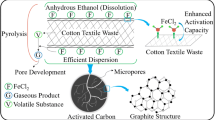
Abstract
Char-based adsorbents (char-FeCl3, char-FeCl2, and char-FeCit) derived from cotton textile waste (CTW) were synthesized by one-step low-temperature pyrolysis approach with different iron salts. The properties of the samples were conducted by BET, SEM, EDS, XRD, XPS, TEM, and FTIR. The results suggested that the surface areas of char-FeCl3 and char-FeCl2 were higher than those of char-FeCit. The presence of Fe2O3 as well as pyrolysis gas (HCl (g) and H2O (g)) could catalyze the formation of porosity. Meanwhile, FeCl3 showed the strongest catalysis effect to decompose cellulose to produce char. The pyrolysis process analysis was investigated by means of thermogravimetry-DSC. FeCl3 and FeCl2 could accelerate the breakage of cellulose structure whereas FeC6H5O7 was not beneficial to form char at low temperature as the incomplete decomposition of citrate. The adsorption property of Cr(VI) for the chars was evaluated. Adsorption processes were fitted well with the Freundlich model, and char-FeCl3 presented the best adsorptive capacity (70.39 mg/g). Thus, this low-temperature pyrolysis method was economical and technologically simplified as well as efficient adsorption capacity of Cr(VI) removal.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Activated carbons have been widely applied as the excellent adsorbents in the water treatment. Traditional activated carbons are produced from coal (Cuhadaroglu and Uygun 2008) and wood (Acharyaa et al. 2009) which are expensive and nonrenewable source. In order to overcome the limitation of exhaustible resources, waste materials have attracted much attention to replace conventional raw materials, such as denim fabric waste (Silva et al. 2018), acrylic textile waste (Nahil and Williams 2010), and polyacrylonitrile (Dong et al. 2015). Particularly, cotton textile waste (CTW) contains cellulose with high-carbon content as a great potential alternative (Chiu and Ng 2012) and cotton-based adsorbent was used in the adsorption of dyes (Chiu and Ng 2012), heavy metal (Muxel et al. 2011), and pharmaceuticals (Sun et al. 2012) in the water treatment as carbon materials.
Commonly, activated carbon is synthesized by physical and chemical activation methods. Physical treatment consists of carbonization and activation, two steps with an activating gas such as CO2 (Daud and Ali 2004) or steam (González et al. 2009). However, physical treatment requires relatively high activated temperature between 800 and 1000 °C. In chemical activation method, some chemical agents including H3PO4, KOH, and ZnCl2 are the most representative activators (Angin 2014; Dutta et al. 2011; He et al. 2016). But, traditional chemical activation also has some drawbacks that contain the damage of equipment resulted from acid or alkali corrosion and high toxicity, which even cause the problem of environmental pollution (Luo et al. 2016). Recently, iron salts are viewed as a novel chemical activator owing to inexpensive and lower toxicity than other chemical agents. In particular, FeCl3 is considered as the most studied activator, which not only have its advantages of catalysis and dehydrogenation but also could bring about remarkable developed porosity (Bedia et al. 2018). Beside, FeCl2 as the catalyst could enhance pyrolysis of raw materials and production of carbon by reported by Wang et al. (2007). However, activated carbon prepared by Fe(NO3)3 and Fe2(SO4)3 exhibits relatively lower porosity than FeCl3 and FeCl2 due to the presence of chloride ions. In addition, organic iron salts like ferric oxalate (FeC2O4) and ferric citrate (FeC6H5O7) (Fu et al. 2014) showed different activation pathways. Moreover, FeC6H5O7 is not harmful to human and is used in photocatalysis research but it is not studied too much as a chemical activator (Wang et al. 2014).
It is widely accepted that activated method required preparation of the adsorbent at relatively high temperatures (> 600 °C). The utilization of low-temperature method has the potential to save energy consumption and be more economical. It was reported that preparation of activated carbon with ZnCl2 at 400 °C shows good surface area and good adsorption to remove Cr(VI) (Rangabhashiyam and Selvaraju 2015a). Kanungo and Mishr (1996) reported that the dehydration of FeCl3 began at around 40 °C, and then decomposition happened at 100 °C, followed by the formation of Fe2O3 during the pyrolysis process. Inspired by transition of iron phase in the range of 40 °C to 300 °C, the feasibility of activated carbon prepared by iron salts at low temperature could be studied. In addition, the mechanism of graphite oxide activation treated by KOH has been investigated below 500 °C (Wu et al. 2016) and Fe2+ has comparable catalysis during the cellulose pyrolysis process with K+ (Wang et al. 2007). On the other hand, carbon contains more C–O and C–H functional groups at low temperatures (300~400 °C) which could provide a platform for removal of adsorbate (Shen and Fu 2018). Hence, three kinds of iron salts (FeCl3, FeCl2, and FeC6H5O7) were used as the representative activators to prepare cotton textile waste–based carbon materials at low pyrolysis temperature.
Chromium is commonly used in industries such as leather, metal plating, battery, and textile (Duranoglu et al. 2012). Cr(VI) could transmigrate freely into the ecosystem and organism because of its high solubility and high mobility in the form of HCrO4− and CrO42− (Liang et al. 2019). It has been noted that Cr(VI) does great harm to human health and has even caused cancers (Salnikow and Zhitkovich 2008). Several methods have been investigated for the removal of Cr(VI), such as ion exchange (Lin and Kiang 2003), biological process (Kumar et al. 2008), extraction (Akama and Sali 2002), and adsorption (Zhang et al. 2015b). Compared to other methods, due to higher efficiency, easier application, and lower cost, the adsorption can be considered as a widely used and promising technique (Liu et al. 2012).
In this study, iron salts were employed to produce the chars derived from CTW via the low-temperature pyrolysis method. The characteristics of chars synthesized by inorganic and organic iron salts were studied by Brunauer-Emmett-Teller (BET), XRD, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and FTIR. Thermogravimetry (TG)-DSC was used to further analyze the effect of inorganic and citrate iron salts on the formation of the chars. Finally, adsorption experiments of Cr(VI) were conducted to evaluate the adsorption efficiency of the prepared chars.
