Mazdai et al. 2003), and fetal liver (Zota et al. 2018). Breastfeeding, however, is the period of life when PBDE intake is at its peak (Jones-Otazo et al. 2005; Johnson-Restrepo and Kannan 2009). PBDEs accumulated in breast milk are directly transferred to infants, who receive very high doses per the unit of mass. Exposure to PBDEs continues through early childhood, but the dominant exposure route is now dust ingestion and inhalation associated with their frequent hand-to-mouth activity and the extensive contact with floors, carpets, and other dusty surfaces (EPA 2008; Stapleton et al. 2008). An interesting survey conducted by Hoffman et al. (2017) on children’s blood and hand wipe samples showed that toddlers who licked their fingers while eating, who played more with plastic toys, and who were more active in general had higher PBDE levels on their hands and in their serum.
Toxicity
Knowledge about the mechanisms of toxic PBDE action and effects on human health is still quite limited. Toxicity has mostly been investigated in animal studies, and several recent studies have evaluated the associations between PBDE concentrations in human tissues (e.g., blood, breast milk) and various health effects (ATSDR 2017).
Animal models report adverse effects at low doses of pentaBDEs and octaBDEs (from 0.6 and 2 mg/kg body weight, respectively) and much higher doses of decaBDEs (80 mg/kg body weight) and include effects on neurobehavioral development and thyroid hormone levels for pentaBDEs, fetal toxicity/teratogenicity for octaBDEs, and morphological effects in the thyroid, liver, and kidney of adult animals for decaBDEs (Darnerud 2003). Carcinogenicity studies have for now been limited to decaBDEs and show some effects only at very high doses, which is probably why the International Agency for Research on Cancer (IARC) still has not classified decaBDEs in respect to its carcinogenicity to humans (Darnerud 2003).
Glazer et al. (2018) reported short- and long-term behavioral impairments in zebrafish embryos exposed to low concentrations of BDE 47 (0.01–0.3 μM) and BDE 99 (0.003–20 μM). They also found that exposure to very low concentrations had no visible effects on larval activity but adult behavior was still strongly affected.
Epidemiological studies point to an association between prenatal PBDE exposure and lower birth weight, lower levels of thyroid-stimulating hormone (TSH), lower intelligence quotient (IQ), increased incidence of hyperactivity disorder, and impaired cognitive, motor, and behavioral neurodevelopment (Gibson et al. 2018). Postnatal exposure is associated with similar effects, including lower IQ and increased incidence of hyperactive or aggressive behavior.
One possible explanation for the observed neurological impairments might be related to changes in the thyroid hormone status. The development of the nervous system highly depends on thyroid hormones, thyroxine (T4) in particular, and is the most sensitive to environmental effects from the last trimester of pregnancy to two years of age. In vitro evidence suggests that PBDEs may disrupt thyroid hormone production by binding to thyroid hormone receptors, because PBDEs and T4 have a similar stereochemical structure (Marchesini et al. 2008). Animal and human studies indicate that PBDEs may alter the circulating levels of thyroid hormones (Costa et al. 2008; Turyk et al. 2008; van der Ven et al. 2008; Meeker et al. 2009; Chevrier et al. 2010).
Chemical analysis
Analytical methods for PBDE determination are generally similar to those used for determining polychlorinated biphenyls, but de Boer and Cofino (2002) pointed that improvements are still needed, especially when it comes to the analysis of BDE 209, which requires a different approach.
PBDE analysis in environmental and human samples takes several steps (sample pretreatment, extraction, extract cleanup, and final instrumental analysis), and we will mention only frequently used techniques in this review, without going into detail.
Sample pretreatment means moisture removal from samples where is necessary and/or convenient. Extraction will highly depend on sample type and available laboratory equipment. The most common extraction methods from solid samples include Soxhlet extraction, ultrasound extraction, accelerated solvent extraction (ASE), microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE) (de la Cal et al. 2003; Regueiro et al. 2006; Wang et al. 2010; Annunciação et al. 2017). For liquid samples, the most common method is liquid-liquid (LLE) (Byczkiewicz and Jabłoński 2015; Darrow et al. 2017; Pei et al. 2018; Kuo et al. 2019) and solid phase extraction (SPE) (Thomsen et al. 2002; Covaci et al. 2003; Thomsen et al. 2007). Extract cleanup depends on the matrix. Sediments and soils may require sulfur removal, whereas biota require lipid removal, which can be done with sulfuric acid treatment, gel permeation chromatography (GPC), or column adsorption chromatography on sorbents like silica, alumina, or Florisil (Boyles et al. 2017; Giulivo et al. 2017; Novak et al. 2017; Persson et al. 2019). Instrumental analysis is based on gas-chromatographic (GC) separation on nonpolar or semi-polar capillary columns with mass spectrometric (MS) detection (Vecchiato et al. 2015; Newton et al. 2015; Martellini et al. 2016; Mcgrath et al. 2016; Pozo et al. 2016; Kademoglou et al. 2017; Liu et al. 2018; Pei et al. 2018; Ohajinwa et al. 2019; Wu et al. 2019).
There are many articles on the determination of PBDEs in a variety of environmental samples, but the determination of one frequently reported congener, BDE 209, is particularly demanding because (1) it is not stable at high temperatures in the GC injector and GC column; (2) it is sensitive to degradation by UV light; (3) in the MS source it behaves differently than chlorinated and lower-brominated compounds (de Boer and Cofino 2002); and (4) it may easily adsorb onto small dust particles in the laboratory, which may result in sample contamination (Covaci et al. 2003). Thermal decomposition of BDE 209 can be avoided by using a short GC column and a thermally inert GC injection port (Beser et al. 2014), but this means that it should be analyzed separately from other PBDEs. Another way to address the difficulties with BDE 209 determination is to use liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Abdallah et al. 2009).
Levels and distribution
To avoid repeating data presented in previous review articles covering massive amounts of earlier data (de Wit 2002; Law et al. 2006, 2014; Mcgrath et al. 2017; Tang and Zhai 2017), we limited our literature search to the last five years of research (2015 to September 2019). This is currently a very active research area, and the number of articles reporting PBDE levels in environmental samples is constantly increasing. Since there is no list of key toxic PBDE congeners to be monitored, reports vary from just one to more than 10 congeners. However, as certain congeners dominated in commercial formulations, they were also more frequently detected in environmental and biota samples than others, which ultimately led to narrowing the range of research to the following congeners: BDE 47, BDE 99, BDE 100, BDE 153, and BDE 154 as representatives of the penta formulation (occasionally including BDE 28 and BDE 138 as well), and BDE 183 and BDE 209 as representatives of the octa and deca formulations, respectively. We decided to take in consideration studies that include at least six congeners. Studies not reporting data on BDE 209 were not excluded from this review, as we are well aware of the difficulties involved in BDE 209 analysis, which has narrowed down the possibility of accurate measurements to a limited number of laboratories.
To make comparison easier between studies, we compared the sums of the mass fractions of all analyzed PBDE congeners in specific research (ΣxPBDE), unless indicated otherwise. For the same reason, we also did our best to present reported data as uniformly as possible. PBDE mass fractions/concentrations in soil and sediment samples were mostly expressed in ng/g dry weight (dw), in air samples in pg/m3, and in dust samples in ng/g of dust. In human and biota samples, mass fractions have been lipid-normalized and reported in ng/g of lipid weight, because these contaminants are highly lipophilic and accumulate in lipids. Some authors (Sühring et al. 2016; Aigars et al. 2017; Novak et al. 2017; Trabalón et al. 2017), however, report mass fractions in biota in ng/g of wet weight (ww), which facilitates assessment of human intake and comparison with Environmental Quality Standards (EQS) in biota set in the EU Directive 2013/39/EU for the evaluation of potential ecotoxicological risk of certain pollutants in aquatic environments. The EQS in biota refers to the sum of mass fractions of six PBDE congeners—BDE 28, 47, 99, 100, 153, and 154—and is set to 0.0085 ng/g ww. This is the limit mass fraction below which no harmful effects are expected in wildlife or humans.
Aquatic environment
Aquatic organisms are highly susceptible to bioaccumulation and biomagnification of organic pollutants, which can cause serious health problems, especially in species at the top of food chains. Accordingly, consumption of fish has been recognized as one of the main sources of human exposure to organic pollutants through food, and in these terms, fish is probably the most investigated sample for PBDE contamination (Sühring et al. 2016; Anh et al. 2017; Govaerts et al. 2018).
