Abstract
Monitoring and evaluating bird exposure to hazardous pollutants in wetlands are receiving considerable attention. In this study, the occurrence of 18 organochlorine pesticides (OCPs) in the muscle of bean geese (Anser fabalis) and common teals (Anas crecca) collected from Honghu Lake Wetland (HLW), Central China was studied. Additionally, an exposure risk assessment model was applied to obtain risk levels of OCPs to these birds through three oral routes (food intake, water drinking and soil ingestion). The results suggested that the most abundant OCPs detected in the muscle of waterbirds were DDTs (7.68–602 ng/g lipid weight), followed by HCHs (1.39–89.8 ng/g lipid weight). A significant difference (p < 0.05) existed between two species, but most of OCPs exhibited no statistically relationship with age or gender (p > 0.05). The compositional patterns of OCPs combined with ratios of certain metabolites to their parent compounds indicated that all OCPs in the HLW were largely from historical usage except heptachlor. The exposure risk assessment revealed that common teals with lighter weight had greater exposure risks than bean geese. Of the OCPs analyzed, DDTs could probably cause harm to target birds studied here. Exposure via food intake was identified to be significant while soil ingestion and water drinking contributed least, but they should still be concerned.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one class of persistent organic pollutants (POPs), organochlorine pesticides (OCPs) were largely applied worldwide as insecticides before 1970s (Jiang et al., 2009). Their presence brought serious environmental problems, thus many OCPs (e.g. dichlorodiphenyltrichloroethane (DDT), hexachlorocyclohexane (HCH)) have been prohibited or restricted for several decades (Jin et al., 2017). However, these pollutants are hard degradable, resulting in their high occurrence in the global soil, air, rivers and lakes (Bozlaker et al., 2009; Luo et al., 2019; Yuan et al., 2013). Moreover, some OCPs, such as dicofol (contains o,p-DDT), lindane (nearly pure γ-HCH), continue to be used illegally in India, Africa and other developing countries (Wang et al., 2016; Zhi et al., 2015). Due to their bioaccumulation ability and ecotoxicity potential, OCPs are still the hazardous pollutants attracting great attention (Lv et al., 2020).
Waterbirds are wetland animals that have frequently been used as bio-monitoring species for POP exposure (Nordstad et al., 2012; Sakellarides et al., 2006; Zapata et al., 2018). Previous works about occurrence and effects of POPs in birds were conducted by collecting their tissues (e.g. liver, muscle, blood, feathers and eggs) (Abbasi et al., 2016). However, the sampling of these organisms may face many practical and ethical issues. Therefore, non-destructive techniques for monitoring and evaluating bird exposure to hazardous pollutants are highly essential (Kocagöz et al., 2014; Yin et al., 2018). Modeling is becoming an inevitable trend without causing harm to birds (Liang et al., 2016). Until now, there have been several bioaccumulation models for estimating concentrations of POPs in birds (Daley et al., 2014; Glaser & Connolly, 2002; Norstrom et al., 2007), but few regarding the risks for exposure to these pollutants. Although Barghi et al. (2018) and Zheng et al. (2018) assessed potential risks of POPs to various bird species, they just compared the observed concentrations with toxicity reference values. In this study, an integrated risk model was applied to quantitatively evaluate risk levels for wetland birds exposed to OCPs through ingestion of food, water and soil. It was successfully used for assessing bird exposure to toxic metals (Liang et al., 2016; Liu et al., 2015; Zarrintab & Mirzaei, 2018), which can provide an effective method for risk management in wetlands.
