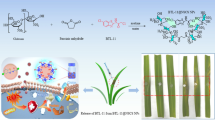
Abstract
During the past decade, nanotechnologies opened a new era in delivery of plant protection products through the development of nanosized controlled release systems, such as polymer nanoparticles, micelles, and so on using a wide variety of materials. To increase the pesticides penetration into the grain under the presowing seed treatment, a new approach based on non-covalent associate preparation with natural polysaccharides and oligosaccharides as delivery systems (DSs) was applied. Earlier, this approach was tested on antidote 1,8-naphthalic anhydride (NA). Enhancement of the NA solubility and penetration into the barley and wheat seeds had been demonstrated. In the present study, these DSs were used to prepare nanocomposites of pesticides (tebuconazole, imidacloprid, imazalil, prochloraz). The composite formation of the pesticides with poly- and oligosaccharides was proved by NMR relaxation method. Enhancement of the pesticides solubility and improvement of its penetration into the seeds of corn and rapeseeds has been detected. The strongest enhancement of penetration ability was observed for arabinogalactan nanocomposites: 5-folds for tebuconazole and imidacloprid, and more than 10-folds for imazalil and prochloraz. Our data show that the effect of polysaccharides and oligosaccharides on the nanopesticide penetration might be associated with the solubility enhancement, affinity of DSs to the surface of grains, and the modification of cell membranes by poly- and oligosaccharides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, the significant progress was achieved in medicine due to applying of supramolecular chemistry methods to design nanosized drug delivery systems (DDS). It allowed to enhance the bioavailability and safety of various drugs (Baker 2013; Polyakov and Kispert 2015). This approach is based on using of water soluble carriers (nanoparticles, liposomes, polymers, micelles, and so on) to increase the solubility of low soluble drug molecules and their permeation ability (Tolstikova and Bryzgalov 2009; Rai and Ingle 2012; Chistyachenko et al. 2015; Selyutina et al. 2016a; Selyutina and Polyakov 2019). The application of nanotechnology in agricultural sector is relatively new and in the early stages of development. Several critical reviews describe the potential of nanotechnology in agriculture, in particular for pesticide delivery (Mukhopadhyay 2014; Çiçek and Nadaroğlu 2015; Meyer et al. 2015; Nuruzzaman et al. 2016; Chhipa 2017). During the past decade, nanotechnologies opened a new era in delivery of plant protection products through the development of nanosized controlled release systems, such as polymer nanoparticles, micelles, and so on using a wide variety of materials (Perlatti et al. 2013). For example, the use of amphiphilic water soluble polymers and micelles as nano-containers for pesticide delivery allows encapsulation of hydrophobic molecules inside a hydrophobic matrix surrounded by a hydrophilic shell. This approach allows to improve pesticide solubility in water, which is an important requirement for pesticide dispersion. In addition, as we have shown in the previous studies, inclusion complexes of “host-guest” type provide protection of unstable organic molecules against harsh environments (UV irradiation, reactive oxygen species, and high temperature), improving chemical stability of “guest” molecules, which leads to prolongation of their activity (Polyakov and Kispert 2015). Also, “nanoencapsulation” allows to achieve high surface to volume ratio in dispersion, and so, decreasing the total amount of requested pesticide. Due to the nanocarrier envelope, various delivery systems for the active ingredients of pesticides can improve the utilization rates of pesticides and prolong their effects. Recent results demonstrate that nanosized delivery systems can significantly improve the controllable release, photostability, and biological activity, which will improve efficiency and reduce pesticide residues (Kah et al. 2018). All these factors result in less negative environmental impact.
Earlier, we have described novel natural delivery systems, polysaccharide arabinogalactan, and saponin glycyrrhizin, as the nanocarriers for plant protection products (Selyutina et al. 2017a), as well as for food antioxidants and drug molecules (Polyakov and Kispert 2015; Khvostov et al. 2017; Zhang et al. 2018). It was demonstrated that these delivery systems form stable water soluble inclusion complexes with variety of hydrophobic molecules, as well as increase their permeability (Selyutina et al. 2016a, 2017a). In the previous study (Selyutina et al. 2017a), this approach was successfully tested on antidote 1,8-naphthalic anhydride (NA) with arabinogalactan (AG), sodium salt of carboxymethylcellulose (Na-CMC), and glycyrrhizin (Gz) as delivery systems. Significant enhancement of the solubility of NA and its penetration into the seeds of barley and wheat had been demonstrated. Also, the interaction (affinity and penetration) of these delivery systems with model lipid membrane had been studied.