Many studies have investigated PBDE levels and distribution in aquatic environments all over the world (Sühring et al. 2016; Aigars et al. 2017; Annunciação et al. 2017; Novak et al. 2017; Markham et al. 2018; Pei et al. 2018). Eljarrat and Barceló (2018) summarized literature data on PBDE levels in river fish samples and compared them to the corresponding EQS limits to see if they were exceeded. Taking into account even the best case scenario, the vast majority of fish samples from Europe exceeded the EQS limits at least hundred times over. Reports for Asia and North America were even worse. Authors also discuss controversy around EQS, implying that it is set too low and that it should be revised as soon as new toxicological data will be available.
Table 1 shows PBDE mass fractions in fish reported on wet weight basis. The highest levels of 11 ng/g ww were found in Norway (Govaerts et al. 2018). If we exclude this extreme, the highest level of 1.3 ng/g ww is two orders of magnitude over the EQS. Moreover, even the lowest reported level (0.03 ng/g ww) is 3.5 times higher than the EQS set for biota.
Recently, Giulivo et al. (2017) compared PBDE levels in sediment and biota samples from three European rivers, one continental (the Sava), one Mediterranean (the Evrotas in Greece), and one Alpine (the Adige, Italy). The biota samples contained all of the analyzed PBDE congeners (BDE 28, 47, 99, 100, 153, 154, 183, and 209), while none of the sediment samples contained BDE 153, 154, or 183. Furthermore, the biota samples had significantly higher sum PBDEs. BDE 209 was the most abundant congener in the continental and Alpine river sediments, while BDE 47 was the most abundant congener in the Mediterranean river sediments as well as fish samples. These findings suggest lower use of decaBDE in commercial mixtures in the Mediterranean.
The samples along the continental, Sava river were collected in four countries through which it passes, including four locations in Croatia. There, the ∑8PBDE ranged from below the limit of detection (< LOD) to 16.7 ng/g dw in sediment, and from 11.9 to 461 ng/g lw in fish, which was higher than in the Mediterranean or the Alpine river. The conversion of the PBDE concentrations in fish samples from the Sava from the lipid to wet weight basis showed that all samples exceeded the EQS threshold. In general, however, all PBDE levels, regardless of the river, were comparable to other European countries. The Sava and the Adige PBDE sediment levels were also comparable to one Australian report (Anim et al. 2017). Giulivo et al. (2017) also reported that BDE 209 contributed with more than 90% to the sum of congeners. In contrast, lower PBDE levels were reported in sediment samples collected from five rivers and eight lakes in Latvia (Aigars et al. 2017). In that study, BDE 209 was not detected in any of the sediment samples but was dominant in fish samples. For comparison, several orders of magnitude higher PBDE levels were detected in sediment samples collected around a flame retardant manufacturing plant in China (Song et al. 2016), along the second largest Chinese Yellow River (Huang He) (Pei et al. 2018), and in the vicinity of a sewage treatment plant on the south coast of Korea (Lee et al. 2018).
Air and soil
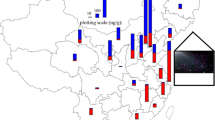
According to literature, atmospheric levels of PBDEs depend on deposition processes, meteorological conditions, long-range atmospheric transport, and the vicinity of PBDE sources to the sampling site (urban/industrial vs. background locations). Between 2011 and 2014, Degrendele et al. (2018) monitored atmospheric PBDE levels (BDE 28, 47, 85, 99, 100, 153, 154, 183, and 209) at a background site in central Europe, which is known to have no sources of PBDEs. Their findings indicated an increase in lower-brominated PBDE congeners in the atmosphere on the global scale, most likely because of debromination of higher brominated congeners by photolysis. As if to confirm that assumption, higher brominated PBDE levels (BDE 99, 100, 153, and 209) decreased over the same period. A similar PBDE profile was reported in background air in Europe by Besis et al. (2017). Pozo et al. (2016), in turn, reported lower air PBDE levels for coastal areas of Sicily. In addition, only three (BDE 47, 99, and 100) of the 26 analyzed PBDEs were detected routinely. To estimate the main factors affecting PBDE air mass concentrations in Spain, Roscales et al. (2018) collected a larger number of seasonal air samples at five urban and seven background sites between 2008 and 2015. The analysis included 14 PBDEs (BDE 28, 47, 66, 85, 99, 100, 153, 154, 183, 184, 191, 196, 197, and 209). Urban air samples had higher median ∑14PBDE than the background samples, but the single highest levels were measured in two samples collected at the same background site in 2014. In both cases, this finding was owed to a rise in BDE 209. This congener also dominated the rest of the air samples, regardless of the sampling site. The authors also reported no significant reduction in PBDE levels in Spanish air over the observed period, despite the European ban and global regulations limiting the production and usage of PBDEs. In comparison to Spain, a heavily industrialized region in Turkey had much higher air PBDE levels (Cetin et al. 2019). Air samples were collected from 23 sites every month for one year, and eight PBDE congeners were analyzed (BDE 28, 47, 99, 100, 153, 154, 183, and 209). Again, BDE 209 was the dominant congener. Air samples collected at industrial/urban locations had the highest PBDE levels, followed by urban, suburban, and rural locations. Seasonal variations were also observed: summer had higher average PBDE air levels (118.5 ± 98.7 pg/m3) than winter (79.7 ± 59.1 pg/m3). This may be owed to greater volatilization from nearby sources at higher temperatures. Another Turkish study of Istanbul air (Kurt-Karakus et al. 2017) reported similar PBDE levels (BDE 17, 28, 47, 66, 85, 99, 100, 138, 153, 154, 183, and 209) for urban (620 pg/m3), suburban (280 pg/m3), and rural (110 pg/m3) neighborhoods.
Air-to-soil transfer is one of the main sources of soil PBDE contamination. Higher brominated PBDE congeners (> 5 bromine atoms) create strong bonds with organic matter in soil. Soils are therefore important secondary sources of pollution (Law et al. 2006; Ma et al. 2019), especially in the vicinity of industrial activities involving PBDEs (Newton et al. 2015; Cetin et al. 2019; Ma et al. 2019; Xu et al. 2019), including e-waste recycling (Wang et al. 2017; Ohajinwa et al. 2019). This was confirmed by Li et al. (2016), who investigated PBDE levels in soil samples collected at urban, rural, background, and industrial/e-waste recycling locations in Japan, China, South Korea, Vietnam, and India. BDE 209 dominated among 23 congeners in the majority of samples, and its mass fraction was more than 20 times higher than that of the sum of the remaining 22 congeners at some sampling locations. Urban locations had the second highest ∑23PBDE, followed by rural, and background locations. By country, soil ∑23PBDE followed this order: Japan > China > South Korea > India > Vietnam in urban (450 > 75 > 39 > 3.4 > 1.1 ng/g, respectively) and rural (161 > 28 > 10 > 0.79 > 0.75 ng/g, respectively) locations. As expected, the highest PBDE levels were observed at e-waste recycling and BFR industrial sites. This industrial impact on PBDE levels has also been reported by Xu et al. (2019). ∑13PBDE in soils collected at three plastic manufacture plants and their vicinity ranged from 2.21 to 18,451 ng/g dw, with an overall mean of 1004 ng/g dw. These levels decreased with the distance from the contaminated area. Again, BDE 209 was the most common and also the most abundant congener.
Significantly lower mass fractions were detected in soils sampled across Azerbaijan (Aliyeva et al. 2018). Only six out of ten analyzed PBDE congeners were detected, and the mean and median of Σ6PBDE were 167 and 91.1 pg/g dw, respectively. Curiously enough, mean Σ6PBDE in soils from industrial sites were not significantly different from mean Σ6PBDE in soils from non-industrial areas. The authors suggested that in their case, PBDE levels were affected by wider regional sources instead of significant point sources inside the country.
There are only a few PBDE soil level studies outside Asia. Eight PBDE congeners of environmental concern (BDE 28, 47, 99, 100, 153, 154, 183 and 209) were analyzed in soils collected in the UK (Drage et al. 2016), France (Gaspéri et al. 2018), and Australia (Mcgrath et al. 2016). In the UK and France, PBDE levels were associated with urbanization. BDE 209 was dominant across all these studies, with the highest levels measured in e-waste recycling sites (Mcgrath et al. 2016). The domination of BDE 209 was also reported by Tombesi et al. (2017) in soils sampled from different locations of Bahía Blanca city and the surrounding region (Argentina).
