Abstract
The Nanhaizi Wetland (NHZW) is a significant part of the Baotou Yellow River National Wetland Park in China, an important migration station and habitat for waterfowl. The Yellow River receives a significant amount of industrial and agricultural wastewater. Therefore, the environmental quality of NHZW directly affects the survival of migratory birds in the Baotou region. We aimed to determine the trace element distribution in tissues and risk of exposure in ruddy shelduck and to provide a scientific basis for bird protection and an environmental quality assessment for the NHZW. In January 2018, we collected water, soil, and 18 dead ruddy shelduck Tadorna ferruginea (nine males and nine females) from the NHZW. We measured concentrations of trace elements (Cd, Pb, Cu, Zn, Hg, and As) in the specimens and modeled the risk of exposure to trace elements. Trace element concentration was greatest in feathers, followed by the kidneys, liver, and muscle, in descending. There was no significant difference in trace element accumulation between sexes. Exposure doses of Hg in water; Cr, Pb, and Cu in soil; and Pb, Cu, and Hg in corn were higher than the tolerable daily intake and may adversely affect ruddy shelduck. The calculated hazard quotients (HQ) for trace elements were ranked as follows: Hg > Cr > Pb > Zn > Cu > As, where Hg and Cr were at high risk levels (HQ > 1).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The wetland ecosystem is of great importance to a variety of wildlife, especially to waterfowls, with appropriate ecological environment. But the growing urbanization and industrialization are increasingly affected the environment of wetland ecosystems, which may pose risks to species (Zeng et al. 2013). Trace element pollution is becoming an increasingly serious problem with the development of industry. It has attracted much attention because of the toxicity, persistence, extensive sources, and non-biodegradable properties of trace elements (Mora 2003). Moreover, trace elements can be transferred to other places through the feces of migrants (Liang et al. 2015). Therefore, it is necessary to monitor the environment in order to control and reduce pollutant emissions. Waterfowl as an important component of wetland ecosystem, trace elements have been enriched and used as pollution indicators in wetland pollution monitoring (Abdullah et al. 2015). In recent years, the risk exposure assessment model for trace elements has provided a scientific method for environmental monitoring and control of trace elements (Liu et al. 2015; Liang et al. 2016).
Baotou, located in the west of Inner Mongolia, northern China, is an integrated industrial city with metallurgy, rare earth metal production, and machinery manufacturing (Li et al. 2010). The Yellow River, which is the largest freshwater ecosystem and the most important water supply in northern China, receives wastewater from industrial and agricultural activities in the Baotou region (Si et al. 2015). The beach lying on both sides of the Baotou National Yellow River Wetland Park covers about 122.22 km2, with the length of 220 km across southern Baotou. It is an important migration station and energy supplement for waterfowl, located at an important crossroads of the East Asian-Australasian flyway and Central Asian flyways (Zhang et al. 2012; Li et al. 2017). Nanhaizi Wetland (NHZW) is an important part of the Baotou National Yellow River Wetland Park and also the main habitat for waterfowl overwintering (Liu et al. 2019). The environmental quality of the wetland will directly affect the health, survival, and population viability of migratory birds.
As the most common waterfowl in the wetland, ducks have been recognized as biomonitors for monitoring and assessing environmental contamination (Liang et al. 2016; Plessl et al. 2017; Wang et al. 2017). Ruddy shelduck is the dominant wintering migratory species in NHZW. It winters in the industrial wastewater area from November to March by eating corns in the farmlands around the wetland and breeds in surrounding area from April to October. In this study, the concentrations of trace elements in the tissues of ruddy shelduck and in the water, soil, and main food (corn) were analyzed, and the exposure risk of ruddy shelduck to trace elements was assessed using a trace element risk assessment model (Liu et al. 2015). The aims of this study were to determine the distribution of trace elements in tissues and the exposure risk of trace elements in ruddy shelducks and to provide a scientific basis for bird protection and an environmental quality assessment for the NHZW.