Glycyrrhizin or glycyrrhizic acid is a triterpene saponin extracted from the licorice root. Its amphiphilic and membranotropic properties make Gz an attractive delivery system. The hydrophilic part of Gz consists of glucose rings; hydrophobic one is a glycyrrhetic acid residue. Owing to this, glycyrrhizic acid is capable to form micelles in water solution and “host-guest” complexes with various hydrophobic molecules (Matsuoka et al. 2016; Wang et al. 2016; Zhang et al. 2018). Previous studies show that formation of Gz complexes with various low soluble drugs results in a significant increase in their solubility, enhancement the therapeutic effect, and consequently, reduce the therapeutic doses (Ragino et al. 2008; Tolstikova and Bryzgalov 2009; Zhang et al. 2018). In addition, the ability of Gz to incorporate into lipid membrane and increase of the membrane permeability for small molecules has been demonstrated (Selyutina et al. 2016a, 2017b).
Arabinogalactan is a natural-branched polysaccharide consisting of arabinose and galactose fragments. AG demonstrates strong binding with different hydrophobic biologically active molecules which results in significant increase of their solubility and oxidative resistance (Polyakov and Kispert 2015; Chistyachenko et al. 2015; Khvostov et al. 2017). AG also increases the permeability of cell membranes for small molecules (Selyutina et al. 2017b).
Carboxymethylcellulose is a linear gel forming polysaccharide widely used in pharmacology21 as well as for enhancement of pesticide sorption capability (Barbosa et al. 2018).
In the present study, we have applied the same approach to increase the effectiveness of various pesticides (TBZ (tebuconazole), ICP (imidacloprid), IMZ (imazalil), PCR (prochloraz)). The formation of stable composites of pesticides under study with AG, Na-CMC, and Gz in water solution was proved by NMR relaxation method. NMR technique was also applied to study the effect of Gz, AG, and Na-CMC on the penetration efficacy of pesticides into the grains of corn and rapeseeds during germination and the interaction of AG with lipid bilayer.
Materials and methods
Materials
Pesticides TBC (tebuconazole), ICP (imidacloprid), IMZ (imazalil), and PCR (prochloraz) were purchased from Jiangsu Huifeng Bio Agriculture Co., Ltd. (China), purity 97–98%, and were used as supplied.
Tebuconazole—(RS)-1p-Chlorophenyl-4,4-dimethyl-3-(1H-1,2,4-triazol-1-ylmethyl) pentan-3-yl—is an effective systemic fungicide for the treatment of seeds of cereals in the fight against phytopathogens transmitted with seeds. It refers to triazols of the third generation. It has a systemic effect and well soluble in organic solvents, but low soluble in water (Paranjape et al. 2014).
Imidacloprid—N-{1-[(6-Chloro-3-pyridyl)methyl]-4,5-dihydroimidazol-2-yl} nitramide—is a systemic insecticide that acts as an insect neurotoxin and belongs to a class of chemicals called the neonicotinoids which act on the central nervous system of insects. Solubility in water is relatively high, 0.51 g/l (20 °C). The main routes of dissipation of imidacloprid in the environment are aqueous photolysis (half-life = 1–4 h) and plant uptake (Paranjape et al. 2014).
Prochloraz—N-propyl-N-[2-(2,4,6-trichlorophenoxy) ethyl] imidazole-1-carboxamide—is contact and as well as system fungicide of the imidazole class with a protective and eradicating effect. Similarly to other azole fungicides, prochloraz is an inhibitor of the enzyme lanosterol 14α-demethylase, which is necessary for the production of ergosterol—an essential component of the fungal cell membrane. The agent is a broad-spectrum, protective, and curative fungicide, effective against Alternaria spp., Botrytis spp., Erysiphe spp., Helminthosporium spp., Fusarium spp., Pseudocerosporella spp., Pyrenophora spp., Rhynchosporium spp., and Septoria spp. (Paranjape et al. 2014).
Imazalil—(±) -1- (β-allyloxy-2,4-dichlorophenylethyl) imidazole—is a systemic fungicide of the imidazole class. Stable to hydrolyze in alkalis and dilute acids in the absence of light, it is moderately hazardous. The substance inhibits sterol biosynthesis in the membranes of phytopathogen cells, well soluble in organic solvents, but low soluble in water (Paranjape et al. 2014).
Arabinogalactan, a water soluble natural polysaccharide from wood of Larix sibirica (Wood Chemistry Co., Russia (purity > 99.5%, moisture 5%)); glycyrrhizic acid, a saponin from licorice root (98%, Shaanxi Pioneer Biotech Co., Ltd., China); and sodium salt of carboxymethylcellulose (CEKOL 700, pharmacopeia purity, http://cpkelco.com/products/cellulose-gum.1) were used for modification of pesticides.