China was ever one of the largest producer and consumer of OCPs as a well-developed agricultural country (Grung et al., 2015). Honghu Lake Wetland (HLW) (113°12′-113°26′E, 29°40′-29°58′N), located in Central China, covers approximately 414 km2. It has been listed as a Wetland of International Importance since 2008. Geographically, the Honghu Lake region is a part of the Jianghan Plain, which is the renowned “land of fish and rice”. Thus, great quantities of pesticides were previously used due to the important role of agriculture in the economy of the region (Li et al., 2001). In addition, new inputs of lindane and dicofol likely presented in this area (Yuan et al., 2013). Also, the HLW is an important stopover and wintering ground for hundred thousands of waterbirds migrating between Australia and East Asia. Two species, bean geese (Anser fabalis) and common teals (Anas crecca), are the most commonly found birds in the HLW. However, they have distinctly different food habits: common teal is an omnivore while bean goose is an herbivore. Recently, these birds have suffered population declines due to illegal hunting and the extensive use of pesticides (Zhou et al., 2018). Therefore, the aims of this research were to: 1) explore the OCP residue concentrations in the muscle of common teals and bean geese collected from HLW; 2) study their compositions and possible sources in all investigated birds; 3) evaluate OCP exposure from oral ingestion and its potential risks to these birds based on an improved exposure risk assessment model.
Materials and methods
Sample collection
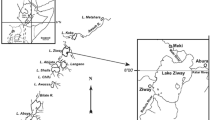
Common teals (CT, n = 19) and bean geese (BG, n = 13) were collected in 2013–2015 from HLW in collaboration with Wildlife Protection Centre, Hubei. They were found dead of serious injures or poisoning. After being transported to the laboratory, these birds were weighted and dissected to remove muscle samples. Then, these samples were bagged and held at − 20 °C before OCP analysis. Meanwhile, age (adult or juvenile) and sex (male or female) were determined by examination of plumage and gonad (Goutner et al., 2011). Additionally, surface water (~ 0.5 m) and sediment (0–15 cm) were simultaneously sampled at five selected sites from the core zone of HLW (Fig. 1). It is the main bird habitat, the area birds are distributed extensively. According to previous research and field investigation, plant samples including potamogeton maackianus (P. maackianus, n = 3), Vallisneria natans (V. natans, n = 2) and sedge (Carex spp., n = 5) were also collected. Among three species, P. maackianus and V. natans are the main food of CT, while Carex spp. is preferred by BG (Hu et al., 2014; Yang et al., 2016). For water samples, they were obtained using amber glass bottles and stored at 4 °C. Sediment and plant samples were kept at − 20 °C in sealed polyethylene bags until further treatment.
Sample extraction and analysis
The extraction, fractionation and analysis of OCPs were described previously (Hu et al., 2018; Yuan et al., 2013). The details of analytical procedures and GC operating conditions are presented in Supporting Information S1. Eighteen OCP compounds (including HCHs: α-, β-, γ- and δ-HCH; HCB; DDTs: p,p′-DDD, o,p′-DDT, p,p′-DDE and p,p′-DDT; HEPTs: heptachlor epoxide and its parent compound heptachlor; ENDs: α-, β-endosulfan and endosulfan sulfate; CHLs: cis-chlordane and trans-chlordane; Aldrins: aldrin and dieldrin) were determined using gas chromatograph and electron capture detector (GC-ECD). Chromatographic separation was achieved by a 30 m length × 0.32 mm i.d. capillary column HP-5.
Quality assurance and quality control (QA/QC)
To evaluate matrix effects and instrumental performance, the surrogates were added to all samples under the same experimental condition. Additionally, one spiked blank and one procedural blank were analyzed in each set of eight samples. The spiked recoveries for the analytes were in the range of 74–102%. No target OCPs were detected in all blanks. The LODs (limits of detection) at a signal-to-noise (S/N) of 3 were 0.01–0.1 ng/l for water, 0.01–0.05 µg/kg dry weight (dw) for sediment and plants, and 0.05–0.64 ng/g lipid weight (lw) for bird samples. For parallel samples (n = 3), their relative standard deviations were 3.6–11.4%. The average recoveries of surrogates, TCmX and PCB209, were 70 ± 15% and 82 ± 13%, respectively. The reported results were corrected by the surrogate recoveries.