Due to long-range atmospheric transport and persistence, PBDEs were also detected in soil samples collected at as remote sites as the Arctic and Antarctica. Their levels measured kept mainly at the pg/g level (Wang et al. 2015; Ma et al. 2019), but Vecchiato et al. (2015) suggested that research stations in Antarctica could be significant sources of PBDEs and similar compounds, where they found soil levels as high as 33 ng/g dw.
Indoor dust
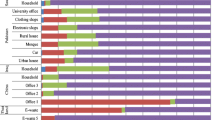
Dust as a significant source of human exposure to PBDEs has attracted a lot of attention over the recent years, especially in terms of PBDE burden in a variety of indoor environments (Zhu et al. 2015; Civan and Kara 2016; Anh et al. 2017; Kurt-Karakus et al. 2017; Muenhor and Harrad 2018; Ohajinwa et al. 2019; Kuo et al. 2019; Rantakokko et al. 2019; Tao et al. 2019). Table 2 summarizes the data published over the last five years. Comparisons between studies can be challenging because of the differences in dust sampling methods, size, and type of the area that has been vacuumed (specific room or entire household), presence of specific products which could increase PBDE levels, and the timing of sampling. Allgood et al. (2017) found that dust settled at elevated surfaces and dust settled on the floor has a different PBDE profile, and that human exposure assessments will much depend on the place from which the accumulated dust is sampled. Furthermore, PBDE levels in dust vary by season. In university laboratories in China, winter recorded the highest levels, and the variations coincided with changes in BDE 209 levels (Jin et al. 2018). Studies from different parts of the world compared PBDE dust levels in a variety of indoor environments, such as households, offices, stores, classrooms, cars, and theaters (Zhu et al. 2015; Sun et al. 2016; Cristale et al. 2016; Kademoglou et al. 2017; Kurt-Karakus et al. 2017; Muenhor and Harrad 2018). Just like in air and soil, their distribution in dust samples also depended on the level of urbanization, i.e., they were higher in urban/industrialized areas than in rural areas. Some studies also suggest that the presence of electronic devices in the indoor environment increases PBDE levels in dust (Sun et al. 2016; Allgood et al. 2017; Muenhor and Harrad 2018; Sugeng et al. 2018).
Historically higher use of PBDEs in the USA and Canada has also reflected on much higher PBDE levels in indoor dust than in Europe and Asia. Allgood et al. (2017) reported very high median mass fractions of Σ10PBDE in dust samples (up to 23,508 ng/g) collected at various locations of the University of California campus in Irvine, USA. In Europe, dust samples collected across UK had PBDE mass fractions nearly as high as those reported in the USA and Canada. This may be related to stringent fire safety regulations in the UK and the widespread use of carpets (Tao et al. 2016; Kademoglou et al. 2017). In other European countries (Turkey, Norway, Sweden, Finland, and Spain) the highest PBDE levels in dust were at least one order of magnitude lower (Civan and Kara 2016; Cristale et al. 2016; Kademoglou et al. 2017; Kurt-Karakus et al. 2017; Rantakokko et al. 2019; Tao et al. 2019).
Venier et al. (2016) compared PBDE indoor levels of three countries: the USA, Canada, and the Czech Republic. Median ∑10PBDE in dust, air, and window films followed this order: USA > Canada > Czech Republic (3650 > 1770 > 163 ng/g; 148 > 60 > 3 pg/m3, and 7.0 > 6.5 > 0.98 ng/m2, respectively). In contrast, Wong et al. (2017) found no significant differences in total PBDE dust levels between Australia, UK, Canada, Sweden, and China.
As concerns PBDE profiles in dust, a number of studies singled out BDE 209 as the dominant congener (Zhu et al. 2015; Cristale et al. 2016; Kim et al. 2016; Venier et al. 2016; Kademoglou et al. 2017; Korcz et al. 2017; Kurt-Karakus et al. 2017; Rantakokko et al. 2019; Tao et al. 2016, 2019). In dust from Australia, the UK, Sweden, and China, BDE 209 dominated in the congener profile, ranging from 50 to 70% of total PBDEs, while in Canada, it accounted for only 20% of total PBDEs (Wong et al. 2017). BDE 209 in house dusts was also reported to vary a lot, especially in studies with a large number of samples. For example, in a study of PBDE levels in 129 house dusts collected in Warsaw, Poland, BDE 209 ranged from 36 to 336,000 ng/g (median 270 ng/g) (Korcz et al. 2017). In indoor dust samples collected in China, BDE 209 ranged from 8.36 to 37,400 ng/g (median 1090 ng/g) (Zhu et al. 2015). Although the components of the decaBDE mixture taking over the dominance in indoor air and dust due to the phase-out of commercial pentaBDE (Björklund et al. 2012), this is not yet true for areas with high historical use of the pentaBDE mixture.
PBDEs in humans
Like other POPs, PBDEs have a tendency to accumulate in lipid-rich compartments. This is why breast milk is the most common and practical tool used for human biomonitoring. It is an ideal bioindicator not only of infant but also of human exposure and the sampling method is not invasive. In a large systematic review, Zhang et al. (2017) summarized global research data on PBDE concentrations in human breast milk specimens collected from 2000 to 2015. The most commonly reported PBDE congeners were BDE 28, 47, 99, 100, 153, 154, and 183. The medians of total PBDE mass fractions ranged from 19.9 to 54.5 ng/g lw in North America, from 0.4 to 6.3 ng/g lw in Europe, and from 1.5 to 11.5 ng/g lw in Asia. In other words, PBDEs were about 20 times higher in North America than in Europe or Asia. These findings strongly suggest that this population is still exposed to some PBDE sources. Furthermore, significantly higher PBDE levels were reported in the breast milk of women living near e-waste recycling plants for more than 20 years than in the breast milk of women living there for less than three years, which confirms that e-waste recycling is an important source of PBDEs (Li et al. 2017).
Many investigations have focused on children as the most vulnerable group. Darrow et al. (2017) reported higher serum PBDE levels in children aged between 1 and 6 years than Cowell et al. (2018) did in children aged between 2 and 9 years. Both studies were conducted in the USA, and in both, BDE 47 was the most abundant congener. Cowell et al. (2018) reported the highest mean concentrations of BDE 47, 99, and 100 at the age of two years, which confirmed that toddler are the most vulnerable group in terms of exposure. They also showed that the levels of PBDE congeners from the pentaBDE mixture significantly decreased over the study period, which is consistent with the fact that its use ceased in the meantime. A similar significant decrease in serum PBDE levels was reported in Australian children after the ban of commercial penta and octaBDE mixtures (Drage et al. 2019). The only research of PBDE levels in children from Europe is the one by Drobná et al. (2019) in 6-year-olds from Slovakia. Their data for individual PBDE congeners (as they did not report the sum) showed relatively low levels compared to other countries. Even so, the authors found an association between PBDE exposure and poor preschool maturity test results.
Information about BDE 209 levels in humans is scarce, because it was not analyzed by most of the human studies. Those few that did include it in analysis did not detect it or the detection rate was very low, mostly because BDE 209 has a much higher limit of detection than other congeners (Darrow et al. 2017; Cowell et al. 2018; Drobná et al. 2019; Kuo et al. 2019). Darnerud et al. (2015) investigated the time trend of BDE 209, 47, 99, 100, and 153, in pooled blood serum samples collected from Swedish first-time mothers between 1996 and 2010. They detected only BDE 153 and 209 in all samples (BDE 209 had the highest mean mass fraction of 1.27 ng/g lw), while other congeners were below the quantitation limit (LOQ) in more than 70% of the samples. Linear regression analysis showed that the levels of BDE 47, 99 and 100 decreased significantly in serum during the study period, BDE 153 showed an increasing trend, while there was no significant trend for BDE 209. In addition, the authors compared serum with breast milk PBDEs in matched samples and found significant correlations between levels of seven BDE congeners (28, 47, 100, 153, 154, 183, and 209). These correlations were weaker for higher brominated BDE congeners (Darnerud et al. 2015). In an investigation of serum PBDE levels of the residents of Washington, median Σ11PBDE (without BDE 209) was 28.70 ng/g lw (Kuo et al. 2019). The highest mass fraction had BDE 209 (617.07 ng/g lw), but it was detected in only 13% of the samples, probably because of its relatively high LOD. The same study reported high levels of BDE 209 in dust samples collected at the workplace of the same people whose serum was analyzed.