Materials and methods
Study area
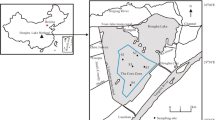
NHZW is located in the Donghe District, Baotou City, Inner Mongolia, covering about 29.92 km2 (approximately 40°30′08″–40°33′32″ N, 109°59′02″–110°02′26″ E) (Liu et al. 2018) (Fig. 1). The Yellow River flows for 7 km through the southern part of the reserve. There is a lake in its territory, Nanhaizi, with an area of about 3.33 km2. The area of wetland expands during the ice and flood seasons or becomes a swamp in other seasons. The reserve has a warm temperate continental monsoon climate, with an average annual temperature of 8.5 °C and an average annual precipitation of 307.4 mm. The coldest temperature was approximate −34.4 °C in January, and the hottest temperature was 38.4 °C in July (Liu et al., 2018). According to the data from NHZW Nature Reserve, there are 207 species of aquatic plants and 228 species of waterfowl including 36 species listed in the International Redbook (Yu et al. 2017).
Sample collection and treatment
In January 2018, 9 water and 9 soil samples and 18 dead ruddy shelducks (9 males and 9 females) were collected in the habitat of the shelduck. Water samples were collected in 500 mL plastic bottles that had been washed with nitric acid. The bottles were cleaned three times with the water at each sampling point before sampling. Samples were collected and immediately acidified with 10 mL HNO3 (1.42 g/mL) and stored at 4 °C. Soil samples were collected from the surface soil (0–20 cm). Four additional samples were collected 50 m away from each original sampling site in different directions. The five soil samples were combined to obtain one sample for each sampling site (Zarrintab and Mirzaei 2017).
Samples of dead ruddy shelduck were collected randomly from the ice and brought back to the laboratory. Liver, kidney, chest muscle, and breast feathers were separated after weighing and measuring. Corn, the main food obtained from the muscular stomach, was placed in polystyrene tubes and kept in the freezer at −20 °C until the next procedure. During dissection, specimens were sexed by observing the sexual organs and color of the neck feathers.
Analytical methods and quality control
An amount of 5 mL of HNO3 and 7 mL of mixed acid (HNO3:HClO4, 5:2, volume ratio) was added to the 100 mL of water samples, which were filtered with 0.45 μm PTFE membrane, for digestion. The soil, corn, and tissue samples were dried to a constant weight in an oven at 80 °C. Feather samples were cut up using ceramic scissors, and soil and corn samples were homogenized uniformly using a porcelain mortar.
After evenly grinding, the soil samples were sieved using a 100-mesh sieve for homogenization. Preprocessed samples were weighed to precisely 0.5 g and were transferred to airtight teflon vessels, and 10 mL HCl(1.19 g / mL) and 13 mL mixed acid (HNO3:HF:HClO4, 5:5:3) were added for digestion using a microwave digestion instrument (Mars 6, CEM Corp, USA). The digested samples were filtrated through a 0.45 μm organic membrane and diluted with ultrapure water to obtain a final volume of 50 mL. Samples of 0.5 g preprocessed corn and tissue were precisely weighed and transferred to airtight teflon vessels, and 12 mL mixed acids (HNO3:HClO4, 3:1) were added for digestion as described earlier.
Concentrations of trace elements in samples were detected using an inductively coupled plasma-optical emission spectrometer (ICP-OES; PerkinElmer, Wellesley, MA, USA). The selection of target elements was determined by referring to previous research literature (Si et al. 2015; Liu et al. 2019). All analytical data were subject to strict quality control. The instruments were calibrated daily with the calibration standards. Precision and accuracy were verified using standard reference materials from the National Institute of Metrology in China (i.e., soil, GBW07402). Accepted recoveries ranged from 94% to 105%. Reagent blanks were also included in each batch of analyses, in order to check for any contamination of the different samples extracts. Average values of three replicates were taken for each determination. Calibration curves were prepared separately for each metal, using different concentrations (i.e., 0.5, 1, 2, 5, and 10 ppm) of standard solutions. The relative coefficients (r2) of calibration curves of each element are all above 0.9995.Chemicals were of guaranteed reagent. All glassware before use were washed with distilled water, soaked in nitric acid (10%) overnight, rinsed in deionized water, and air-dried. The detection limits were 0.01 μg/g for Cr, Cu, and Zn 0.001 μg/g and for Pb, Hg, and As.