Data analysis
The SPSS 16.0 statistical software (SPSS Inc., Illinois) was used for data analysis. OCP concentrations were expressed in ng/g lw for bird samples, ng/l for water, µg/kg dw for sediments and plants. When OCPs were not detected, a value of LOD/2 was assigned for statistical evaluation. Statistical significance (p < 0.05) of differences between species, ages and sexes were performed with a one-way ANOVA test.
Exposure risk assessment
An integrated exposure model derived from Ecological Risk Assessment and Toxicological Benchmarks for Wildlife: 1996 Revision was applied to study the exposure of waterbirds to OCPs in the HLW. Although birds are exposed to POPs by various exposure routes, oral ingestion is the most important pathway (Maul et al., 2018). The routes of oral exposure for birds include food uptake, water drinking and soil ingestion (either deliberate or incidental) (Liu et al., 2015). Thus, the food consumption rate (Id, g/d dw) for birds is estimated as follows:
where BW is body weight (g) of exposed birds. In this study, BW used the average value of birds, which was 383 g for CT and 1750 g for BG according to their body weight measurement.
Similarly, water consumption rate (Iw, l/d) is calculated using the following allometric regression model based on body weight (kg).
Some species, especially herbivores, may ingest soil or sediment attached to the food when foraging (Liang et al., 2016). The soil consumption rate (Is, g/d) is obtained by the following formula:
where P is the proportion of soil or sediment accounted in food. The value of P was 0.19 for CT and 0.08 for BG derived from their food items in stomachs (Hu et al., 2014).
where Ej (mg/kg/d), Ii(l/d or kg/d), Cij (mg/l or mg/kg) and m is oral exposure dose, medium consumption rate (i), concentration of pollutant (j) in medium (i) and the number of medium (e.g. soil, water, food), respectively. In particular, the waterbirds of this study were more likely to take in sediment attached to their food aquatic plants. Consequently, soil exposure doses mentioned below were calculated on the basis of the OCP concentrations in sediment.
For chemicals that lead to adverse effects, the tolerable daily intake can be derived using the following equation:
where NOAELj (mg/kg/d), LOAELj (mg/kg/d), and TDIj (mg/kg/d) is no observed adverse effect level, lowest observed adverse effect level, and tolerable daily intake of chemical (j), respectively. The UF is uncertainty factor, which was set as 10 to have the most conservative TDI in this study (CCME, 1998).
To evaluate potential exposure risks caused by OCPs to bird population, the hazard quotient (HQ) is computed as a ratio between the oral exposure dose and the tolerable daily intake.
HQj > 1 indicates that the exposure can result in adverse effects to birds. However, HQj < 1 suggests no potential risks to birds.
Results and discussion
OCP levels in waterbirds
The mean and range levels of individual OCPs in the muscle of common teals and bean geese from HLW are expressed in Table 1. For both two bird species, β-HCH, δ-HCH, p,p′-DDT, p,p′-DDE and HCB were found in every muscle sample. However, the lowest frequency measured was 32% for o,p′-DDT and α-endosulfan in CT and 46% for β-endosulfan in BG. The total contents of OCPs (∑OCPs, sum of the 18 OCPs) were 28.3–696 ng/g lw in CT (mean: 276 ng/g lw) and 40.1–302 ng/g lw in BG (mean: 124 ng/g lw). This result suggested higher OCP levels in CT than those in BG. Further statistical analysis showed that a significant difference between total OCP levels of these two species was observed (p = 0.02, F = 6.41), mainly contributing to their different dietary habits. CT is an omnivore feeding on plant food (69%), and insects, shrimps (12%), while BG is an herbivore preying on aquatic plants (Hu et al., 2014). Among all OCPs, DDTs were the most abundant, with the concentrations of 9.34–602 ng/g lw in CT and 7.68–235 ng/g lw in BG, which constituted 65 ± 25% and 62 ± 27% of total OCPs (Fig. S1), respectively. Coinciding with our study, high concentrations and proportions of DDTs were recorded in birds from other regions (Luo et al., 2009; Luzardo et al., 2014; Wu et al., 2019). It is highly attributable to their relatively higher octanol–water partition coefficients (log Kow = 5.3–6.2) and the extensive application in history (Wang et al., 2011). HCHs followed, and the levels ranged from 1.39 to 89.8 ng/g lw, accounting for 12 ± 12% of total OCPs on average. This seems reasonable since HCHs are less lipophilic and bioaccumulative, and have shorter half-lives in biological systems relative to DDTs (Rajaei et al., 2010). HCB and HEPTs contributed almost equally to total OCPs, their concentrations were 0.23–150 ng/g lw and 0.12–83.3 ng/g lw, respectively. Concerning the other OCPs, CHLs, ENDs and Aldrins occurred at much lower levels of non-detectable (nd)-8.90, nd-32.5 and nd-23.1 ng/g lw, respectively, only contributing less than 5% to total OCPs.