Materials and methods
Chemical and materials
CTW was collected in Wuxi, Jiangsu Province, China. Ferric chloride hexahydrate (FeCl3·6H2O), ferrous chloride tetrahydrate (FeCl2·4H2O), ferric citrate (FeC6H5O7), hydrochloric acid (HCl), nitric acid (HNO3), sodium hydroxide (NaOH), and potassium dichromate (K2Cr2O7) were purchased from Sinopharm Chemical Reagent Co., Shanghai, China. All experiment procedures were performed by using deionized water (Milli-Q Advantage A10, USA).
Preparation
The CTW was firstly rinsed through deionized water to remove impurities and dried at 50 °C overnight. And CTW was cut into the size of 0.5~1 cm and fully impregnated with 25 mL solutions of different iron salts (FeCl3, FeCl2, and FeC6H5O7) in a mass ratio of 3:1 (iron salts:CTW) at 60 °C in a drying oven for 24 h, respectively. Then, these dried mixtures were pyrolyzed at 300 °C for 60 min with a heating rate of 10 °C/min under N2 flow of 100 mL/min in a furnace and the samples were cooled to room temperature. After that, the samples were soaked in 50 mL boiling HCl solution (1.2 mol/L) and washed with deionized water until the pH of elution was close to neutral. Finally, the resulting chars were dried at 80 °C for 12 h and the prepared chars obtained by FeCl3, FeCl2, and FeC6H5O7 were denoted as char-FeCl3, char-FeCl2, and char-FeCit, respectively.
Characterization
The surface area and porosity of chars were investigated with nitrogen adsorption/desorption isotherms at 77 K using a surface area analyzer (Autosorb-iQ 2MP; Quantachrome, USA). The surface area was calculated using the BET equation, and the pore size distributions were calculated by density functional theory (DFT). The total pore volume was defined at the relative pressure (P/P0) of 0.99. The crystal structures of chars were acquired using powder XRD patterns over a wide range of angles from 10 to 90° (D8 ADVANCE; Bruker, Germany). XPS was carried out using a Kratos Axis Ultra spectrometer with a monochromatic X-ray source of Al Kα operation. The surface morphologies of CTW as well as chars were analyzed by a scanning electron microscope (S4800; Hitachi, Japan), and energy-dispersive spectrometry (EDS) spectrum was obtained by an energy-dispersive spectrometer (EDAX; Genesis, USA). TEM images of chars were performed by a TEM instrument (Tecnai-G2-F20; FEI, USA). The surface functional groups of samples were explored by a FTIR spectrometer (iS10; Nicolet, China) ranging from 4000 to 400 cm−1. The pyrolysis characteristics of CTW and mixtures of CTW with iron salts (CTW + FeCl3, CTW + FeCl2, and CTW + FeCit) were performed using a TG analyzer (STA 8000; PerkinElmer, USA).
Adsorption experiments
To evaluate the effect of initial pH, 0.1 g of char was added into the Cr(VI) solution (50 mL, 150 mg/L) and the pH values were adjusted to 2~12 with HNO3 (0.1 mol/L) and NaOH (0.1 mol/L). The prepared solution was agitated at a speed of 150 rpm at 25 °C for 12 h. After that, the mixture was passed through a 0.45-μm filter and the concentration of filtered liquor was analyzed by a UV–vis spectrophotometer (Jingke/UV-754, China). The adsorbed capacity of Cr(VI) was calculated using Eq. (1)
where C0 and Ce (mg/L) are the initial and equilibrium concentrations of Cr(VI), respectively; V (L) is the volume of Cr(VI) solution; and m (g) is the weight of adsorbent.
The adsorption kinetics of Cr(VI) onto chars were studied; 0.2 g of char was mixed with Cr(VI) solution (100 mL, 150 mg/L, initial pH of 2.0) in centrifuge tubes and oscillated at 25 °C with 150 rpm. The concentration of Cr(VI) solution was measured at disparate time periods (10 min, 30 min, 60 min, 120 min, 240 min, 480 min, 720 min, and 1440 min).
The adsorption isothermal model of Cr(VI) onto chars was analyzed; 0.1 g of char was introduced into the Cr(VI) solution (50 mL, 80~300 mg/L, initial pH at 2.0). After 12 h of oscillation at different temperatures (15 °C, 25 °C, and 35 °C), the concentration of Cr(VI) solution was measured.
Results and discussion
The N2 adsorption/desorption isotherms
The N2 adsorption/desorption isotherms and pore distributions of the chars are depicted in Fig. 1. As shown in Fig. 1a, according to the IUPAC classification, the N2 adsorption/desorption isotherms of both char-FeCl3 and char-FeCl2 exhibited type II with H3 hysteresis loops illustrating the presence of micropores and a large number of mesopores (Bai et al. 2012). For char-FeCit, the isotherm showed almost a straight line with the lowest adsorption capacity, leading to the relatively low specific surface area and pore volume. According to Fig. 1b, char-FeCl3 and char-FeCl2 had both a small number of micropores and the medium pores with the main proportion, especially between 8 and 25 nm. The porosity of char-FeCl2 was comparable to that of char-FeCl3 due to their similar catalytic effect (Wang et al. 2008). Meanwhile, the pore volume of char-FeCl3 was distinctly greater than that of char-FeCl2. Besides, char-FeCit displayed the lowest porosity which almost had no micropores and mesopores. It could be assumed that the great different properties in three samples were related to disparate thermolysis of inorganic iron salts and iron citrate which have an effect on the CTW (Rudnev et al. 2018).
Furthermore, char-FeCl3 presented the maximum surface area (78 m2/g) and the largest total pore volume (0.315 m3/g) among the three samples. The obtained surface areas were comparable to the biochars reported by Hanoğlu et al. (2019) (26.4 m2/g) from textile fibers. Char-FeCl3 exhibited the multifarious porosity because some iron species might form to develop a pore structure during the pyrolysis process (Oliveira et al. 2009). The BET surface area and total pore volume of char-FeCl2 were 43 m2/g and 0.119 m3/g, respectively, indicating that the addition of FeCl2 could also strengthen the generation of pores during the pyrolysis process of CTW. Besides, the yield of char-FeCl2 (48.6%) was higher than that of char-FeCl3 (43.9%). However, char-FeCit displayed the lowest BET (13 m2/g) and the lowest Vtot (0.033 m3/g).