Final remarks
PBDE findings reported in the last five years confirm that measures taken to minimize the use of these contaminants did not result in lower PBDE levels in the environment. Research has also showed that PBDE levels always reflect the proximity of a contamination sources, such as manufacturing or sewage treatment plants, landfills, and/or e-waste recycling industries. By far, the highest mass fractions of PBDEs, especially of BDE 209, have been reported in soil samples collected at industry locations in China (up to 19 μg/g dw).
Indoor dust PBDE levels are far higher in North America and the UK than in the rest of the world as a consequence of intensive BFR use in the past, and although this trend is declining, human exposure is still notable. Exposure through contaminated food has also been evidenced. Most of the fish analyzed in the last five years contained PBDE levels above the EU limit. In addition, the levels of lower-brominated PBDE congeners seem to persist or even increase in the environment as a result of the degradation of higher brominated congeners, mainly BDE 209 (Zeng et al. 2010).
All these findings indicate that the efforts made so far to eliminate PBDEs from the environment and reduce their negative impact on humans are not sufficient and that PBDEs shall remain a significant global environmental problem for many years to come.
This is why new measures need to be taken to remove the remaining greatest source of contamination, solid waste, and e-waste in particular. These measures should define efficient means to achieve a sustainable waste management goal. Informal recycling, one of the major issues of waste management in developing countries, needs to be integrated into the formal waste management sector. This is crucial for the health of people involved in recycling, but also for the environment and mankind in general.
References
Abbasi G, Buser AM, Soehl A, Murray MW, Diamond ML (2015) Stocks and flows of PBDEs in products from use to waste in the U.S. and Canada from 1970 to 2020. Environ Sci Technol 49(3):1521–1528. https://doi.org/10.1021/es504007v
Abdallah MA, Harrad S, Covaci A (2009) Isotope dilution method for determination of polybrominated diphenyl ethers using liquid chromatography coupled to negative ionization atmospheric pressure photoionization tandem mass spectrometry: validation and application to house dust. Anal Chem 81(17):7460–7467. https://doi.org/10.1021/ac901305n
ATSDR (2017) Agency for Toxic Substances and Disease Registry; Division of toxicology and human health sciences. Public Health Statement. www.atsdr.cdc.gov/
Aigars J, Suhareva N, Poikane R (2017) Distribution of polybrominated diphenyl ethers in sewage sludge, sediments, and fish from Latvia. Environments 4(1):12. https://doi.org/10.3390/environments4010012
Aliyeva G, Sinnott-Clark CA, Škrdlíková L, Kukučka P, Klanova J, Halsall C (2018) A contemporary assessment of polybrominated diphenyl ethers (PBDE) in the ambient air and soil of Azerbaijan. Environ Sci Pollut Res 25(32):31863–31873. https://doi.org/10.1007/s11356-017-0573-2
Allgood JM, Jimah T, McClaskey CM, La Guardia MJ, Hammel SC, Zeineddinea MM, Tanga IW, Runnerstroma MG, Ogunseitan OA (2017) Potential human exposure to halogenated flame-retardants in elevated surface dust and floor dust in an academic environment. Environ Res 153:55–62. https://doi.org/10.1016/j.envres.2016.11.010
Andersson O, Blomkvist G (1981) Polybrominated aromatic pollutants found in fish in Sweden. Chemosphere 10(9):1051–1060. https://doi.org/10.1016/0045-6535(81)90216-2
Anh HQ, Nam VD, Tri TM, Ha NM, Ngoc NT, Mai PTN, Anh DH, Minh NH, Tuan NA, Minh TB (2017) Polybrominated diphenyl ethers in plastic products, indoor dust, sediment and fish from informal e-waste recycling sites in Vietnam: a comprehensive assessment of contamination, accumulation pattern, emissions, and human exposure. Environ Geochem Health 39(4):935–954. https://doi.org/10.1007/s10653-016-9865-6
Anim AK, Drage DS, Goonetilleke A, Mueller JF, Ayoko GA (2017) Distribution of PBDEs, HBCDs and PCBs in the Brisbane river estuary sediment. Mar Pollut Bull 120(1–2):165–173. https://doi.org/10.1016/j.marpolbul.2017.05.002
Annunciação DLR, Almeida FV, Sodré FF (2017) Method development and validation for the determination of polybrominated diphenyl ether congeners in Brazilian aquatic sediments. Microchem J 133:43–48. https://doi.org/10.1016/j.microc.2017.03.009
Antignac JP, Main KM, Virtanen HE, Boquien CY, Marchand P, Venisseau A, Guiffard I, Bichon E, Wohlfahrt-Veje E, Legrand A, Boscher C, Skakkebæk NE, Toppari J, Le Bizec B (2016) Country-specific chemical signatures of persistent organic pollutants (POPs) in breast milk of French, Danish and Finnish women. Environ Pollut 218:728–738. https://doi.org/10.1016/j.envpol.2016.07.069
Beser MI, Beltrán J, Yusà V (2014) Design of experiment approach for the optimization of polybrominated diphenyl ethers determination in fine airborne particulate matter by microwave-assisted extraction and gas chromatography coupled to tandem mass spectrometry. J Chromatogr A 1323:1–10. https://doi.org/10.1016/j.chroma.2013.10.081
Besis A, Lammel G, Kuku P, Samara C (2017) Polybrominated diphenyl ethers (PBDEs) in background air around the Aegean: implications for phase partitioning and size distribution. Environ Sci Pollut Res 24:28102–28120. https://doi.org/10.1007/s11356-017-0285-7
Björklund JA, Sellström U, de Wit CA, Aune M, Lignell S, Darnerud PO (2012) Comparisons of polybrominated diphenyl ether and hexabromocyclododecane concentrations in dust collected with two sampling methods and matched breast milk samples. Indoor Air 22(4):279–288. https://doi.org/10.1111/j.1600-0668.2011.00765.x
Boyles E, Nielsen CK (2017) PBDEs and dechloranes in raccoons in the midwestern United States. Bull Environ Contam Toxicol 98(6):758–762. https://doi.org/10.1007/s00128-017-2072-z
Boyles E, Tan H, Wu Y, Nielsen CK, Shen L, Reiner EJ, Chen D (2017) Halogenated flame retardants in bobcats from the midwestern United States. Environ Pollut 221:191–198. https://doi.org/10.1016/j.envpol.2016.11.063
Bramwell L, Harrad S, Abou-Elwafa AM et al (2017) Predictors of human PBDE body burdens for a UK cohort. Chemosphere 189:186–197. https://doi.org/10.1016/j.chemosphere.2017.08.062
Byczkiewicz M, Jabłoński M (2015) Determining polybrominated diphenyl ethers in surface waters of western pomerania using gas chromatography with electron capture detection. Pol J Environ Stud 24(3):961–968. https://doi.org/10.15244/pjoes/34010
Cetin B, Yurdakul S, Odabasi M (2019) Spatio-temporal variations of atmospheric and soil polybrominated diphenyl ethers (PBDEs) in highly industrialized region of Dilovasi. Sci Total Environ 646:1164–1171. https://doi.org/10.1016/j.scitotenv.2018.07.299
Chen T, Huanga M, Li J et al (2019) Polybrominated diphenyl ethers and novel brominated flame retardants in humanmilk fromthe general population in Beijing, China: occurrence, temporal trends, nursing infants' exposure and risk assessment. Sci Total Environ 689:278–286. https://doi.org/10.1016/j.scitotenv.2019.06.442
Chevrier J, Harley KG, Bradman A, Gharbi M, Sjödin A, Eskenazi B (2010) Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect 118(10):1444–1449. https://doi.org/10.1289/ehp.1001905
Civan MY, Kara UM (2016) Risk assessment of PBDEs and PAHs in house dust in Kocaeli, Turkey: levels and sources. Environ Sci Pollut Res 23(23):23369–23384. https://doi.org/10.1007/s11356-016-7512-5
Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A (2008) Polybrominated diphenyl ether ( PBDE ) flame retardants: environmental contamination, human body burden and health effects. Acta Biomed 79:172–183
Covaci A, Voorspoels S, de Boer J (2003) Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples—a review. Environ Int 29(6):735–756. https://doi.org/10.1016/S0160-4120(03)00114-4
Cowell WJ, Sjödin A, Jones R, Wang Y, Wang S, Herbstman JB (2018) Temporal trends and developmental patterns of plasma polybrominated diphenyl ether concentrations over a 15- year period between 1998 and 2013. J Expo Sci Environ Epidemiol 29:49–60. https://doi.org/10.1038/s41370-018-0031-3
Cristale J, Hurtado A, Gómez-Canela C, Lacorte S (2016) Occurrence and sources of brominated and organophosphorus flame retardants in dust from different indoor environments in Barcelona, Spain. Environ Res 149:66–76. https://doi.org/10.1016/j.envres.2016.05.001
Darnerud PO (2003) Toxic effects of brominated flame retardants in man and in wildlife. Environ Int 29(6):841–853. https://doi.org/10.1016/S0160-4120(03)00107-7
Darnerud PO, Lignell S, Aune M, Isaksson M, Cantillana T, Redeby J, Glynn A (2015) Time trends of polybrominated diphenylether (PBDE) congeners in serum of Swedish mothers and comparisons to breast milk data. Environ Res 138:352–360. https://doi.org/10.1016/j.envres.2015.02.031
Darrow LA, Jacobson MH, Preston EV, Lee GE, Panuwet P, Hunter RE Jr, Marder ME, Marcus M, Barr DB (2017) Predictors of serum polybrominated diphenyl ether (PBDE) concentrations among children aged 1–5 years. Environ Sci Technol 51:645–654. https://doi.org/10.1021/acs.est.6b04696
Dassanayake RMAPS, Wei H, Chen RC, Li A (2009) Optimization of the matrix solid phase dispersion extraction procedure for the analysis of polybrominated diphenyl ethers in human placenta. Anal Chem 81(23):9795–9801. https://doi.org/10.1021/ac901805d
De Boer J, Cofino WP (2002) First world-wide interlaboratory study on polybrominated diphenylethers (PBDEs). Chemosphere 46(5):625–633. https://doi.org/10.1016/S0045-6535(01)00226-0
de la Cal A, Eljarrat E, Barceló D (2003) Determination of 39 polybrominated diphenyl ether congeners in sediment samples using fast selective pressurized liquid extraction and purification. J Chromatogr A 1021(1–2):165–173. https://doi.org/10.1016/j.chroma.2003.09.023
De Wit CA (2002) An overview of brominated flame retardants in the environment. Chemosphere 46:583–624 https://doi.org/10.1016/S0045-6535(01)00225-9
Degrendele C, Wilson J, Kukučka P, Klánová J, Lemmel G (2018) Are atmospheric PBDE levels declining in central Europe? Examination of the seasonal and semi-long-term variations, gas—particle partitioning and implications for long-range atmospheric transport. Atmos Chem Phys 18(17):12877–12890 https://www.atmos-chem-phys.net/18/12877/2018/
Drage DS, Newton S, De Wit CA, Harrad S (2016) Concentrations of legacy and emerging flame retardants in air and soil on a transect in the UK West Midlands. Chemosphere 148:195–203. https://doi.org/10.1016/j.chemosphere.2016.01.034
Drage DS, Harden FA, Jeffery T, Mueller JF, Hobson P, Toms L-ML (2019) Human biomonitoring in Australian children: brominated flame retardants decrease from 2006 to 2015. Environ Int 122:363–368. https://doi.org/10.1016/j.envint.2018.11.044
Drobná B, Fabišiková A, Čonka K, Gago F, Oravcová P, Wimmerová S, Oktapodas Feiler M, Šovčíková E (2019) PBDE serum concentration and preschool maturity of children from Slovakia. Chemosphere 233:387–395. https://doi.org/10.1016/j.chemosphere.2019.05.284
EFSA panel on contaminants in the food chain (CONTAM) (2011) Scientific opinion on polybrominated diphenyl ethers (PBDEs) in food. EFSA J 9(5):2156. https://doi.org/10.2903/j.efsa.2011.2156
Eljarrat E, Barceló D (2018) How do measured PBDE and HCBD levels in river fish compare to the European Environmental Quality Standards? Environ Res 160:203–211. https://doi.org/10.1016/j.envres.2017.09.011
Fair PA, White ND, Wolf B, Arnott SA, Kannan K, Karthikraj R, Vena JE (2018) Persistent organic pollutants in fish from Charleston Harbor and tributaries, South Carolina, United States: a risk assessment. Environ Res 167:598–613. https://doi.org/10.1016/j.envres.2018.08.001
Farrar NJ, Smith KEC, Lee RGM, Thomas GO, Sweetman AJ, Jones KC (2004) Atmospheric emissions of polybrominated diphenyl ethers and other persistent organic pollutants during a major anthropogenic combustion event. Environ Sci Technol 38:1681–1685. https://doi.org/10.1021/es035127d
Fischer D, Hooper K, Athanasiadou M, Athanassiadis I, Bergman A (2006) Children show highest levels of polybrominated diphenyl ethers in a California family of four: a case study. Environ Health Perspect 114(10):1581–1584. https://doi.org/10.1289/ehp.8554
Fraser AJ, Webster TF, McClean MD (2009) Diet contributes significantly to the body burden of PBDEs in the general U.S. population. Environ Health Perspect 117(10):1520–1525. https://doi.org/10.1289/ehp.0900817
Gaspéri J, Ayrault S, Moreau-guigon E et al (2018) Contamination of soils by metals and organic micropollutants: case study of the Parisian conurbation. Environ Sci Pollut Res 25:23559–23573. https://doi.org/10.1007/s11356-016-8005-2
Gibson EA, Siegel EL, Eniola F, Herbstman JB, Factor-Litvak P (2018) Effects of polybrominated diphenyl ethers on child cognitive, behavioral, and motor development. Int J Environ Res Public Health 15(8):1636. https://doi.org/10.3390/ijerph15081636
Giulivo M, Capri E, Kalogianni E, Milacic R, Majone B, Ferrari F, Eljarrat E, Barceló D (2017) Occurrence of halogenated and organophosphate flame retardants in sediment and fish samples from three European river basins. Sci Total Environ 586:782–791. https://doi.org/10.1016/j.scitotenv.2017.02.056
Glazer L, Wells CN, Drastal M, Odamah KA, Galat RE, Behl M, Levin ED (2018) Developmental exposure to low concentrations of two brominated flame retardants, BDE-47 and BDE-99, causes life-long behavioral alterations in zebrafish. NeuroToxicology 66:221–232. https://doi.org/10.1016/j.neuro.2017.09.007
Govaerts A, Verhaert V, Covaci A, Jaspers VLB, Berg OK, Addo-Bediako A, Jooste A, Bervoets L (2018) Distribution and bioaccumulation of POPs and mercury in the Ga-Selati River (South Africa) and the rivers Gudbrandsdalslågen and Rena (Norway). Environ Int 121:1319–1330. https://doi.org/10.1016/j.envint.2018.10.058
Guo W, Holden A, Crispo S et al (2016) PBDE levels in breast milk are decreasing in California. Chemosphere 150:505–513. https://doi.org/10.1016/j.chemosphere.2015.11.032
Harrad S, Hazrati S, Ibarra C (2006) Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: implications for human exposure. Environ Sci Technol 40(15):4633–4638. https://doi.org/10.1021/es0609147
Herbstman JB, Sjödin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR (2008) Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect 116(10):1376–1382. https://doi.org/10.1289/ehp.11379
Hoffman K, Webster TF, Sjödin A, Stapleton HM (2017) Toddler’s behavior and its impacts on exposure to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol 27(2):193–197. https://doi.org/10.1038/jes.2016.11
Ilankoon IMSK, Ghorbani Y, Chong MN, Herath G, Moyo T, Petersen J (2018) E-waste in the international context—a review of trade flows, regulations, hazards, waste management strategies and technologies for value recovery. Waste Manag 82:258–275. https://doi.org/10.1016/j.wasman.2018.10.018