Preparation of grain samples and penetration measurement
Corn and rapeseed grains (7.5 g) were placed in a glass tube with 0.5 ml of pesticide dispersion in water (5 mg/ml of each compound) in the absence and in the presence of modification agents: AG, Gz, or Na-CMC (0.1% and 1%). The dispersions were prepared by sonification technique during 30 min. Then, the tube was rotated for 15 min for homogeneous distribution of pesticides on the grains. After that, the grains were placed in Petri dish in humid atmosphere for 3 days for germination. After germination, the grains were dried and washed with acetone to obtain solution of pesticides that remained on the surface of grains. Then, washed grains were dried, milled, and mixed with acetone for 24 h to extract pesticides from the inside of grains. Grain solids were removed by centrifugation. The amount of pesticides in “washed” and “extracted” samples in acetone was analyzed by means of NMR technique. 1H NMR spectra were recorded on Bruker Avance III 500 MHz spectrometer. Deuterated solvent (Acetone-D6 (99.8% D), Aldrich) was used as received.
Solubility enhancement and binding of pesticide molecules with delivery systems
The formation of inclusion complexes of pesticides with delivery systems after dissolution of solid mixtures in water was investigated by the NMR relaxation technique (James 1975; Deese et al. 1982; Pastor et al. 2002; Gabrielska et al. 2006). Earlier, we have successfully applied this approach to study inclusion complexes of various drugs with Gz and AG (Chistyachenko et al. 2015; Khvostov et al. 2017; Zhang et al. 2018). The solid mixture of pesticides with delivery systems was prepared with the ratio 1:10 in by grinding in agate mortar. Then, the solid mixtures were dissolved in water (D2O, 10 mg/ml) at magnetic mixer during 3 h at room temperature, and the insoluble precipitate was removed by centrifugation. The clear nanopesticide solutions were analyzed by NMR to determine pesticides’ solubility and to measure spin-spin relaxation time. Spin-spin relaxation time T2 was measured by the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence. Deuterated solvent (D2O (99.9% D), Aldrich) was used as received.
Preparation of liposome samples
Liposomes were formed from dipalmitoylphosphatidylcholine (DPPC, A.V.T. Pharmaceutical Co. (Shanghai), purity > 99%, Fig. 1). Powder components were pre-dissolved in chloroform. After solvent evaporation, the dry lipid film was hydrated with D2O. The final concentration of lipid was 13 mM. The suspension was then sonicated (about 37 kHz, 1 h) to obtain unilamellar liposomes. NMR spectra were recorded for samples of 0.5 ml of vesicle suspension supplemented with 4 mM PrCl3 in 5-mm NMR tubes. After addition of PrCl3, the liposome suspension was supplemented with AG. Addition of PrCl3 allows to separate the signals of outer and inner N+(CH3)3-groups of DPPC (Gabrielska et al. 2006).
Spin-spin relaxation time T2 was measured by the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence. Diffusion of liposomes was studied by pulse field gradient spin-echo sequence. Diffusion coefficients were determined for different lipid functional groups (-N+(CH3)3-group in lipid polar head and terminal CH3-groups in hydrophobic tails); average values and errors were calculated.
Statistical analysis
All experiments were repeated thrice, and resulted values were calculated as an arithmetic mean, and resulted error was calculated as mean value of least squares errors; the differences were considered statistically significant at p < 0.01 using a t test.
Results and discussion
NMR study of pesticide penetration into the seeds of rape and corn
The NMR method is a powerful analytical tool allowing to determine with high confidence the content of active components in plant extracts, as well as the content of active components remained on the grain surface. In the present study, we have applied NMR technique to measure the amount of pesticides in extracts from treated seeds. As an example, Fig. 2 shows 1H NMR spectra of individual pesticides and the spectrum of the extract from corn seeds treated with arabinogalactan-modified pesticide suspension after germination for 3 days and washing off the surface.
The following samples were studied: (1) solution after washing off the surface of rape and corn seeds treated with mixture of pure pesticide suspension after germination for 3 days; (2) extract from rape and corn seeds treated with mixture of pure pesticide suspension after germination; (3) solution after washing off the surface of rape and corn seeds treated with suspension of pesticides in the presence of 0.1% or 1% of delivery systems (Gz, AG, or Na-CMC) after germination; (4) extract from rape and corn seeds treated with suspension of pesticides in the presence of delivery systems.