Applied exposure risk assessment model
The models utilized in the study were mainly based on Sample et al. (1996). Generally, wildlife is exposed to trace elements mainly through three routes: ingestion, dermal contact, and inhalation. Ingestion is the major route used to assess exposure in wildlife; the other routes are usually ignored (Liang et al. 2016). Trace element exposure in birds through oral ingestion can be quantified by the following equations (Liu et al. 2015).
where Idf is the food consumption rate per day (dry weight, g/d); food consumption rates are estimated from allometric regression models (Nagy 1987); and BW refers to bodyweight (g). According to our measurements, the average BW of the ruddy shelduck was 1494 g.
where Iw is the water consumption rate (mL/d), and the unit of BW is kg. Water consumption rate was estimated from allometric regression models (Calder and Braun, 1983).
where Is is the soil consumption rate (g/day) and P is the proportion of soil in the food. The 8.2% of soil consumed by the Canada goose Branta canadensis (Beyer et al. 1994) was used for the ruddy shelduck in our study.
where Ej is the oral exposure dose of trace element (j) (mg/kg/day), m is the number of absorbing medium (food and soil in our study), Ii is the consumption rate of the medium (i) (g/d or mL/d), and Cij is the concentration of the metal (j) in the medium (i) (mg/kg or mg/l).
Trace element exposure risk (risk of an adverse effect) was evaluated by comparing the intake dose to the tolerable daily intake (TDI). The TDI can be calculated by Eq. (5) (CCME 1998).
where TDIj is tolerable daily intake of trace element (j) (mg/kg/d); LOAELj is the lowest observed adverse effect level of trace element (j) (mg/kg/d); NOAELj is the no observed adverse effect level (mg/kg/d), and UF is an uncertainty factor. The LOAEL and NOAEL values were obtained from avian toxicity tests (Sample et al. 1996). The UF was used to account for the uncertainty of risk. The total UF used in calculating a TDI may not be less than 10 in order to extrapolate for a long-term exposure concentration without an effect. The selected UF may be higher than 10, depending on the type, amount, and quality of data available (CCME, 1998). In the present study, UF = 10 was chosen as the most conservative TDI (mcTDI). The most dangerous TDI (mdTDI) was obtained at a UF value of 100.
Imitating a human health risk assessment model (USEPA 1989), a hazard quotient (HQ) was employed to estimate the exposure risk to the birds of each trace element.
where HQj is the hazard quotient of trace element (j). In our study, trace element exposure risk to birds was separated into four levels: no risk (HQ < 1), low risk (1 < HQ < 2), moderate risk (2 < HQ < 3), and high risk (HQ > 3) (Liu et al. 2015).
Statistical analysis
All data were log transformed to obtain a normal distribution. Shapiro-Wilk’s and Levene’s tests were used to test the normality of data and the homogeneity of variances, respectively. One-way analysis of variance (with LSD post hoc pair wise test) was used to compare the content of trace elements in different tissues. Pearson’s correlations in trace element concentrations among different materials were also conducted by using a statistical significance level of 0.05. Data are presented in mean ± standard deviation (SD). SPSS software version 19.0 was used for all statistical analyses.
Results and discussion
Trace element concentrations
The concentrations of six trace elements in water, soil, and corn samples from NHZW are shown in Table 1. Except for As, the concentrations of all elements in the water exceeded class I of the Chinese environmental quality standards for surface water (“St”); this may be related to local industrial pollution (Si et al. 2015). Four trace elements in soil (Cr, Pb, Cu, and Zn) exceeded their background values. This result is in accordance with a previous finding (Han et al. 2018) and is related to the addition of industrial wastewater into the river. In addition, the concentration of Pb and Hg in corn exceeded the Chinese hygienic standard for grain (“HSG”). This is related to the long-term irrigation of corn crops with polluted Yellow River water, leading to trace element accumulation in local farmland soils and crops (Si et al. 2015).
Trace elements in tissues
Accumulation of all trace elements in different tissues was ranked in this order: feather > kidney > liver > muscle (Table 2). Contamination by Cr, Zn, and As was significantly higher in the feathers than in other tissues, which is consistent with the finding of Tsipoura et al. (2011). Most contaminants exhibit the highest contents in feathers, because birds accumulate trace elements in feathers and then eliminate them during molting (Burger, 1993; Liu et al. 2019).