Jin M, Yin J, Zheng Y et al (2018) Pollution characteristics and sources of polybrominated diphenyl ethers in indoor air and dustfall measured in university laboratories in Hangzhou, China. Sci Total Environ 624:201–209. https://doi.org/10.1016/j.scitotenv.2017.12.117
Johnson-Restrepo B, Kannan K (2009) An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 76(4):542–548. https://doi.org/10.1016/j.chemosphere.2009.02.068
Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B (2005) Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol 39(14):5121–5130. https://doi.org/10.1021/es048267b
Kademoglou K, Xu F, Padilla-Sanchez JA, Haug LS, Covaci A, Collins CD (2017) Legacy and alternative flame retardants in Norwegian and UK indoor environment: implications of human exposure via dust ingestion. Environ Int 102:48–56. https://doi.org/10.1016/j.envint.2016.12.012
Khairy MA, Luek JL, Dickhut R, Lohmann R (2016) Levels, sources and chemical fate of persistent organic pollutants in the atmosphere and snow along the western Antarctic Peninsula. Environ Pollut 216:304–313. https://doi.org/10.1016/j.envpol.2016.05.092
Kim S, Kim K, Hong S (2016) Overview on relative importance of house dust ingestion in human exposure to polybrominated diphenyl ethers ( PBDEs ): international comparison and Korea as a case. Sci Total Environ 571:82–91. https://doi.org/10.1016/j.scitotenv.2016.07.068
Korcz W, Struciński P, Góralczyk K, Hernik A, Łyczewska M, Matuszak M, Czaja K, Minorczyk M, Ludwicki JK (2017) Levels of polybrominated diphenyl ethers in house dust in Central Poland. Indoor Air 27(1):128–135. https://doi.org/10.1111/ina.12293
Kuo L, Cade SE, Cullinan V, Schultz IR (2019) Polybrominated diphenyl ethers (PBDEs) in plasma from E-waste recyclers, outdoor and indoor workers in the Puget Sound, WA region. Chemosphere 219:209–216. https://doi.org/10.1016/j.chemosphere.2018.12.006
Kurt-Karakus PB, Alegria H, Jantunen L, Birgul A, Topcu A, Jones KC, Turgut C (2017) Polybrominated diphenyl ethers (PBDEs) and alternative flame retardants (NFRs) in indoor and outdoor air and indoor dust from Istanbul-Turkey: levels and an assessment of human exposure. Atmos Pollut Res 8(5):801–815. https://doi.org/10.1016/j.apr.2017.01.010
La Guardia MJ, Hale RC, Harvey E (2006) Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol 40(20):6247–6254. https://doi.org/10.1021/es060630m
Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA (2006) Levels and trends of brominated flame retardants in the European environment. Chemosphere 64(2):187–208. https://doi.org/10.1016/j.chemosphere.2005.12.007
Law RJ, Covaci A, Harrad S, Herzke D, Abdallah MA, Fernie K, Toms LM, Takigami H (2014) Levels and trends of PBDEs and HBCDs in the global environment: status at the end of 2012. Environ Int 65:147–158. https://doi.org/10.1016/j.envint.2014.01.006
Lee HJ, Jeong HJ, Jang YL, Kim GB (2018) Distribution, accumulation, and potential risk of polybrominated diphenyl ethers in the marine environment receiving effluents from a sewage treatment plant. Mar Pollut Bull 129(1):364–369. https://doi.org/10.1016/j.marpolbul.2018.02.050
Leung A, Luksemburg W, Wong AS, Wong MH (2007) Spatial distribution of polybrominated diphenyl ethers and polychlorinated dibenzo-p-dioxins and dibenzofurans in soil and combusted residue at Guiyu, an electronic. Environ Sci Technol 41(8):2730–2737. https://doi.org/10.1021/es0625935
Li W, Ma W, Jia H, Hong WJ, Moon HB, Nakata H, Minh NH, Sinha RK, Chi KH, Kannan K, Sverko E, Li YF (2016) Polybrominated diphenyl ethers ( PBDEs ) in surface soils across five asian countries : levels, spatial distribution and source contribution. Environ Sci Technol 50(23):12779–12788. https://doi.org/10.1021/acs.est.6b04046
Li X, Tian Y, Zhang Y et al (2017) Accumulation of polybrominated diphenyl ethers in breast milk of women from an e-waste recycling center in China. J Environ Sci 52:305–313. https://doi.org/10.1016/j.jes.2016.10.008
Liu J, Lu G, Zhang F, Nkoom M, Yan Z, Wu D (2018) Polybrominated diphenyl ethers (PBDEs) in a large, highly polluted freshwater lake, China: occurrence, fate, and risk assessment. Int J Environ Res Public Health 15(7). https://doi.org/10.3390/ijerph15071529
Lorber M (2008) Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol 18(1):2–19. https://doi.org/10.1038/sj.jes.7500572
Lunder S, Hovander L, Athanassiadis I, Bergman Å (2010) Significantly higher polybrominated diphenyl ether levels in young US children than in their mothers. Environ Sci Technol 44(13):5256–5262. https://doi.org/10.1021/es1009357
Ma X, Wang Z, Yu L, Yao W, Xiao L, Yao Z, Na G, Wang YW, Jiang G (2019) Mirror image between gas – particle partitioning and soil – moss distribution of polybrominated diphenyl ethers in the polar regions. Sci Total Environ 656:1199–1206. https://doi.org/10.1016/j.scitotenv.2018.11.452
Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, Takeuchi M, Irth H, Murk AJ (2008) Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol 232:150–160. https://doi.org/10.1016/j.taap.2008.06.014
Markham E, Brault EK, Khairy M, Robuck AR, Goebel ME, Cantwell MG, Dickhut RM, Lohmann R (2018) Time trends of polybrominated diphenyl ethers (PBDEs) in antarctic biota. ACS Omega 3(6):6595–6604. https://doi.org/10.1021/acsomega.8b00440
Martellini T, Diletti G, Scortichini G, Lolini M, Lanciotti E, Katsoyiannis A, Cincinelli A (2016) Occurrence of polybrominated diphenyl ethers (PBDEs) in foodstuffs in Italy and implications for human exposure. Food Chem Toxicol 89:32–38. https://doi.org/10.1016/j.fct.2015.12.026
Matovu H, Sillanpää M, Ssebugere P (2019) Polybrominated diphenyl ethers in mothers’ breast milk and associated health risk to nursing infants in Uganda. Sci Total Environ 692:1106–1115
Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM (2003) Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect 111(9):1249–1252. https://doi.org/10.1289/ehp.6146
Mcgrath TJ, Morrison PD, Sandiford CJ et al (2016) Widespread polybrominated diphenyl ether (PBDE) contamination of urban soils in Melbourne, Australia. Chemosphere 164:225–232. https://doi.org/10.1016/j.chemosphere.2016.08.017
Mcgrath TJ, Ball AS, Clarke BO (2017) Critical review of soil contamination by polybrominated diphenyl ethers (PBDEs) and novel brominated flame retardants (NBFRs); concentrations, sources and congener profiles. Environ Pollut 230:741–757. https://doi.org/10.1016/j.envpol.2017.07.009
Meeker JD, Johnson PI, Camann D, Hauser R (2009) Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci Total Environ 407(10):3425–3429. https://doi.org/10.1016/j.scitotenv.2009.01.030
Muenhor D, Harrad S (2018) Polybrominated diphenyl ethers (PBDEs) in car and house dust from Thailand: implication for human exposure. J Environ Sci Heal Part A 53(7):629–642. https://doi.org/10.1080/10934529.2018.1429725
Newton S, Sellstro U, De Wit CA (2015) Emerging flame retardants, PBDEs, and HBCDDs in indoor and outdoor media in Stockholm, Sweden. Environ Sci Technol 49:2912–2920. https://doi.org/10.1021/es505946e
Nicklisch SCT, Bonito LT, Sandin S, Hamdoun A (2017) Geographic differences in persistent organic pollutant levels of Yellowfin tuna. Environ Health Perspect 125(6):067014. https://doi.org/10.1289/EHP518
Novak P, Zuliani T, Milačič R, Ščančar J (2017) Development of an analytical method for the determination of polybrominated diphenyl ethers in mussels and fish by gas chromatography—inductively coupled plasma mass spectrometry. J Chromatogr A 1524:179–187. https://doi.org/10.1016/j.chroma.2017.09.059