As the result, all delivery systems have shown their effectiveness as absorption and permeation enhancers. As an example, Fig. 3 demonstrates the effect of Gz, AG, and Na-CMC on total amount of pesticides (extract + washing off the surface) detected after permeation of treated corn seeds.
All results of this study are summarized in Tables 1 and 2. Table 1 shows the relative amounts (mole ratios) of different pesticides detected in extracts from treated corn seeds after permeation as well as total amount of pesticides (extract + washing off the surface). The corn seeds were treated by the mixture of pure pesticides, as well as in the presence of 0.1% of delivery systems. The experiment shows that the increase the concentration of delivery systems up to 1% does not lead to an increase in the effect within the measurement error (approximately 20%).
Table 2 shows the experimental results with rapeseeds. The rapeseeds were also treated by the mixture of pure pesticides, as well as in the presence of 0.1% of delivery systems. The experiment shows that the increase the concentration of delivery systems up to 1% does not lead to an increase in the effect within the measurement error (approximately 20%).
As can be seen from the presented data, in all systems, there is a significant increase in both the adhesion of the active substances to the surface and their penetration into the grains.
The analysis of the data of Tables 1 and 2 and Fig. 2 shows that the polysaccharide arabinogalactan exerts the greatest influence on the adhesion of the molecules of pesticides. It should be noted that some pesticides (IMZ, PCR) do not penetrate into the grain in pure form. However, they are found in the grains in significant amounts after treatment with suspensions containing delivery systems. We also note that it was precisely for IMZ and PHR that the solubility enhancement in the presence of AG was greatest, and, according to NMR relaxation data, the most stable inclusion complexes with arabinogalactan were formed.
Solubility enhancement and NMR relaxation study of binding of pesticide molecules with delivery systems
Two main mechanisms can be considered to understand the reason of pesticide permeability enhancement in the presence of delivery systems, namely inclusion complexes formation with increased solubility and adhesion to the surface, and membrane modification by the delivery systems. Earlier, we have demonstrated that both of these mechanisms are working for Gz and AG as the drug delivery systems (Dushkin et al. 2008; Selyutina et al. 2016a, 2017b; Khvostov et al. 2017; Zhang et al. 2018). In the present study, we have answered the following questions: does Gz, AG, and Na-CMC form inclusion complexes with pesticides under study, and does the solubility of pesticides enhance due to complexation with delivery systems?
To answer the first question, we have applied NMR relaxation method (Deese et al. 1982; Pastor et al. 2002). The NMR relaxation technique is a unique and powerful tool to study rotational mobility of organic molecules. Spin-spin relaxation times (T2) are very sensitive to molecular motions and are inversely proportional to the rotational correlation time. This approach is widely used to probe changes in the environment or state (free or bound) of the molecules. In particular, NMR relaxation technique can be used to prove the intermolecular complexes formation of hydrophobic molecules (Chistyachenko et al. 2015; Khvostov et al. 2017; Zhang et al. 2018). In the present study, NMR relaxation technique was applied to get the evidence of inclusion complex formation of various pesticides with delivery systems in water. To analyze the relaxation times, signals were used in the aromatic region of the NMR spectrum (6–9 ppm, Fig. 1) to avoid overlapping with signals from the complexant protons. One percent complexant solution was used for all experiments. The initial ratio of the components in the dry mix is 1/10. The results are summarized in Table 3.
As an example of the use of the NMR relaxation method to prove the interaction of a guest molecule with delivery system, Fig. 4 shows the kinetics of the NMR signal decay in the CPMG experiment for TBC in the absence and in the presence of AG. Relaxation times of the order of several tens of milliseconds are typical for complexes of AG with hydrophobic molecules (Dushkin et al. 2008; Chistyachenko et al. 2015; Khvostov et al. 2017).
Kinetics of NMR signal decay (in logarithmic scale) and relaxation times T2 of TBC protons (1-H, 8.5 ppm at Fig.1) in D2O at T = 30 °C
The strongest effect on relaxation time of pesticide protons was observed for Gz solution. It is known that due to its amphiphilicity, glycyrrhizin is able to form micelles in aqueous solutions at concentration > 1 mM (~ 0.1%) (Matsuoka et al. 2016; Wang et al. 2016; Petrova et al. 2017). It was demonstrated earlier that the relaxation times 5–10 ms are characteristic values for Gz micelles (Petrova et al. 2017). It means that all pesticide molecules are effectively penetrated into Gz micelles. However, the solubility enhancement was detected only for TBC, about 1.9 times.