In this study, the concentration of As in the feathers of ruddy shelduck was lower than in the feathers of Canada goose and whooper swan (Cygnus cygnus) and also below the level where biological impacts would be anticipated (2–10 μg/g) (Eisler 1988).
Hexavalent Cr at high concentration is a mutagen, teratogen, and carcinogen, but trivalent Cr has low toxicity and is an essential trace element. Contamination by Cr is associated with chromite processing and the presence of human activities (Burle et al. 1991). In this study, the average concentration of Cr in the feathers of ruddy shelduck was 3.74 μg/g, which was higher than that found in other ducks such as mallard (Anas platyrhynchos), spot-billed duck (A. poecilorhyncha) (Kim and Oh 2014), whooper swan (Wang et al. 2017), and Canada goose (Tsipoura et al. 2011). The Cr concentration of 2.8 μg/g in the feathers may have adverse effects on the embryo development, hatching success rate, and viability of birds (Kertész and Fáncsi 2003; Abdullah et al. 2015). Based on the high concentration level of Cr found in the feathers, ruddy shelduck inhabiting NHZW is at risk of exposure.
Zinc is one of the important essential trace elements, but excessive intake can lead to nephrotoxicity and affect reproduction (Carpenter et al. 2004). In this study, the concentrations of Zn in the feathers and kidneys of ruddy shelduck were significantly higher than those in other tissues, which is similar to reports on other ducks (Kalisińska et al. 2004; Tsipoura et al. 2011). However, its concentration was also higher than that found in whooper swan (Wang et al. 2017), mallards (Plessl et al. 2017), and waterfowl in Korea (Kim et al. 2014); thus more attention is required on the pollution of Zn in NHZW.
Mercury is mainly concentrated in the feathers of birds. Chronic Hg poisoning, even at very low concentrations (e.g., 0.04 μg/g), has been shown to reduce the reproductive success rate of the common loon Gavia immer (Evers et al. 2008). In this study, the concentration of Hg (0.229 μg/g) in the feathers of ruddy shelduck was higher than that found in whooper swan (0.196 μg/g) and Canada goose (0.200 μg/g) (Tsipoura et al. 2011; Wang et al. 2017). Concentrations of Hg in the muscle and liver were higher than those reported for mallards (Plessl et al. 2017). We propose that more attention should be given to the problem of Hg contamination in NHZW.
Copper is one of the essential trace elements associated with the respiratory electron transport chain and metabolism of oxygen (Janssens et al. 2003), but long-term excessive intake can lead to toxicological alterations in birds. Henderson and Hen (1975) found that a Cu concentration of 187–323 μg/g in the liver of Canada goose led to acute poisoning. In this study, the average concentration of Cu in the liver tissues of ruddy shelduck was significantly higher than in other tissues, which is similar to Plessl’s (2017) findings. The concentration of Cu in the liver tissues of ruddy shelduck was higher than in the liver of mallards in Korea (3.88 μg/g) and Iran (11.9 μg/g) (Kim and Oh 2012; Mansouri and Majnoni 2014) but has not yet reached the toxicity range of Canadian geese.
Lead was mainly distributed in the kidney, liver, and muscle tissue of ruddy shelduck; levels in these tissues were significantly higher than in the feathers. The average concentrations of Pb in the liver and kidney were also higher than the threshold values of 1.5 μg/g in the liver and 3.0 μg/g in the kidney of the ducks (Guitart et al. 1994; Clark et al. 2003). Therefore, Pb may pose a threat to the health and survival of ruddy shelducks in NHZW.
Correlations
The correlation relationships of the trace element contents were all extremely significant among different materials (p < 0.01 between water and soil, water and corn, soil and corn, respectively) (Table 3). The correlation relationships among kidney, liver, muscle, and feather were very significant as well (p < 0.01). Notably, the trace element concentration in the four tissues was extremely significantly correlated with that in soil and corn (all p < 0.01), and the water had a significant correlation between feather and muscle as well (p < 0.05). It is suggested that trace elements in tissues of ruddy shelducks may come from the surrounding environment.