Ohajinwa CM, Van Bodegom PM, Xie Q et al (2019) Hydrophobic organic pollutants in soils and dusts at electronic waste recycling sites: occurrence and possible impacts of polybrominated diphenyl ethers. Int J Environ Res Public Health 16(3):360. https://doi.org/10.3390/ijerph16030360
Pei J, Yao H, Wang H, Li H, Lu S, Zhang X, Xiang X (2018) Polybrominated diphenyl ethers (PBDEs) in water, surface sediment, and suspended particulate matter from the Yellow River, China: levels, spatial and seasonal distribution, and source contribution. Mar Pollut Bull 129(1):106–113. https://doi.org/10.1016/j.marpolbul.2018.02.017
Persson J, Wang T, Hegberg J (2019) Temporal trends of decabromodiphenyl ether and emerging brominated flame retardants in dust, air and window surfaces of newly built low-energy preschools Josefin Persson. Indoor Air 29(2):263–275. https://doi.org/10.1111/ina.12528
Pozo K, Palmeri M, Palmeri V, Estellano VH, Mulder MD, Efstathiou CI, Sará GL, Romeo T, Lammel G, Focardi S (2016) Assessing persistent organic pollutants (POPs) in the Sicily Island atmosphere, Mediterranean, using PUF disk passive air samplers. Environ Sci Pollut Res 23:20796–20804. https://doi.org/10.1007/s11356-016-7131-1
Rantakokko P, Kumar E, Braber J, Huang T, Kiviranta H, Cequier E, Thomsen C (2019) Concentrations of brominated and phosphorous flame retardants in Finnish house dust and insights into children’s exposure. Chemosphere 223:99–107. https://doi.org/10.1016/j.chemosphere.2019.02.027
Regueiro J, Llompart M, García-Jares C, Cela R (2006) Determination of polybrominated diphenyl ethers in domestic dust by microwave-assisted solvent extraction and gas chromatography-tandem mass spectrometry. J Chromatogr A 1137(1):1–7. https://doi.org/10.1016/j.chroma.2006.09.080
Robinson BH (2009) E-waste: an assessment of global production and environmental impacts. Sci Total Environ 408(2):183–191. https://doi.org/10.1016/j.scitotenv.2009.09.044
Roscales JL, Muñoz-Arnanz J, Ros M, Vicente A, Barrios L, Jiménez B (2018) Assessment of POPs in air from Spain using passive sampling from 2008 to 2015. Part I: spatial and temporal observations of PBDEs. Sci Total Environ 634:1657–1668. https://doi.org/10.1016/j.scitotenv.2018.03.043
Rose M, Bennett DH, Bergman AKE, Fängström B, Pessah IN, Hertz-Picciotto I (2010) PRDEs in 2-5 year-old children from California and associations with diet and indoor environment. Environ Sci Technol 44(7):2648–2653. https://doi.org/10.1021/es903240g
Schecter A, Päpke O, Harris TR, Tung KC, Musumba A, Olson J, Birnbaum L (2006) Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ Health Perspect 114(10):1515–1520. https://doi.org/10.1289/ehp.9121
Shin MY, Lee S, Kim HJ, Lee JJ, Choi G, Choi S, Kim S, Kim SY, Park J, Moon HB, Choi K, Kim S (2016) Polybrominated diphenyl ethers in maternal serum, breast milk, umbilical cord serum, and house dust in a south Korean birth panel of mother-neonate pairs. Int J Environ Res Public Health 13(8). https://doi.org/10.3390/ijerph13080767
Sjödin A, Hagmar L, Klasson-Wehler E, Björk J, Bergman A (2000) Influence of the consumption of fatty Baltic Sea fish on plasma levels of halogenated environmental contaminants in Latvian and Swedish men. Environ Health Perspect 108(11):1035–1041. https://doi.org/10.1289/ehp.001081035
Song S, Shao M, Tang H, He Y, Wang W, Liu L, Wu J (2016) Development, comparison and application of sorbent-assisted accelerated solvent extraction, microwave-assisted extraction and ultrasonic-assisted extraction for the determination of polybrominated diphenyl ethers in sediments. J Chromatogr A 1475:1–7. https://doi.org/10.1016/j.chroma.2016.10.077
Stapleton MH, Kelly SM, Allen JG, Mcclean MD, Webster TF (2008) Measurement of polybrominated diphenyl ethers on hand wipes estimating exposure from hand-to-mouth contact. Environ Sci Technol 42(9):3329–3334. https://doi.org/10.1021/es7029625
Sugeng EJ, MDe C, PEG L, Van De BM (2018) Electronics, interior decoration and cleaning patterns affect flame retardant levels in the dust from Dutch residences. Sci Total Environ 645:1144–1152. https://doi.org/10.1016/j.scitotenv.2018.07.127
Sühring R, Busch F, Fricke N, Kötke D, Wolschke H, Ebinghaus R (2016) Distribution of brominated flame retardants and dechloranes between sediments and benthic fish—a comparison of a freshwater and marine habitat. Sci Total Environ 542:578–585. https://doi.org/10.1016/j.scitotenv.2015.10.085
Sun J, Wang Q, Zhuang S, Zhang A (2016) Occurrence of polybrominated diphenyl ethers in indoor air and dust in Hangzhou , China: Level, role of electric appliances, and human exposure. Environ Pollut 218:942–949. https://doi.org/10.1016/j.envpol.2016.08.042
Tang J, Zhai JX (2017) Distribution of polybrominated diphenyl ethers in breast milk, cord blood and placentas: a systematic review. Environ Sci Pollut Res 24(27):21548–21573. https://doi.org/10.1007/s11356-017-9821-8
Tang S, Tan H, Liu X, Chen D (2019) Legacy and alternative flame retardants in house dust and hand wipes from South China. Sci Total Environ 656:1–8. https://doi.org/10.1016/j.scitotenv.2018.11.369
Tao F, Abdallah MA, Harrad S (2016) Emerging and legacy flame retardants in UK indoor air and dust: evidence for replacement of PBDEs by emerging flame retardants? Environ Sci Technol 50(23):13052–13061. https://doi.org/10.1021/acs.est.6b02816
Tao F, Sellström U, De Wit CA (2019) Organohalogenated flame retardants and organophosphate esters in office air and dust from Sweden. Environ Sci Technol 53(4):2124–2133. https://doi.org/10.1021/acs.est.8b05269
Terry P, Towers CV, Liu LY, Peverly AA, Chen J, Salamova A (2017) Polybrominated diphenyl ethers (flame retardants) in mother-infant pairs in the southeastern U.S. Int J Environ Health Res 27(3):205–214. https://doi.org/10.1080/09603123.2017.1332344
Thomsen C, Småstuen Haug L, Leknes H, Lundanes E, Becher G, Lindstoom G (2002) Comparing electron ionization high-resolution and electron capture low-resolution mass spectrometric determination of polybrominated diphenyl ethers in plasma, serum and milk. Chemosphere 46(5):641–648. https://doi.org/10.1016/S0045-6535(01)00228-4
Thomsen C, Liane VH, Becher G (2007) Automated solid-phase extraction for the determination of polybrominated diphenyl ethers and polychlorinated biphenyls in serum-application on archived Norwegian samples from 1977 to 2003. J Chromatogr B 846(1–2):252–263. https://doi.org/10.1016/j.jchromb.2006.09.011
Tiwari M, Kumar S, Rahul S, Ajmal CBPY (2018) Polybrominated diphenyl ethers ( PBDEs ) in core sediments from creek ecosystem: occurrence, geochronology, and source contribution. Environ Geochem Health 40(6):2587–2601. https://doi.org/10.1007/s10653-018-0125-9
Tombesi N, Pozo K, Álvarez M, Přibylová P, Kukučka P, Audy O, Klánová J (2017) Tracking polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in sediments and soils from the southwest of Buenos Aires Province, Argentina (south eastern part of the GRULAC region). Sci Total Environ 575:1470–1476. https://doi.org/10.1016/j.scitotenv.2016.10.013
Toms LML, Sjödin A, Harden F, Hobson P, Jones R, Edenfield E, Mueller JF (2009) Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2-5 years of age) than in infants and adults. Environ Health Perspect 117(9):1461–1465. https://doi.org/10.1289/ehp.0900596
Trabalón L, Vilavert L, Domingo JL,Pocurull E, Borrull F, Nadal M (2017) Human exposure to brominated flame retardants through the consumption of fish and shellfish in Tarragona County (Catalonia, Spain). Food Chem Toxicol 104:48–56. https://doi.org/10.1016/j.fct.2016.11.022