As one can see from Table 3, the branched polymer arabinogalactan forms more strong complexes (lower values of T2) than linear polymer Na-CMC. The less soluble pesticide molecules (TBC, IMZ, and PCR) form more strong complexes than ICP. The difference in relaxation times of pesticide protons in the complex and in free form is a consequence of the intermolecular interaction of the active substances with the polysaccharide. The relaxation times observed for TBC, PCR, and IMZ molecules in the presence of AG approximately coincide with the relaxation time of the AG itself, which indicates the formation of a stable complex under the given conditions. At the same time, for molecules of ICP, only a small fraction of them is in the complex. An estimation of the change in the solubility of the compounds under study based on the intensity of the corresponding signals in the NMR spectrum shows an increase in solubility for TBC 1.5 times, for PCR 5.5 times, and for IMZ 2.5 times, which correlates with the stability of the inclusion complexes.
The Na-CMC molecule is a linear polysaccharide, so it binds less effectively to the hydrophobic molecules than the polysaccharide arabinogalactan. This difference is clearly visible from the change in proton relaxation times—these changes are less pronounced than with AG. Nevertheless, the greatest effect is observed, as in the case of AG, for PCR, and IMZ molecules. And it was for these compounds that an increase in solubility was observed in the presence of Na-CMC: for PCR 4.4-fold and for IMZ 2.5-fold, which also correlates with the stability of inclusion complexes.
Although at this moment, several examples of solubility increase of drug molecules in the presence of Gz and AG are known (Dushkin et al. 2008; Polyakov and Kispert 2015; Khvostov et al. 2017; Meteleva et al. 2018); our study is the first example of such observation for pesticide molecules.
AG interaction with lipid bilayer
For more deep understanding of the observed enhancement of pesticide penetration into the seeds, the interaction of AG with model lipid bilayer on the example of unilamellar liposomes was studied. Lipid bilayer was chosen as a model of the cell membrane. In most cases, in order for a plant protection compound to reach its site of action within a plant, it must cross several barriers. It must cross the cell wall, the cell membrane (plasma membrane), and even perhaps organellar membranes within the plant cell. Cell wall has a cellulose and matrix polymer structure. It contains large amounts of water, partly free, partly bound. Also, measurements of its permeability show that globular particles up to 7 nm in diameter, corresponding to proteins with amass of about 60 kDa, could move freely in the empty spaces of cell wall mesh (Carpita et al. 1979; Mohr and Schopfer 1995). At the present work, we focused on the study of the model of AG interaction with cell plasma membrane. Phospholipids are one of the major components of plant plasma membranes, and phosphatidylcholine is their most common phospholipid (Murphy et al. 2016). The phospholipid bilayer is often used to study the interaction of pesticides and herbicides with a plant cell membrane (Simon 1974; Tsuchiya 2015; Lebecque et al. 2019).
The NMR relaxation and diffusion measurement and the measurement of the phase transition temperature of lipids were done to understand the AG influence on lipid dynamics in bilayer. It was found that addition of 0.2 mM of AG to the liposome suspension results in the increase of the spin-spin relaxation times for observed functional groups of DPPC (Fig. 1). Also, it was found that the addition of AG lowers phase transition temperatures for all functional groups of protons measured by NMR on 1°K. All results are summarized in Table 4. The kinetics of echo signal decay for pure liposomes is bi-exponential. According to Ellena et al. (1993), spin-spin relaxation times characterize the lateral diffusion of phospholipids in liposomes. The short component is caused by vesicle tumbling (Grell 1981) and it was not significantly affected by AG. The increase in spin-spin relaxation times and in phase transition temperature demonstrates the disordering effect of AG on lipid bilayer. It should be noticed that the observed effect is opposite to effect of GA which was shown to decrease the spin-spin relaxation times of phospholipids by incorporation in lipid bilayer (Selyutina et al. 2016b).