XRD and XPS
To understand the effect of iron phase evolution on the pyrolysis process, Fig. 2 illustrates the XRD patterns of chars before acid washing. The diffraction pattern of char-FeCl3 was found to be similar to that of char-FeCl2. The patterns of char-FeCl3 and char-FeCl2 showed the peaks at 2θ of 15.7°, 20.5°, 37.7°, 38.8°, 42.2°, 43.4°, 51.8°, 57.8°, and 67.5° were assigned to FeCl2·2H2O; the peaks at 2θ of 32.4° and 33.6° were attributed to the Fe2O3 particles; and the peaks at 2θ of 31.2° were ascribed to FeOOH (Liu et al. 2014). These results suggested that the char might occur in the following reactions ((2)–(4)) (Cazetta et al. 2016):
The reactions showed that Fe(OH)3 was caused by the hydrolysis of FeCl3, and then converted to FeOOH at the pyrolysis temperature around 100 °C. Next, Fe2O3 was produced from FeOOH when the char was heated to 200 °C. It was proved that iron compounds had loaded on the interior pore structure of char-FeCl3 and catalyzed to form char at a low temperature of 300 °C. Meanwhile, the reduction of FeCl3·6H2O to FeCl2·2H2O together with HCl gas was generated to catalyze pore structure (Nicolas Louvain et al. 2013; Oliveira et al. 2009).
Furthermore, it was observed in char-FeCl2 that the peak of FeCl2·2H2O was caused by dehydration of FeCl2·4H2O. In addition, dehydrochlorination of FeCl2·4H2O was transformed to FeOOH and further formed into Fe2O3 species. The observation established that the diffraction intensity of char-FeCl2 was stronger than that of char-FeCl3, which probably was attributed to the better impregnation of FeCl2 (Ohmukai et al. 2008), whereas the pore volume of char-FeCl3 was better than that of char-FeCl2 in Fig. 1b, which is mainly due to the stronger catalysis from Fe3+ (Liu et al. 2009).
In contrast, the pattern of char-FeCit demonstrated the peaks at 2θ of 29.5° and 43.2° were attributed to Fe3O4; the peaks at 2θ of 30.0° and 60.5° were assigned to FeOOH; and the peaks at 2θ of 40.0° and 57.8° were ascribed to Fe2O3. The results implied that the decomposition of FeC6H5O7 led to the formation of FeOOH and further decomposition and conversion into Fe3O4 species. The converted Fe3O4 played an activated role which facilitated the further formation of pore (Zhang et al. 2016).
XPS survey was performed that aimed at the surface of chars before pickling as shown in Fig. 3a–c. For char-FeCl3 and char-FeCl2, two samples had similar characteristic peaks. The peaks at the binding energy of 710.97 eV and 724.07 eV were ascribed to Fe(III) 2p3/2 and Fe(III) 2p1/2, respectively. And of the two chars, the peak at 715.12 eV was the satellite peak of Fe(III) 2p3/2. At the same time, Fe(II) 2p1/2 was observed at the peaks of 729.47 eV which was coincided to FeCl2. Moreover, the peaks at 710.97 eV and 724.07 eV can be corresponding to Fe2O3 (Ohmukai et al. 2008). Additionally, it was estimated that the Fe(III) and Fe(II) area ratio was about 7:1 which corresponded to relative Fe2O3 and FeCl2 contents of char-FeCl3 and char-FeCl2. It was confirmed that Fe2O3 was mainly produced by the decomposition of FeCl3·6H2O or FeCl2·4H2O, then further impacting the pore development of chars. Oppositely, the peak positions of Fe 2p3/2 and Fe 2p1/2 are 711.27 eV and 724.57 eV, respectively, which were signed to the Fe3O4 in the char-FeCit (Mills and Sullivan 1983). The composition of iron compounds was further determined by XPS analysis. Herein, it was confirmed that the decomposition of inorganic iron salts could induce the formation of Fe2O3, while the ferric citrate pyrolysis resulted in the existence of Fe3O4.
SEM and TEM
The surface morphology was shown by SEM for the comparison of the chars after acid washing. It could be clearly observed in Fig. 4a that raw CTW gave a smooth and soft surface without any pores in its structure. After pyrolysis, the chars exhibited pore structure, indicating that the low-temperature (300 °C) pyrolysis process could led to the development of porosity of raw materials as shown in Fig. 4b–d.
From Fig. 4b and c, the chars appeared to have a similar pore structure between char-FeCl3 and char-FeCl2 which have a rough and accidented surface, and the char-FeCl3 appeared to have more abundant multiholes than char-FeCl2 which contributed to the increase of the surface area of char-FeCl3. The observed results could be explained that FeCl3 had a stronger effect on the decomposition of cellulose of CTW, facilitating the development of better pore channel (Liu et al. 2009). Besides, Cl− would combined with chars and cross-linking reactions occurred during the pyrolysis process of CTW with FeCl3 or FeCl2 (Xu et al. 2019). As can be seen in Fig. 4d, char-FeCit showed an entirely different morphology, and the agglomeration phenomenon with the irregular cavity character was observed. The porous structure of char-FeCit was underdeveloped presumably due to the insufficient pyrolysis temperature to decompose citrate and impact the pyrolysis of CTW (Bao et al. 2019).
The SEM-EDS images in Fig. 5a–c display that the C content occupied the dominant location (63.99~79.77%), demonstrating that it was feasible to produce chars at the low-temperature approach. And, a low content of iron existed when iron oxide was loaded on the surface of the three chars after acid washing and iron compounds were covered by chars on the surface’s pore structure successfully (Zhang et al. 2015a). The oxygen content mainly came from functional groups of corrupted cellulose as well as formed iron oxide particles. And, the char-FeCit existed incompletely decomposed citrate which displayed the highest oxygen content.