Trace element exposure
The values of mcTDI and mdTDI are shown in Table 4, and exposure doses of Cr, Pb, Cu, Zn, Hg, and As to ruddy shelduck are shown in Fig. 2. The Cr exposure doses in corn were higher than mdTDI, while total exposure doses (from soil, water, and corn) were higher than mcTDI, mainly because of the relatively higher exposure dose of Cr in the soil. Therefore, Cr may be having negative effects on migratory birds living in this area. The exposure doses of Pb in soil and corn both exceeded mdTDI, and its total exposure doses were higher than mdTDI but lower than mcTDI. Therefore, we believe that the intake of Pb through soil and corn may have a significant impact on ruddy shelduck in NHZW. The exposure doses of Cu and As in water, soil, and corn were lower than mdTDI, and total exposure doses were lower than mcTDI, which indicates that Cu and As exposure through water, soil, and corn was relatively safe during the study period in this region. The exposure doses of Zn in water and soil were lower than mdTDI, and exposure doses in corn were higher than mdTDI, while total exposure doses were higher than mdTDI. Therefore, Zn may be having negative effects on ruddy shelduck. Except in soil, the exposure dose of Hg in water was higher than mdTDI, and the exposure dose of Hg in corn exceeded the mcTDI; hence, the total exposure dose of Hg was significantly higher than mcTDI. Therefore, we believe that the intake of Hg through water and corn may have a significant impact on migratory birds in NHZW, and closer attention should be given to these trace elements.
Trace element exposure risk
The order of HQ values for the six trace elements is as follows, from largest to smallest: Hg > Cr > Pb > Zn > Cu > As (Fig. 3). Hg posed the highest risk to ruddy shelduck, probably because the exposure dose of Hg from corn was much higher than its mdTDI. The exposure doses of corn have exceeded mcTDI. The phenomenon indicates that the food pathway plays a major role in Hg exposure to ruddy shelducks, and the food is an important factor in the study of trace element exposure to migratory birds, which is similar to the results of Liu et al. (2015). Additionally, exposure doses of Hg in the water exceeded mdTDI, and its concentration in water exceeded the Chinese environmental quality standards, indicating that industrial wastewater was released into the agricultural irrigation system. The discharge of industrial wastewater into the river not only pollutes the wetland environment; this water is then also used to irrigate the crops in the wetland. This irrigation is required because of the semiarid climate of the region. Excessive accumulation of metals in agricultural soils through irrigation may result not only in soil contamination but also lead to a concentration of Hg in corn crops; this is consistent with the finding reported by Si et al. (2015). Consequently, Hg poses the largest threat to these ruddy shelduck who feed on contaminated crops and may negatively affect other migratory birds in the area. Water is therefore an important route through which trace elements can affect migratory birds.
The exposure dose of Cr in the soil exceeded mcTDI and posed a moderate risk to ruddy shelduck. Exposure to Cr was greatest from the soil; this is consistent with previous findings (Liu et al. 2015; Liang et al. 2016), indicating that soil is an important route to consider when studying trace element exposure in migratory birds (Zarritab et al. 2018). The concentrations of Cr in the water and soil were several times higher than the Chinese environmental quality standard and background values, indicating that the NHZW is polluted by Cr. Therefore, the impact of Cr on all migratory species in the area needs further attention.
The other four elements Pb, Cu, Zn, and As posed no risk to ruddy shelducks (HQ < 1), but the exposure doses of Pb in the soil and Pb and Zn in the corn exceeded mdTDI, and the concentrations of Pb and Cu in water, corn, and soil exceeded the Chinese environmental quality standards or local background values. Therefore, further attention should be given to their effects on migratory birds. Levels of Cu and As were considered relatively safe in this study.
Benthic invertebrates such as shrimp, screw, and plant algae are also food for waterfowl. This additional route may enhance the accumulation of trace elements in these birds (Liu et al. 2015). Therefore, the overall risk that trace elements pose to migratory birds may be higher if benthic invertebrates are considered, and these invertebrates should be considered in further studies.
Conclusions
In this study, we collected samples of water, soil, and dead ruddy shelducks (resident birds in NHZW, Baotou) and quantitatively analyzed trace element concentrations in the samples. Feathers were the most enriched tissue (feather > kidney > liver > muscle). The risk assessment model of trace element exposure to migratory birds in the wetland ecosystem was comprehensively applied. The exposure risk of six trace elements to ruddy shelducks in the NHZW was Hg > Cr > Pb > Zn > Cu > As. Mercury and Cr posed high or moderate exposure risk to ruddy shelduck.