The next stage was diffusion study of DPPC liposomes in the presence of AG. NMR pulse-field gradient echo sequence allows to study the diffusion of vesicles as a whole. Diffusion coefficients (D) of lipids in the presence of different concentrations of AG and AG own diffusion coefficients are given in Table 5. Increasing the diffusion coefficient of liposomes in the presence of arabinogalactan, which due to its size (M ~ 17 kDa) is not expected to integrate into the liposome, lets us to make the assumption that arabinogalactan is able to extract lipids from liposomes. Since the observed signal is an average signal from all lipids in the solution, it could be suggested that free lipids with the diffusion coefficient in orders of magnitude higher than diffusion coefficient of liposomes appeared in solution. A similar effect was observed when cyclodextrin was added to the liposome solution (Huang and London 2013). To test this assumption, additional experiments were conducted. They showed that with an increase in the concentration of arabinogalactan, the apparent diffusion coefficient of lipids continues to grow to 3.3 × 10−10 m2/s with the addition of 2 mM AG. The diffusion coefficient of pure AG is equal to 1.07 × 10−10 m2/s in a solution of liposomes and 1.36 × 10−10 m2/s in an aqueous medium (at a concentration of 0.2 mM), so the diffusion coefficient of AG does not change significantly in liposome suspension. AG effect on the properties of the lipid membrane is very significant. So when the concentration of AG is equal to 2 mM, the difference in chemical shifts from protons of the outer and inner surfaces of the liposome in the NMR spectrum disappears. This indicates the penetration of shift reagent ions into the liposome. This can be caused both by the formation of pores in the membrane and by complete destruction of liposomes under these conditions. Thus, these experiments prove the hypothesis about the extraction of lipids from liposomes in the presence of arabinogalactan. We also see an increase in the relaxation times for the sample of lipids with AG, which is consistent with the hypothesis expressed.
In the previous work, it was found that glycyrrhizic acid penetration causes the changes in the membrane permeability (Chhipa 2017; Khvostov et al. 2017; Mukhopadhyay 2014]. This ability could also give a contribution in the observed increase of pesticide penetration into the seeds. In the present study, it was demonstrated that though arabinogalactan do not incorporate into lipid membrane, it demonstrated an affinity to the lipid bilayer surface and could extract phospholipids from bilayer. So, these two delivery systems demonstrate completely different mechanisms of interaction with the lipid membrane, but both of them results in the increase of the pesticide penetration into the seed in comparison with pure substances.
Conclusion
Thus, glycyrrhizic acid (natural saponin from the licorice root), sodium salt of carboxymethylcellulose, and arabinogalactan (natural larch polysaccharide) in a concentration of 0.1% can be used as modifying additives in seed dressers of cereal crops to improve the adhesion and penetration of active substances. In the present work, we had studied the penetration of pesticides tebuconazole, imidacloprid, imazalil, and prochloraz into the grain of corn and rapeseeds. To increase the pesticide solubility, adhesion to the surface, and membrane permeability under the presowing seed treatment, we have applied new approach based on non-covalent associate preparation with natural water soluble polysaccharides and oligosaccharides as delivery systems for active substances. The formation of stable composites of pesticides under study with polysaccharide arabinogalactan, sodium salt of carboxymethylcellulose, and glycyrrhizin as delivery systems in water solution was proved by NMR relaxation method. We have found that the solubility enhancement correlates with complex stability and hydrophobicity of pesticides. There is an increase in the penetration of pesticides into grains in the presence of all delivery vehicles, but the greatest effect is observed in the presence of arabinogalactan. Our data show that the effect of polysaccharides and oligosaccharides on the penetration efficacy of nanopesticides under the presowing seed treatment might be associated with the detected solubility enhancement and affinity of delivery systems to the surface of grains, as well as due to modification of cell membranes by poly- and oligosaccharides. The interaction of delivery systems with cell membrane has been previously studied by measurement of lipid mobility in model lipid bilayer (Selyutina et al. 2017a, b). All delivery systems have good affinity to outer surface of membrane. But only glycyrrhizin molecules are able to penetrate into the cell membrane. In the present work, it was demonstrated that arabinogalactan interacts with the membrane surface and extracts lipid from bilayer. This interaction with membrane surface could explain the present observation of strong effect of AG even at concentration of 0.1% on pesticides permeation into the seeds of rape and corn. It could be caused by the strong adhesion of AG to the grain surface. The study of the mechanisms of nanopesticide interaction with biological membranes is important not only for understanding the penetration enhancement of active substances into the plant (systemic action), but also for the development of new plant protection products of contact action. We believe that this approach can be applied to enhance the activity and reduce the effective dose of other plant protection agents as well as to reduce harmful action of plant protection products on ecosystem and to increase of food quality.