Figure 6 depicts the TEM image to further characterize the morphologies of the char after acid washing. Some channels of char-FeCl3 and char-FeCl2 had appeared and tended to have an amorphous structure owing to the release of volatiles during the pyrolysis process as shown in Fig. 6a and b. The change of iron phase could increase the porosity during the catalytic pyrolysis process. Additionally, it was noticed that char-FeCl3 possessed more chaotic and compact than char-FeCl2. It was due to that Fe3+ had lower accessibility for carbonization of CTW at low temperature (Hamid et al. 2015). However, it was observed in Fig. 6c that few evident bright spots were determined which were assigned to pore structure. The accumulation of iron particles was spread throughout into char-FeCit.
FTIR
Figure 7 presents FTIR spectra of the chars which could analyze the surface functional groups. The bands at around 3428 cm−1 of char-FeCl3 and char-FeCl2 and at 3372 cm−1 of char-FeCit were ascribed to the O–H stretching vibration of carboxyl and phenol groups. And, a portion of O–H groups was derived from FeOOH and FeCl2·2H2O. Besides, the functional groups of O–H vibration might be involved in the adsorption of metal ions as a participant (Rangabhashiyam and Selvaraju 2015b). The band at between 2920 cm−1 and 2900 cm−1 was assigned to the C–H stretching vibration of aromatic ring. The band of 1700 cm−1 was associated to the C=O vibration of char-FeCl3 and char-FeCl2 in esters or carboxylic groups. Moreover, the band of 1046~1293 cm−1 was attributed to the C–O vibration and the C=C vibration of aromatic ring was presented at the band of 1590 cm−1 (Nahil and Williams 2010; Sun et al. 2012). The stretching vibration of C–O groups in char-FeCl3 and char-FeCl2 obviously became weaker that corroborated the cellulose from CTW which mostly decomposed and tended to carbonization (Tang et al. 2016). Besides, the band at 670 cm−1 was ascribed to the C–Cl groups, indicating that the char-FeCl3 and char-FeCl2 might form a C–Cl bond to grow cross-linking reaction (Lee et al. 2014).
Thermal behavior by TG-DSC analysis
To understand the thermal behavior of the three prepared chars, TG was performed. Figure 8 a–d displays the TG-DSC curve of CTW and mixtures of CTW and iron salts (FeCl3, FeCl2, and FeC6H5O7) under nitrogen to analyze the relation between CTW and iron salts. The TG of CTW (Fig. 8a) could be divided into two stages in the temperature ranges of 0~400 °C. At the primary process below 235 °C, there was the evaporation of moisture mainly with a slight weight loss. The second process with a mass loss of 66.5% was the main pyrolysis process between 235 and 400 °C, attributing to a large number of the collapse of cellulose chains which is accompanied with the release of volatile gases (Mostashari and Mostashari 2008). The DSC enabled to find the endothermic peak at 215 °C which was due to the loss of moisture.
After adding iron salts, it is shown vividly in Fig. 8b–d that the pyrolysis temperature of weight loss is significantly different. For the TG curve of CTW + FeCl3 (Fig. 8b), there were three weight loss steps. The initial weight loss (23.5%) at 0~152 °C was considered for removal of water from CTW, corresponding to an endothermic peak at 97 °C in DSC curve. It was suggested that the addition of FeCl3 promoted the loss of water molecules from CTW. The second weight loss (11.5%) in the range of 152~190 °C occurred in which Fe3+ entered the CTW, then strongly fractured the hydrogen bond and glycosidic bond between cellulose (Li et al. 2015), being assigned to the endothermic peak of DSC curve (165 °C). And, the release of H2O molecule was derived from reduction of FeCl3·6H2O to FeCl2·2H2O at this temperature stage (Li et al. 2011). The decreasing weight loss was attributed to decomposition of CTW, breaking the structure of the cellulose. And, the volatile matters escaped and left the space in the char matrix. Compared to the TG of CTW, the images provided some interesting finding that the initial pyrolysis temperature was advanced significantly from 235 to 152 °C, which indicated that FeCl3 led to acceleration of the decomposition of cellulose and the pore development was advanced (Rufford et al. 2011). The last stage with the mass loss of 7% at the temperature range of 190 °C and 400 °C had further the decomposition of CTW as well as the production of pyrolysis gas (HCl (g) and H2O (g)). The slope of TG curves became smooth, and the transition of iron phase was caused by the decomposition of FeCl2·2H2O to Fe2O3 (Zhu et al. 2014), which was in accordance with the XRD patterns of char-FeCl3 (Fig. 2). The existence of Fe2O3 species was favorable to catalyze pore structure during the catalytic pyrolysis process (Gong et al. 2009). All of these indicated that doping with FeCl3 as a catalyst accelerated the pyrolysis process of CTW and change the characteristics, creating a pore structure of char-FeCl3.
From Fig. 2c, the TG of CTW + FeCl2 showed three stages. A weight loss of 10% occurred at 0~146 °C which can be attributed to moisture evaporation. The next weight loss of 13.2% was observed between 146 and 204 °C owing to the decomposition of cellulose. It was indicated that FeCl2 could be used as a catalyst and was beneficial to the decomposition reaction during the pyrolysis process (Yuan et al. 2010). Furthermore, the loss of mass was about 7.6% at the temperature of 204~400 °C which was ascribed to transformation of FeCl2 to Fe2O3 to facilitate the formation of chars (Nicolas Louvain et al. 2013). In DSC, the initial endothermic peaks at 143 °C belonged to removal of water molecule and the second endothermic peak at 192 °C belonged to decomposition of CTW. The use of ferrous chloride could lead to the disruption of the intermolecular hydrogen bond of cellulose (Yu et al. 2011). When cellulose was decomposed at 146~400 °C, it promoted the release of volatiles and decomposition of CTW because of the addition of FeCl2; in the meantime, the cellulose had trended to collapse to form pore channel. Furthermore, CTW + FeCl3 and CTW + FeCl2 had the same trend during the mass loss process. And, the endothermic peak of CTW + FeCl2 became stronger in comparison with that of CTW + FeCl3, which was related to the stronger cleavage degree of cellulose bonds (Hamid et al. 2015). In a word, it was confirmed that FeCl2 played an advantageous role in developing char in the pyrolysis process.