The main routes of exposure to trace elements were via corn and soil. Water also was an important route, and it should be assessed in determining trace element exposure in migratory birds. This study advocates the need to mitigate against the contamination of the Yellow River, wetlands, and crops and to routinely monitor and manage Yellow River pollution. Our findings have important implications for the development of migratory bird conservation strategies in the NHZW ecosystem. Further, because the Yellow River wetland in Baotou is located at an important crossroads of the East Asian-Australasian and Central Asian flyways, they have broader implications for the protection of this wetland.
References
Abdullah M, Fasola M, Muhammad A, Malik SA, Bostan N, Bokhari H, Kamran MA, Shafqat MN, Alamdar A, Khan M, Ali N, Eqani SAMAS (2015) Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: a case study from severely contaminated areas. Chemosphere 119:553–561
Beyer WN, Connor EE, Gerould S (1994) Estimates of soil ingestion by wildlife. J Wildl Manag 58:375–382
Burger J (1993) Metals in avian feathers: bioindicators of environmental pollution. Rev Environ Toxicol 5:203–311
Calder WA 3rd, Braun EJ (1983) Scaling of osmotic regulation in mammals and birds. Am J Phys 244(5):R601–R6066
Carpenter JW, Andrews GA, Beyer WN (2004) Zinc toxicosis in a free-flying trumpeter swan (Cygnus buccinator). J Wildl Dis 40(4):769–774
CCME (1998) Protocol for the derivation of Canadian tissue residue guidelines for the protection of wildlife that consume aquatic biota. Canadian Council of Ministers of the Environment, Winnipeg
Eisler R (1988) Arsenic hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish and Wildlife Service biology report 85(1.12)
Guitart R, To-Figueras J, Mateo R, Bertolero A, Cerradelo S, Martínez-Vilalta A (1994) Lead poisoning in waterfowl from the Ebro Delta, Spain: calculation of lead exposure thresholds for mallards. Arch Environ Contam Toxicol 27(3):289–293
Han XD, Li HJ, Su MX, An PL (2018) Spatial network analysis of surface soil pollution from heavy metals and some other elements: a case study of the Baotou region of China. J Soils Sediments 19(2):629–640
Janssens E, Dauwe T, Pinxten R, Bervoets L, Blust R, Eens M (2003) Effects of heavy metal exposure on the condition and health of nestlings of the great tit (Parus major), a small songbird species. Environ Pollut 126(2):267–274
Kalisińska E, Salicki W, Mysłek P, Kavetska KM, Jackowski A (2004) Using the mallard to biomonitor heavy metal contamination of wetlands in North-Western Poland. Sci Total Environ 320(2–3):145–161
Kertész V, Fáncsi T (2003) Adverse effects of (surface water pollutants) Cd, Cr and Pb on the embryogenesis of the mallard. Aquat Toxicol 65(4):425–433
Kim J, Oh JM (2012) Metal levels in livers of waterfowl from Korea. Ecotoxicol Environ Saf 78:162–169
Kim J, Oh JM (2014) Concentration of trace elements in feathers of waterfowl, Korea. Environ Monit Assess 186(12):8517–8525
Li JX, Hong M, Yin XQ, Liu JL (2010) Effects of the accumulation of the rare earth elements on soil macrofauna community. J Rare Earth 28(6):957–964
Li SH, Meng WY, Chen LX, LI YF, Gao RY, RuWD, Sun MH, Dai Q, Zhang GG, Lu J (2017) The spring waterbird community and home range of the whooper swan Cygnus cygnus at the upper and middle reaches of Yellow River in Inner Mongolia. Chinese Journal of Ecology 36(7): 1910–1916
Liang J, Liu J, Yuan X, Zeng G, Yuan Y, Wu H, Li F (2016) A method for heavy metal exposure risk assessment to migratory herbivorous birds and identification of priority pollutants/areas in wetlands. Environ Sci Pollut Res 23(12):11806–11813