Tue NM, Takahashi S, Subramanian A, Sakai S, Tanabe S (2013) Environmental contamination and human exposure to dioxin-related compounds in e-waste recycling sites of developing countries. Environ Sci Process Impacts 15(7):1326–1331. https://doi.org/10.1039/c3em00086a
Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R Jr., Anderson HA (2008) Hormone disruption by PBDEs in adult male sport fish consumers. Health Perspect 116(12):1635–1641943. https://doi.org/10.1289/ehp.l
UNEP (2001) Stockholm Convention on Persistent Organic Pollutants (POPs). http://www.pops.int/TheConvention/ThePOPs/tabid/673/Default.aspx
UNEP (2015) Guidance for the inventory of polybrominated diphenyl ethers (PBDEs) listed under the Stockholm convention on persistent organic pollutants. UN Environ Program 127:66–75. https://doi.org/10.1093/toxsci/kfs077
US Environmental Protection Agency (EPA) (2008) Child-Specific Exposure Factors Handbook. National Center for Environmental Assessment, Washington, p 448 EPA/600/R-06/096F
US Environmental Protection Agency (EPA) (2010) An exposure assessment of polybrominated diphenyl ethers (PBDE). National Center for Environmental Assessment, Washington, p 378. https://doi.org/10.1080/15287390903212436
US Environmental Protection Agency (EPA) (2014) An alternatives assessment for the flame retardant decabromodiphenyl ether (DecaBDE). National Center for Environmental Assessment, Washington
Van der Ven LTM, van de Kuil T, Verhoef A et al (2008) A 28-day oral dose toxicity study enhanced to detect endocrine effects of a purified technical pentabromodiphenyl ether (pentaBDE) mixture in Wistar rats. Toxicology 245(1–2):109–122. https://doi.org/10.1016/j.tox.2007.12.016
Vecchiato M, Zambon S, Argiriadis E et al (2015) Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in Antarctic ice-free areas: influence of local sources on lakes and soils. Microchem J 120:26–33. https://doi.org/10.1016/j.microc.2014.12.008
Venier M, Vojta Š, Be J et al (2016) Brominated flame retardants in the indoor environment—comparative study of indoor contamination from three countries. Environ Int 94:150–160. https://doi.org/10.1016/j.envint.2016.04.029
Wang P, Zhang Q, Wang Y, Wang T, Li X, Ding L, Jiang G (2010) Evaluation of Soxhlet extraction, accelerated solvent extraction and microwave-assisted extraction for the determination of polychlorinated biphenyls and polybrominated diphenyl ethers in soil and fish samples. Anal Chim Acta 663(1):43–48. https://doi.org/10.1016/j.aca.2010.01.035
Wang Z, Na G, Ma X, Ge L, Lin Z, Yao Z (2015) Characterizing the distribution of selected PBDEs in soil, moss and reindeer dung at Ny-Ålesund of the Arctic. Chemosphere 137:9–13. https://doi.org/10.1016/j.chemosphere.2015.04.030
Wang Y, Hou M, Zhao H, Zhang Q, Wu X (2017) Factors influencing the diurnal atmospheric concentrations and soil-air exchange of PBDEs at an e-waste recycling site in China. Atmos Pollut Res 9:166–171. https://doi.org/10.1016/j.apr.2017.09.003
Webster L, Tronczynski J, Bersuder P, Vorkamp KLP (2010) Determination of polybrominated diphenyl ethers (PBDEs) in sediment and biota. ICES Tech Mar Environ Sci 46:1–16
Wei H, Turyk M, Cali S, Dorevitch S, Erdal S, Li A (2009) Particle size fractionation and human exposure of polybrominated diphenyl ethers in indoor dust from Chicago. J Environ Sci Health A Tox Hazard Subst Environ Eng 44(13):1353–1361. https://doi.org/10.1080/10934520903213251
Whitehead T, Metayer C, Buffler P, Rappaport SM (2011) Estimating exposures to indoor contaminants using residential dust. J Expo Sci Environ Epidemiol 21(6):549–564. https://doi.org/10.1038/jes.2011.11
Wong F, Suzuki G, Michinaka C, Yuan B, Takigami H, de Wit CA (2017) Dioxin-like activities, halogenated flame retardants, organophosphate esters and chlorinated paraffins in dust from Australia, the United Kingdom, Canada, Sweden and China. Chemosphere 168:1248–1256. https://doi.org/10.1016/j.chemosphere.2016.10.074
Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey E, La Guardia M, Mcclean MD, Webster TF (2007) Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ Sci Technol 41(5):1584–1589. https://doi.org/10.1021/es0620282
Wu XM, Bennett DH, Moran RE, Wu X, Tulve NS, Clifton MS, Colon M, Weathers W, Sjodin A, Jones R, Hertz-Picciotto I (2015) Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents , and older adults: an exposure assessment study. Environ Health 14(23):1–11. https://doi.org/10.1186/s12940-015-0002-2
Wu Y, Miller GZ, Gearhart J, Romanak K, Lopez-Avila V, Venier M (2019) Children’s car seats contain legacy and novel flame retardants. Environ Sci Technol Lett 6(1):14–20. https://doi.org/10.1021/acs.estlett.8b00568
Xu B, Wu M, Wang M, Pan C, Qiu W, Tang L, Xu G (2018) Polybrominated diphenyl ethers (PBDEs) and hydroxylated PBDEs in human serum from Shanghai, China: a study on their presence and correlations. Environ Sci Pollut Res 25:3518–3526. https://doi.org/10.1007/s11356-017-0709-4
Xu J, Qian W, Li J, Zhang X, He J, Kong D (2019) Polybrominated diphenyl ethers (PBDEs) in soil and dust from plastic production and surrounding areas in eastern of China. Environ Geochem Health 41:2315. https://doi.org/10.1007/s10653-019-00247-0
Zacs D, Rjabova J, Ikkere LE, Bavrins K, Bartkevics V (2018) Brominated flame retardants and toxic elements in the meat and liver of red deer (Cervus elaphus), wild boar (Sus scrofa), and moose (Alces alces) from Latvian wildlife. Sci Total Environ 621:308–316. https://doi.org/10.1016/j.scitotenv.2017.11.247
Zhang J, Qi S et al (2011) Organochlorine pesticides (OCPs) in soils and sediments, southeast China: a case study in Xinghua Bay. Mar Pollut Bull 62(6):1270–1275. https://doi.org/10.1016/j.marpolbul.2011.03.010
Zhang J, Chen L, Xiao L, Ouyang F, Zhang Q-Y, Luo Z-C (2017) Polybrominated diphenyl ether concentrations in human breast milk specimens worldwide. Epidemiology 28:89–97. https://doi.org/10.1097/EDE.0000000000000714
Zeng X, Massey Simonich SL, Robrock KR, Korytár P, Alvarez-Cohen L, Barofsky DF, (2010) Application of a congener-specific debromination model to study photodebromination, anaerobic microbial debromination, and FE0 reduction of polybrominated diphenyl ethers . Environ Toxicol Chem 29 (4):770-778. http://doi.org 10.1002/etc.119.
Zhu NZ, Liu LY, Ma WL, Li WL, Song WW, Qi H, Li YF (2015) Polybrominated diphenyl ethers (PBDEs) in the indoor dust in China: levels, spatial distribution and human exposure. Ecotoxicol Environ Saf 111:1–8. https://doi.org/10.1016/j.ecoenv.2014.09.020
Zota AR, Rudel RA, Morello-Frosch RA, Brody JG (2008) Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol 42(21):8158–8164. https://doi.org/10.1021/es801792z
Zota AR, Mitro SD, Robinson JF, Hamilton EG, Park JS, Parry E, Zoeller RT, Woodruff TJ (2018) Polybrominated diphenyl ethers (PBDEs) and hydroxylated PBDE metabolites (OH-PBDEs) in maternal and fetal tissues, and associations with fetal cytochrome P450 gene expression. Environ Int 112:269–278. https://doi.org/10.1016/j.envint.2017.12.030
Acknowledgments
Studies of brominated flame retardants have been funded by the Croatian Science Foundation (project HrZZ-UIP-2017-05-6713).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philipp Gariguess
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Klinčić, D., Dvoršćak, M., Jagić, K. et al. Levels and distribution of polybrominated diphenyl ethers in humans and environmental compartments: a comprehensive review of the last five years of research. Environ Sci Pollut Res 27, 5744–5758 (2020). https://doi.org/10.1007/s11356-020-07598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07598-7