The TG of CTW + FeCit in Fig. 8d had three weight loss steps. In the first step, the weight loss (10%) was ascribed to liberation of absorbed water in the temperature range of 0 °C and 194 °C. In addition, volatilization of crystalline water led to the appearance of endothermic peaks at 114 °C. Compared with the formers, the initial mass loss temperature of CTW + FeCit possibly resulted from the complexation between Fe3+ and C6H5O73− in aqueous solution (Balachandran 2014), inhibiting the decomposition of ferric citrate. The main weight loss corresponded to the decomposition of FeCit and translation to citraconic and itaconic anhydrides through decarboxylation and dehydration in steps 2 (194~340 °C) and 3 (340~400 °C) (Zhu et al. 2017). The endothermic peak at 218 °C was attributed to the decomposition of CTW. The decomposition of FeCit involved many complex chemical reactions at 300 °C, and the final decomposition product could be corresponded to the organic acids and Fe3O4. The mass loss behavior of CTW with citrate salts appeared to be similar to the results proposed by Zhou et al. (2012) using calcium citrate as the carbon source to prepare porous carbon. Thus, it was suggested that citrate was mainly converted to organic acids before 300 °C and had weak ability to break the structure of cellulose, then decompose CTW at the pyrolysis temperature of 300 °C. The result showed that char-FeCit formed poor pore structure.
Adsorption capacity assessment
The effects of initial pH
The effects of initial pH on the removal of Cr(VI) onto chars are displayed in Fig. 9. The adsorption capacity of Cr(VI) was dependent on initial pH, and the highest removal efficiency was obtained at pH 2. As the pH increased, the predominant ion was HCrO4− which gradually converted to CrO42− and Cr2O72− on the adsorption reaction. In addition, the increased negative charge density with the higher OH− ions hindered the diffusion of Cr(VI) ions (Liu et al. 2012). Therefore, the efficiency of removal for Cr(VI) declined obviously from pH 4 to 12 due to the electrostatic repulsion between the surface of chars and CrO42− (Goswami et al. 2014).
Adsorption kinetics
The kinetic models were analyzed using both the pseudo-first-order and the pseudo-second-order models by the following equations ((5) and (6)):
Pseudo-first-order model equation
Pseudo-second-order model equation
where qt and qe (mg/g) are the adsorption amounts of Cr(VI) at time t and equilibrium, respectively; k1 (1/min) is the pseudo-first-order rate constant; and k2 (g/(mg/min)) is the pseudo-second-order rate constant.
Figure 10 shows the influence of agitation time of the chars on the adsorption of Cr(VI). With the increasing time, the adsorption efficiency of the chars for Cr(VI) removal went up quickly at first 30 min and then tended to be stable until 720 min, because the adsorption sites of chars were occupied by the adsorbed Cr(VI) (Demiral et al. 2008). As can be seen from Table 1, the adsorption kinetics of Cr(VI) sorption onto chars were both well fitted with pseudo-first-order and pseudo-second-order models, suggesting that chemisorption and physisorption played important roles in the Cr(VI) removal (Liang et al. 2019). The adsorption capacity of three chars ranked from high to low as char-FeCl3 > char-FeCl2 > char-FeCit. On the one hand, the Cr(VI) removal was subjected to oxidation–reduction reaction with the functional groups like O–H and C–O to reduce Cr(VI) and oxidize the chars which led to the expected improved removal efficiency of Cr(VI) (Li et al. 2019). On the other hand, the difference of adsorption capacity was related to the specific surface area of the chars (Bedia et al. 2018). The increasing volume of mesopores was supportive to offer channels and active sites for the movement and adsorption of Cr(VI) (Tang et al. 2016). Besides, the SEM (Fig. 4c) of char-FeCit presented that some cavities might provide a good chance for the Cr(VI) to be adsorbed (Rangabhashiyam and Selvaraju 2015b). This reason gave an explanation for the feasible adsorption capacity from char-FeCit.
Adsorption isotherms
The adsorption isotherms of Cr(VI) onto chars at different initial concentrations were fitted using the Langmuir and Freundlich models.
The nonlinear form of the Langmuir and Freundlich equations can be expressed as (7) and (8)
where qe (mg/g) is the adsorption amount of Cr(VI), qm (mg/g) is the maximum adsorption capacity, Ce (mg/L) is the equilibrium concentration, KL (L/mg) is the Langmuir constant, and KF (mg/g(L/mg)1/n) is the Freundlich constant.
Figure 11 depicts the Cr(VI) adsorption isotherms onto the chars. The adsorption isotherm explained the relationship between Cr(VI) adsorbed and the concentration in the equilibrium solution. The values of relevant parameters are listed in Table 2, and the maximum adsorption capacity was calculated via the Langmuir model. In the adsorption isotherm study, the chars better fitted with the Freundlich model than the Langmuir model according to the correlation coefficient (R2), indicating that removal of Cr(VI) onto chars was multilayer coverage (Liu et al. 2012). The separation factor of KL was between 0 and 1, demonstrating that the adsorption of Cr(VI) onto chars prepared by low pyrolysis temperature was favorable. The value of n between 2 and 10 suggested that the chars were effective adsorbents for Cr(VI) removal. With the increasing temperature, molecular motion was stronger, leading to the increased capacity of Cr(VI) removal, which indicated that the process of Cr(VI) removal with chars could be promoted (Duranoglu et al. 2012).
The maximum adsorption capacity of Cr(VI) onto chars was competitive among the previously reported absorbents derived from different waste materials in Table 3. Compared to the other adsorbents prepared by the relatively high pyrolysis temperature (> 400 °C), the maximum adsorptive capacity of the chars in this study at low-temperature pyrolysis conditions (300 °C) was better. Considering low-energy consumption and environmental friendly method, char-FeCl3, char-FeCl2, and char-FeCit were considered as the feasible and efficient adsorbents for Cr(VI) removal.