References
Baker JR (2013) Why I believe nanoparticles are crucial as a carrier for targeted drug delivery. Wiley Interdisc Rev Nanomed Nanobiotechnol 5:423–429. https://doi.org/10.1002/wnan.1226
Barbosa DHO, de Moura MR, Aouada FA (2018) Polysaccharide-based nanocomposite hydrogels with zeolite: evaluation of the sorption process of pesticide paraquat. Química Nova 41:380–385. https://doi.org/10.21577/0100-4042.20170188
Carpita N, Sabularse D, Montezinos D, Delmer DP (1979) Determination of the pore size of cell walls of living plant cells. Science 205:1144–1147. https://doi.org/10.1126/science.205.4411.1144
Chhipa H (2017) Nanofertilizers and nanopesticides for agriculture. Environ Chem Lett 15:15–22. https://doi.org/10.1007/s10311-016-0600-4
Chistyachenko YS, Meteleva ES, Pakharukova MY, et al (2015) A physicochemical and pharmacological study of the newly synthesized complex of albendazole and the polysaccharide arabinogalactan from larch wood. In: Current Drug Delivery. http://www.eurekaselect.com/131369/article. Accessed 3 Oct 2019
Çiçek S, Nadaroğlu H (2015) The use of nanotechnology in the agriculture
Deese AJ, Dratz EA, Hymel L, Fleischer S (1982) Proton NMR T1, T2, and T1 rho relaxation studies of native and reconstituted sarcoplasmic reticulum and phospholipid vesicles. Biophys J 37:207–216. https://doi.org/10.1016/S0006-3495(82)84670-5
Dushkin AV, Meteleva ES, Tolstikova TG, Tolstikov GA, Polyakov NE, Neverova NA, Medvedeva EN, Babkin VA (2008) Mechanochemical preparation and pharmacological activities of water-soluble intermolecular complexes of arabinogalactan with medicinal agents. Russ Chem Bull 57:1299–1307. https://doi.org/10.1007/s11172-008-0167-8
Ellena JF, Lepore LS, Cafiso DS (1993) Estimating lipid lateral diffusion in phospholipid vesicles from carbon-13 spin-spin relaxation. J Phys Chem 97:2952–2957. https://doi.org/10.1021/j100114a021
Gabrielska J, Gagoś M, Gubernator J, Gruszecki WI (2006) Binding of antibiotic amphotericin B to lipid membranes: a 1H NMR study. FEBS Lett 580:2677–2685. https://doi.org/10.1016/j.febslet.2006.04.021
Grell E (ed) (1981) Membrane Spectroscopy. Springer-Verlag, Berlin Heidelberg
Huang Z, London E (2013) Effect of cyclodextrin and membrane lipid structure upon cyclodextrin–lipid interaction. Langmuir 29:14631–14638. https://doi.org/10.1021/la4031427
James TL (1975) Nuclear magnetic resonance in biochemistry. Elsevier. https://doi.org/10.1016/B978-0-12-380950-6.X5001-4
Kah M, Walch H, Hofmann T (2018) Environmental fate of nanopesticides: durability, sorption and photodegradation of nanoformulated clothianidin. Environ Sci Nano 5:882–889. https://doi.org/10.1039/C8EN00038G
Khvostov MV, Borisov SA, Tolstikova TG, Dushkin AV, Tsyrenova BD, Chistyachenko YS, Polyakov NE, Dultseva GG, Onischuk AA, An’kov S (2017) Supramolecular complex of ibuprofen with larch polysaccharide arabinogalactan: studies on bioavailability and pharmacokinetics. Eur J Drug Metab Pharmacokinet 42:431–440. https://doi.org/10.1007/s13318-016-0357-y
Lebecque S, Lins L, Dayan FE et al (2019) Interactions between natural herbicides and lipid bilayers mimicking the plant plasma membrane. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.00329
Matsuoka K, Miyajima R, Ishida Y et al (2016) Aggregate formation of glycyrrhizic acid. Colloids Surf A Physicochem Eng Asp 500:112–117. https://doi.org/10.1016/J.COLSURFA.2016.04.032
Meteleva ES, Chistyachenko YS, Suntsova LP, Tsyganov MA, Vishnivetskaya GB, Avgustinovich DF, Khvostov MV, Polyakov NE, Tolstikova TG, Mordvinov VA, Dushkin AV, Lyakhov NZ (2018) Physicochemical properties and anti-opisthorchosis effect of mechanochemically synthesized solid compositions of praziquantel with glycyrrhizic acid disodium salt. Dokl Biochem Biophys 481:228–231. https://doi.org/10.1134/S1607672918040142
Meyer WL, Gurman P, Stelinski LL, Elman NM (2015) Functional nano-dispensers (FNDs) for delivery of insecticides against phytopathogen vectors
Mohr H, Schopfer P (1995) Plant physiology. Springer
Mukhopadhyay SS (2014) Nanotechnology in agriculture: prospects and constraints. Nanotechnol Sci Appl 7:63–71. https://doi.org/10.2147/NSA.S39409
Murphy AS, Peer WA, Schulz B (2016) The plant plasma membrane - ReadingSample