Results of differences in muscle levels of OCPs due to age and sex are described in Fig. 2. By comparison, adult common teals showed significantly higher levels than juvenile ones for DDTs, HCB and ENDs (p < 0.02, F > 1.50). However, the other OCPs (HCHs, HEPTs, CHLs and Aldrins) could not reveal significant differences (p > 0.05, F < 3.35). Similarly, no age-related difference was observed for bean geese (p > 0.05, F < 1.06). Regarding the gender, there were also no noticeable differences in OCP concentrations between males and females for both two species (p > 0.05, F < 2.19) (Fig. 2). These findings were consistent with those reported in great cormorant from Greece (Goutner et al., 2011), yellow-legged gulls from Spain (Vizuete et al., 2018), common kingfisher in South China (Wu et al., 2019), common moorhen and common teals in Central China (Hu et al., 2018), where the absence of age or gender difference was ascribed to the low number of samples or (and) the timing of the collections. These could be somewhat reflected by our data reported here. For example, there are only one female, two males and two birds of unknown sex for juvenile bean geese. In addition, birds at low trophic levels usually have a low intake of organochlorine compounds (OCs) from their food (Bustnes et al., 2003). Thus, these birds can reach steady-state equilibrium (OCs intake equivalent to elimination yearly) with their environment at early stage of reproduction (Vizuete et al., 2018). In this work, birds feed mainly on aquatic plants, and they seem to have been in equilibrium before sampling, which might weaken the relationship between age and OC burdens.
For comparison purposes, the concentrations in the muscle of waterbirds from HLW were expressed in two weight basis, lipid weight and wet weight. The overall levels of DDTs in our study (9.34–602 ng/g lw; 0.92–59.6 ng/g ww) were much lower than the reported values in the muscle of common teals in Iran (mean 561 ± 320 ng/g ww) (Rajaei et al., 2010), similar or slight higher than those in eurasian coots from Turkey (mean 40 ± 11 ng/g lw) (Kocagöz et al., 2014), common teals in Central China (7.58–243 ng/g lw) (Hu et al., 2018), water cocks in South China (2.4–11 ng/g ww) (Zhang et al., 2011) and ducks in North China (0.3–2.8 ng/g ww) (Hu et al., 2010). For HCHs, their contents (1.39–89.8 ng/g lw; 0.14–8.89 ng/g ww) in the HLW were comparable to those in the muscle of ducks in North China (0.9–5.0 ng/g ww) (Hu et al., 2010), common teals in Iran (mean 8 ng/g ww) (Rajaei et al., 2010) and water cocks in South China (1.9–2.7 ng/g ww) (Zhang et al., 2011), while significantly greater than the levels measured in eurasian coots from Turkey (mean 3 ± 0.1 ng/g lw) (Kocagöz et al., 2014). Other OCPs such as HEPTs and HCB (HEPTs: 1.96–83.3 ng/g lw; HCB: 1.40–150 ng/g lw) had similar ranges with data for muscle of common teals in Central China (HEPTs: 1.03–43.2 ng/g lw; HCB: 0.64–58.8 ng/g lw) (Hu et al., 2018), but had relatively lower levels than common teals in Iran (HCB: nd-120 ng/g ww) (Rajaei et al., 2010).