Conclusion
Different iron salts served as activating agents to prepare the cotton textile waste–based char adsorbent at the low pyrolysis temperature of 300 °C. It was demonstrated that FeCl3 and FeCl2 had similar characteristics on the breakage of the cellulose and the formation of iron oxides to develop pores during the pyrolysis process. Moreover, the C–Cl bond structure was beneficial to form chars. The best surface area had been produced from char-FeCl3 owing to the highest catalysis effect at low pyrolysis temperature. FeC6H5O7 could promote the decomposition of CTW, but char-FeCit showed unnoticeable porous structures because of the complexation between Fe3+ and C6H5O73− and inefficient decomposition of citrate. The adsorption behavior of the chars was mostly completed in a short time, which was conformed to pseudo-first-order and pseudo-second-order models while chemisorption and physisorption occurred in the adsorption process. The adsorption equilibrium data followed the Freundlich isotherm model. The order of the adsorption efficiency in relation to BET was char-FeCl3 > char-FeCl2 > char-FeCit. In addition, surface functional groups also provided a platform to improve the adsorption efficiency of Cr(VI). Therefore, it can be concluded that the low-temperature approach to synthesize char-based adsorbents could be employed.
References
Acharyaa J, Sahub JN, Mohantyc CR, Meikap BC (2009) Removal of lead(II) from wastewater by activated carbon developed from tamarind wood by zinc chloride activation. Chem Eng J 149:249–262. https://doi.org/10.1016/j.cej.2008.10.029
Akama Y, Sali A (2002) Extraction mechanism of Cr(VI) on the aqueous two-phase system of tetrabutylammonium bromide and (NH4)2SO4 mixture. Talanta 57:681–686
Angin D (2014) Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 115:804–811. https://doi.org/10.1016/j.fuel.2013.04.060
Bai J, Wang Q, Jiao G (2012) Study on the pore structure of oil shale during low-temperature pyrolysis. Energy Procedia 17:1689–1696. https://doi.org/10.1016/j.egypro.2012.02.299
Balachandran M (2014) Bio-processing of indian coals by microorganisms: an investigation. J Environ Res Dev 9:209–215
Bao S et al (2019) In-situ porous nano-Fe3O4/C composites derived from citrate precursor as anode materials for lithium-ion batteries. Mater Chem Phys 225:379–383. https://doi.org/10.1016/j.matchemphys.2018.12.072
Bedia J, Belver C, Ponce S, Rodriguez J, Rodriguez JJ (2018) Adsorption of antipyrine by activated carbons from FeCl3-activation of Tara gum. Chem Eng J 333:58–65. https://doi.org/10.1016/j.cej.2017.09.161
Cazetta AL, Pezoti O, Bedin KC, Silva TL, Paesano Junior A, Asefa T, Almeida VC (2016) Magnetic activated carbon derived from biomass waste by concurrent synthesis: efficient adsorbent for toxic dyes. ACS Sustain Chem Eng 4:1058–1068. https://doi.org/10.1021/acssuschemeng.5b01141
Chiu K-L, Ng DHL (2012) Synthesis and characterization of cotton-made activated carbon fiber and its adsorption of methylene blue in water treatment. Biomass Bioenergy 46:102–110. https://doi.org/10.1016/j.biombioe.2012.09.023
Cuhadaroglu D, Uygun OA (2008) Production and characterization of activated carbon from a biuminous coal by chemical activation. Afr J Biotechnol 7:3703–3710. https://doi.org/10.1016/j.protis.2008.04.002
Daud WMAW, Ali WSW (2004) Comparison on pore development of activated carbon produced from palm shell and coconut shell. Bioresour Technol 93:63–69. https://doi.org/10.1016/j.biortech.2003.09.015
Demiral H, Demiral İ, Tümsek F, Karabacakoğlu B (2008) Adsorption of chromium(VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem Eng J 144:188–196. https://doi.org/10.1016/j.cej.2008.01.020
Dong X, Lu C, Zhou P, Zhang S, Wang L, Li D (2015) Polyacrylonitrile/lignin sulfonate blend fiber for low-cost carbon fiber. RSC Adv 5:42259–42265. https://doi.org/10.1039/c5ra01241d
Duranoglu D, Trochimczukb AW, Bekera U (2012) Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile-divinylbenzene copolymer. Chem Eng J 187:193–202. https://doi.org/10.1016/j.cej.2012.01.120
Dutta S, Bhattacharyya A, Ganguly A, Gupta S, Basu S (2011) Application of response surface methodology for preparation of low-cost adsorbent from citrus fruit peel and for removal of methylene blue. Desalination 275:26–36. https://doi.org/10.1016/j.desal.2011.02.057
Enniya I, Rghioui L, Jourani A (2018) Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain Chem Pharm 7:9–16. https://doi.org/10.1016/j.scp.2017.11.003
Fu KF et al (2014) Physicochemical and adsorptive properties of activated carbons from Arundo donax Linn utilizing different iron salts as activating agents. J Taiwan Inst Chem Eng 45:3007–3015. https://doi.org/10.1016/j.jtice.2014.08.026
Gong X, Guo Z, Wang Z, Gong X, Guo Z, Wang Z (2009) Variation of char structure during anthracite pyrolysis catalyzed by Fe2O3 and its influence on char combustion reactivity. Energy Fuel 23:4547–4552
González JF, Román S, Encinar JM, Martínez G (2009) Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J Anal Appl Pyrolysis 85:134–141. https://doi.org/10.1016/j.jaap.2008.11.035
Goswami M, Borah L, Mahanta D, Phukan P (2014) Equilibrium modeling, kinetic and thermodynamic studies on the adsorption of Cr(VI) using activated carbon derived from matured tea leaves. J Porous Mater 21:1025–1034. https://doi.org/10.1007/s10934-014-9852-1