Liang J, Liu JY, Yuan XZ, Zeng GM, Lai X, Li XD, Wu HP, Yuan YJ, Li F (2015) Spatial and temporal variation of heavy metal risk and source in sediments of Dongting Lake wetland, mid-South China. J Environ Sci Health A Tox Hazard Subst Environ Eng 50(1):100–108
Liu J, Liang J, Yuan X, Zeng G, Yuan Y, Wu H, Huang X, Liu J, Hua S, Li F, Li X (2015) An integrated model for assessing heavy metal exposure risk to migratory birds in wetland ecosystem: a case study in Dongting Lake wetland, China. Chemosphere 135:14–19
Liu L, Zhang L, Sun Y, Si WT, Men ZH, Zhang MD, Liu XG (2018) Relationship of heavy metal contents between the feathers of three Ardeidae species and environment at the Nanhaizi Wetland in Baotou City. Chinese Journal of Zoology 53(4):628–640
Liu L, Liu XG, Sun Y, Pu ZH, Xu HY, Li WX, Wang ZH (2019) Trace elements in the feathers of waterfowl from Nanhaizi Wetland, Baotou, China. Bull Environ Contam Toxicol 102(6):778–783
Mansouri B, Majnoni F (2014) Comparison of the metal concentrations in organs of two bird species from western of Iran. Bull Environ Contam Toxicol 92(4):433–439
Mora MA (2003) Heavy metals and metalloids in egg contents and eggshells of passerine birds from Arizona. Environ Pollut 125:393–400
Nagy KA (1987) Field metabolic rate and food requirement scaling in mammals and birds. Ecol Monogr 57:111–128
Plessl C, Jandrisits P, Krachler R, Keppler BK, Jirsa F (2017) Heavy metals in the mallard Anas platyrhynchos from eastern Austria. Sci Total Environ 580:670–676
Sample BE, Opresko DM, Suter GW II (1996) Toxicological benchmarks for wildlife: 1996 revision. Laboratory, Oak Ridge National
Si W, Liu J, Cai L, Jiang H, Zheng C, He X, Wang J, Zhang X (2015) Health risks of metals in contaminated farmland soils and spring wheat irrigated with Yellow River Water in Baotou, China. Bull Environ Contam Toxicol 94(2):214–219
Tsipoura N, Burger J, Newhouse M, Jeitner C, Gochfeld M, Mizrahi D (2011) Lead, mercury, cadmium, chromium, and arsenic levels in eggs, feathers, and tissues of Canada geese of the New Jersey meadowlands. Environ Res 111(6):775–784
USEPA (1989) Risk assessment guidance for superfund volume I: human health evaluation manual (part a), interim final, EPA/540/1–89/ 002, United States Environmental Protection Agency, Washington, D.C
Wang F, Xu S, Zhou Y, Wang P, Zhang X (2017) Trace element exposure of whooper swans (Cygnus cygnus) wintering in a marine lagoon (swan lake), northern China. Mar Pollut Bull 119(2):60–67
Yu W, Miao CL, Liu L, Bo NL (2017) Birds of Nanhaizi Wetland in Inner Mongolia. China Forestry Publishing House, Beijing
Zarrintab M, Mirzaei R (2017) Evaluation of some factors influencing on variability in bioaccumulation of heavy metals in rodents species: Rombomys opimus and Rattus norvegicus from Central Iran. Chemosphere 169:194–203
Zeng G, Chen M, Zeng Z (2013) Risks of neonicotinoid pesticides. Science 340:1403
Zhang YM, Jia YF, Jiao SW, Zeng Q, Feng DD, Guo YM, Lei GC (2012) Wuliangsuhai wetlands: a critical habitat for migratory waterbirds. J Resour Ecol 3:316–323
Funding
The study was supported by the National Natural Science Foundation of China (No. 31560598; No. 81760590), the Inner Mongolia Natural Science Foundation (No. 2019MS03094), the Inner Mongolia Study Abroad Back Area Staff Research Start Up Fund Project (No. 2015-339), and the Inner Mongolia Natural Science Foundation (No. 2019MS03094).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, L., Du, C., Sun, Y. et al. Trace element distribution in tissues and risk of exposure of ruddy shelduck wintering in Nanhaizi Wetland, Baotou, China. Environ Sci Pollut Res 27, 6429–6437 (2020). https://doi.org/10.1007/s11356-019-07132-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07132-4