Nuruzzaman M, Rahman MM, Liu Y, Naidu R (2016) Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J Agric Food Chem 64:1447–1483. https://doi.org/10.1021/acs.jafc.5b05214
Paranjape K, Gowariker V, Krishnamurthy VN, Gowariker S (2014) The pesticide encyclopedia. CABI
Pastor RW, Venable RM, Feller SE (2002) Lipid bilayers, NMR relaxation, and computer simulations. Acc Chem Res 35:438–446. https://doi.org/10.1021/ar0100529
Perlatti B, Bergo PL d S, Silva MF d GF d et al (2013) Polymeric nanoparticle-based. A controlled release purpose for agrochemicals. insecticides - development of safer and more effective technologies, Insecticides. https://doi.org/10.5772/53355
Petrova SS, Schlotgauer AA, Kruppa AI, Leshina TV (2017) Self-association of glycyrrhizic acid. NMR Study. Zeitschrift für Physikalische Chemie 231:839–855. https://doi.org/10.1515/zpch-2016-0845
Polyakov NE, Kispert LD (2015) Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr Polym 128:207–219. https://doi.org/10.1016/j.carbpol.2015.04.016
Ragino YI, Vavilin VA, Salakhutdinov NF, Makarova SI, Stakhneva EM, Safronova OG, Lyakhovich VV, Nikitin YP, Tolstikov GA (2008) Antioxidant and endothelium-stabilizing effects of simvaglyzin on rabbits with experimental hypercholesterolemia. Bull Exp Biol Med 146:206–209. https://doi.org/10.1007/s10517-008-0252-x
Rai M, Ingle A (2012) Role of nanotechnology in agriculture with special reference to management of insect pests. Appl Microbiol Biotechnol 94:287–293. https://doi.org/10.1007/s00253-012-3969-4
Selyutina OY, Apanasenko IE, Khalikov SS, Polyakov NE (2017a) Natural poly- and oligosaccharides as novel delivery systems for plant protection compounds. J Agric Food Chem 65. https://doi.org/10.1021/acs.jafc.7b02591
Selyutina OY, Apanasenko IE, Shilov AG, Khalikov SS, Polyakov NE (2017b) Effect of natural polysaccharides and oligosaccharides on the permeability of cell membranes. Russ Chem Bull 66:129–135. https://doi.org/10.1007/s11172-017-1710-2
Selyutina OY, Polyakov NE, Korneev DV, Zaitsev BN (2016a) Influence of glycyrrhizin on permeability and elasticity of cell membrane: perspectives for drugs delivery. Drug Deliv 23:848–855. https://doi.org/10.3109/10717544.2014.919544
Selyutina OY, Apanasenko IE, Kim AV, Shelepova EA, Khalikov SS, Polyakov NE (2016b) Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloids Surf B: Biointerfaces 147:459–466. https://doi.org/10.1016/j.colsurfb.2016.08.037
Selyutina OY, Polyakov NE (2019) Glycyrrhizic acid as a multifunctional drug carrier – from physicochemical properties to biomedical applications: a modern insight on the ancient drug. Int J Pharm 559:271–279. https://doi.org/10.1016/J.IJPHARM.2019.01.047
Simon EW (1974) Phospholipids and plant membrane permeability. New Phytol 73:377–420. https://doi.org/10.1111/j.1469-8137.1974.tb02118.x
Tolstikova TG, Bryzgalov MVK (2009) The complexes of drugs with carbohydrate-containing plant metabolites as pharmacologically promising agents. In: Mini-Reviews in Medicinal Chemistry. http://www.eurekaselect.com/85315/article. Accessed 3 Oct 2019
Tsuchiya H (2015) Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 20:18923–18966. https://doi.org/10.3390/molecules201018923
Wang Y, Zhao B, Wang S, Liang Q, Cai Y, Yang F, Li G (2016) Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Delivery 23:1623–1635. https://doi.org/10.3109/10717544.2015.1135489
Zhang Q, Polyakov NE, Chistyachenko YS et al (2018) Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Delivery 25:198–209. https://doi.org/10.1080/10717544.2017.1422298
Funding
The reported study was funded by Russian Foundation for Basic Research and Government of the Novosibirsk region according to the research projects № 15-29-05792 and 18-416-540007 and by Russian Ministry of Science and Education (project № 0304-2017-0009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Giovanni Benelli
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Selyutina, O.Y., Khalikov, S.S. & Polyakov, N.E. Arabinogalactan and glycyrrhizin based nanopesticides as novel delivery systems for plant protection. Environ Sci Pollut Res 27, 5864–5872 (2020). https://doi.org/10.1007/s11356-019-07397-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07397-9