Hamid SBA, Teh SJ, Lim YS (2015) Catalytic hydrothermal upgrading of α-cellulose using iron salts as a Lewis acid. BioResources 10:5974–5986. https://doi.org/10.15376/biores.10.3.5974-5986
Hanoğlu A, Cay A, Yanık J (2019) Production of biochars from textile fibres through torrefaction and their characterisation. Energy 166:664–673. https://doi.org/10.1016/j.energy.2018.10.123
He J et al (2016) Preparation of highly porous carbon from sustainable α-cellulose for superior removal performance of tetracycline and sulfamethazine from water. RSC Adv 6:28023–28033. https://doi.org/10.1039/c6ra00277c
Kanungo SB, Mishr SK (1996) Thermal dehydration and decomposition of FeCl3·xH2O. J Therm Anal 46:1487–1500
Kumar R, Bishnoi NR, Garima, Bishnoi K (2008) Biosorption of chromium(VI) from aqueous solution and electroplating wastewater using fungal biomass. Chem Eng J 135:202–208. https://doi.org/10.1016/j.cej.2007.03.004
Lee SM, Choi WJ, Hwang K, Kim J-H, Lee J-Y (2014) Effect of catalyst concentration and reaction time on one-step synthesized hypercrosslinked polyxylene. Macromol Res 22:481–486. https://doi.org/10.1007/s13233-014-2065-2
Li W-H, Yue Q-Y, Gao B-Y, Wang X-J, Qi Y-F, Zhao Y-Q, Li Y-J (2011) Preparation of sludge-based activated carbon made from paper mill sewage sludge. Desalination 278:179–185. https://doi.org/10.1016/j.desal.2011.05.020
Li J, Zhang X, Zhang M, Xiu H, He H (2015) Ultrasonic enhance acid hydrolysis selectivity of cellulose with HCl-FeCl3 as catalyst. Carbohydr Polym 117:917–922. https://doi.org/10.1016/j.carbpol.2014.10.028
Li B et al (2019) Facile modification of activated carbon with highly dispersed nano-sized α-Fe2O3 for enhanced removal of hexavalent chromium from aqueous solutions. Chemosphere 224:220–227. https://doi.org/10.1016/j.chemosphere.2019.02.121
Liang H, Song B, Peng P, Jiao G, Yan X, She D (2019) Preparation of three-dimensional honeycomb carbon materials and their adsorption of Cr(VI). Chem Eng J 367:9–16. https://doi.org/10.1016/j.cej.2019.02.121
Lin SH, Kiang CD (2003) Chromic acid recovery from waste acid solution by an ion exchange. Chem Eng J 92:193–199. https://doi.org/10.1016/S1385-8947(02)00140-7
Liu L, Sun J, Cai C, Wang S, Pei H, Zhang J (2009) Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation. Bioresour Technol 100:5865–5871. https://doi.org/10.1016/j.biortech.2009.06.048
Liu W, Zhang J, Zhang C, Ren L (2012) Preparation and evaluation of activated carbon-based iron-containing adsorbents for enhanced Cr(VI) removal: mechanism study. Chem Eng J 189–190:295–302. https://doi.org/10.1016/j.cej.2012.02.082
Liu WJ, Tian K, He YR, Jiang H, Yu HQ (2014) High-yield harvest of nanofibers/mesoporous carbon composite by pyrolysis of waste biomass and its application for high durability electrochemical energy storage. Environ Sci Technol 48:13951–13959. https://doi.org/10.1021/es504184c
Louvain N et al (2013) One-shot versus stepwise gas–solid synthesis of iron trifluoride: investigation of pure molecular F2 fluorination of chloride precursors. CrystEngComm 15:3664–3671. https://doi.org/10.1039/c3ce27033e
Luo Y, Street J, Steele P, Entsminger E, Guda V (2016) Activated carbon derived from pyrolyzed pinewood char using elevated temperature, KOH, H3PO4, and H2O2. BioResources 11:10433–10447. https://doi.org/10.15376/biores.11.4.10433-10447
Mills P, Sullivan JL (1983) A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy. J Phys D Appl Phys 16:723–732
Mostashari SM, Mostashari SZ (2008) Combustion pathway of cotton fabrics treated by ammonium sulfate as a flame-retardant studied by TG. J Therm Anal Calorim 91:437–441
Muxel AA, Gimenez SMN, de Souza Almeida FA, da Silva Alfaya RV, da Silva Alfaya AA (2011) Cotton Fiber/ZrO2, a new material for adsorption of Cr(VI) ions in water. Clean: Soil, Air, Water 39:289–295. https://doi.org/10.1002/clen.201000165
Nahil MA, Williams PT (2010) Activated carbons from acrylic textile waste. J Anal Appl Pyrolysis 89:51–59. https://doi.org/10.1016/j.jaap.2010.05.005
Ohmukai Y, Hasegawa I, Fujisawa H, Okuma O, Mae K (2008) Production of an iron-loaded carbonaceous material through pyrolyzing biomass impregnated with FeCl2. Fuel 87:2041–2049. https://doi.org/10.1016/j.fuel.2007.12.013
Oliveira LC, Pereira E, Guimaraes IR, Vallone A, Pereira M, Mesquita JP, Sapag K (2009) Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents. J Hazard Mater 165:87–94. https://doi.org/10.1016/j.jhazmat.2008.09.064
Rangabhashiyam S, Selvaraju N (2015a) Adsorptive remediation of hexavalent chromiumfromsynthetic wastewater by a natural and ZnCl2 activated Sterculia guttata shell. J Mol Liq 207:39–49. https://doi.org/10.1016/j.molliq.2015.03.018
Rangabhashiyam S, Selvaraju N (2015b) Efficacy of unmodified and chemically modified Swietenia mahagoni shells for the removal of hexavalent chromium from simulated wastewater. J Mol Liq 209:487–497. https://doi.org/10.1016/j.molliq.2015.06.033
Rudnev VS, Morozova VP, Lukiyanchuk IV, Tkachenko IA, Adigamova MV, Nedozorov PM (2018) The effect of iron precursors in an electrolyte on the formation, composition, and magnetic properties of oxide coatings on titanium. Prot Met Phys Chem 53:1005–1014. https://doi.org/10.1134/s2070205117060193
Rufford TE, Hulicova-Jurcakova D, Zhu Z, Lu GQ (2011) A comparative study of chemical treatment by FeCl3, MgCl2, and ZnCl2 on microstructure, surface chemistry, and double-layercapacitance of carbons from waste biomass. J Mater Res 25:1451–1459. https://doi.org/10.1557/JMR.2010.0186s
Salnikow K, Zhitkovich A (2008) Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol 21:28–44. https://doi.org/10.1021/tx700198a
Shen Y, Fu Y (2018) KOH-activated rice husk char via CO2 pyrolysis for phenol adsorption. Mater Today Energy 9:397–406. https://doi.org/10.1016/j.mtener.2018.07.005
Shouman MA, Fathy NA, Khedr SA, Attia AA (2013) Comparative biosorption studies of hexavalent chromium ion onto raw and modified palm branches. Adv Phys Che 2013:1–9. https://doi.org/10.1155/2013/159712
Silva TL, Cazetta AL, PSC S, Zhang T, Asefa T, Almeida VC (2018) Mesoporous activated carbon fibers synthesized from denim fabric waste: efficient adsorbents for removal of textile dye from aqueous solutions. J Clean Prod 171:482–490. https://doi.org/10.1016/j.jclepro.2017.10.034
Sun Y, Yue Q, Gao B, Li Q, Huang L, Yao F, Xu X (2012) Preparation of activated carbon derived from cotton linter fibers by fused NaOH activation and its application for oxytetracycline (OTC) adsorption. J Colloid Interface Sci 368:521–527. https://doi.org/10.1016/j.jcis.2011.10.067
Tang S, Chen Y, Xie R, Jiang W, Jiang Y (2016) Preparation of activated carbon from corn cob and its adsorption behavior on Cr(VI) removal. Water Sci Technol 73:2654–2661. https://doi.org/10.2166/wst.2016.120
Wang S, Liu Q, Liao Y, Luo Z, Cen K (2007) A study on the mechanism research on cellulose pyrolysis under catalysis of metallic salts. Korean J Chem Eng 24:336–340
Wang M, Ren K, Wang L (2008) Iron-catalyzed ligand-free carbon-selenium (or tellurium) coupling of arylboronic acids with diselenides and ditellurides. Adv Synth Catal 351:1586–1594. https://doi.org/10.1002/adsc.200900095
Wang L, Yao Y, Sun L, Mao Y, Lu W, Huang S, Chen W (2014) Rapid removal of dyes under visible irradiation over activated carbon fibers supported Fe(III)–citrate at neutral pH. Sep Purif Technol 122:449–455. https://doi.org/10.1016/j.seppur.2013.11.029
Wu S, Chen G, Kim NY, Ni K, Zeng W, Zhao Y, Tao Z, Ji H, Lee Z, Zhu Y (2016) Creating pores on graphene platelets by low-temperature KOH activation for enhanced electrochemical performance. Small 12:2376–2384. https://doi.org/10.1002/smll.201503855
Xu Z, Tian D, Sun Z, Zhang D, Zhou Y, Chen W, Deng H (2019) Highly porous activated carbon synthesized by pyrolysis of polyester fabric wastes with different iron salts: pore development and adsorption behavior. Colloids Surf A Physicochem Eng Asp 565:180–187. https://doi.org/10.1016/j.colsurfa.2019.01.007
Yu Q, Zhuang X, Yuan Z, Qi W, Wang Q, Tan X (2011) The effect of metal salts on the decomposition of sweet sorghum bagasse in flow-through liquid hot water. Bioresour Technol 102:3445–3450. https://doi.org/10.1016/j.biortech.2010.10.084
Yuan J, Giordano C, Antonietti M (2010) Ionic liquid monomers and polymers as precursors of highly conductive, mesoporous, graphitic carbon nanostructures. Chem Mater 22:5003–5012. https://doi.org/10.1021/cm1012729
Zhang X, Li YX, Li GY, Hu CW (2015a) Preparation of Fe/activated carbon directly from rice husk pyrolytic carbon and its application in catalytic hydroxylation of phenol. RSC Adv 5:4984–4992. https://doi.org/10.1039/c4ra13248c
Zhang YJ, Ou JL, Duan ZK, Xing ZJ, Wang Y (2015b) Adsorption of Cr(VI) on bamboo bark-based activated carbon in the absence and presence of humic acid. Colloid Surf A 481:108–116. https://doi.org/10.1016/j.colsurfa.2015.04.050
Zhang S et al (2016) The role and mechanism of K2CO3 and Fe3O4 in the preparation of magnetic peanut shell based activated carbon. Powder Technol 295:152–160. https://doi.org/10.1016/j.powtec.2016.03.034
Zhou QQ, Chen XY, Wang B (2012) An activation-free protocol for preparing porous carbon from calcium citrate and the capacitive performance. Microporous Mesoporous Mater 158:155–161. https://doi.org/10.1016/j.micromeso.2012.03.031
Zhu X, Liu Y, Luo G, Qian F, Zhang S, Chen J (2014) Facile fabrication of magnetic carbon composites from hydrochar via simultaneous activation and magnetization for triclosan adsorption. Environ Sci Technol 48:5840–5848. https://doi.org/10.1021/es500531c
Zhu J, Xu D, Wang C, Qian W, Guo J, Yan F (2017) Ferric citrate-derived N-doped hierarchical porous carbons for oxygen reduction reaction and electrochemical supercapacitors. Carbon 115:1–10. https://doi.org/10.1016/j.carbon.2016.12.084
Funding
This work was financially supported by the National Natural Science Foundation of China (21707090), Chinese Postdoctoral Science Foundation (2017M611590), and Shanghai Natural Science Foundation (14ZR1428900).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, Z., Gu, S., Sun, Z. et al. Synthesis of char-based adsorbents from cotton textile waste assisted by iron salts at low pyrolysis temperature for Cr(VI) removal. Environ Sci Pollut Res 27, 11012–11025 (2020). https://doi.org/10.1007/s11356-019-07588-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07588-4