Components and sources analysis of OCPs in waterbirds
The accumulation patterns of DDTs in the muscle of CT and BG from HLW are given in Fig. 3. Similar congener patterns were observed for both two bird species. Considering that p,p′-DDE has higher persistence and biomagnification potentials compared to others (Luzardo et al., 2014). Of course, p,p′-DDE dominated among the DDTs studied here, which had a range of 0.28–579 ng/g lw in CT (mean: 127 ng/g lw) and 1.40–182 ng/g lw in BG (mean: 50.9 ng/g lw), averagely accounting for 71.0% and 66.5% of total DDTs, respectively. The significantly high burden of p,p′-DDE was also documented in bird tissues in many earlier reports (Wu et al., 2019; Zheng et al., 2018). For other DDTs, their concentrations followed the order of p,p′-DDT > o,p′-DDT > p,p′-DDD, with average proportions of 23.5%, 3.9% and 1.6% in CT and 24.6%, 5.7% and 3.2% in BG, respectively. Contrary to previously reported results (Barghi et al., 2018; Luo et al., 2009; Luzardo et al., 2014), p,p′-DDT here was detected in all samples and had higher contributions than o,p′-DDT. One possible explanation is that p,p′-DDT has a longer half-life than o,p′-DDT (Torre et al., 2016). On the other hand, this accumulation pattern is probably related to its pollution sources in the region. Commonly, technical DDT comprises primarily of p,p′-DDT (~ 75%), with a less amount of o,p′-DDT (~ 15%) (Pan et al., 2016). Conversely, DDT-containing dicofol has less p,p′-DDT (~ 1.7%) compared to o,p′-DDT (~ 11.4%) (Bai et al., 2015). Thus, the higher proportions of p,p′-DDT than o,p′-DDT combined with low detectable of o,p′-DDT recorded in our samples revealed notable contributions from technical DDT rather than dicofol. Moreover, the values of p,p′-DDT to p,p′-DDE were 0.03–7.43 (after removing extreme values 168 and 31.2), with an average ratio of 0.34, demonstrating that DDTs mainly originated from historical application of technical DDT (Xu et al., 2017), but new inputs also existed in the HLW. The ratios of o,p′-DDT/p,p′-DDT (range: 0.00–1.22) for samples with p,p′-DDT/p,p′-DDE > 1 were close to that of technical DDT (0.2–0.3) (Lv et al., 2020), further suggesting that recent DDT input was most probably from technical DDT. Even after the ban of technical DDT for agricultural application in 1983, it continued to be used as an additive for antifouling paints in fishing boats until 2014 (Zhi et al., 2015). According to statistics, the amount of technical DDT used in the production of antifouling paints between 1950s and 2005 was about 250 tons/yr (Niu et al., 2016). Available data showed that there were 14,000 fishing population and 2,000 fishing boats in Honghu region (Li et al., 1983). Besides, technical DDT is still permitted for dicofol production and disease vector control, which was verified by Lv et al. (2020). From the above, we can conclude that technical DDT usage in the past predominated in the HLW. Recent inputs of technical DDT in antifouling paints and disease vector control might also be the sources of DDTs in this area.
As shown in Fig. 3, the relative abundance of four HCH isomers to total HCHs in CT was ranked as: β-HCH (34.7%) > α-HCH (22.6%) > γ-HCH (21.8%) > δ-HCH (20.9%). Likewise, β-HCH was dominant in BG, with an average contribution of 41.5%. The predomince of β-HCH in HCH family in bird samples was expected, since it is the most stable isomer and could be more readily transformed from α-HCH and γ-HCH (Wang et al., 2016). A previous study reported that the half-life of β-HCH was up to 7.6 years in fatty tissues (Kuang et al., 2020). HCHs were commonly used in two formulations: lindane (pure γ-HCH: > 99%) and technical HCH (γ-HCH: 10–12%; β-HCH: 5–12%; α-HCH: 60–70%) (Devi et al., 2015). Differed from the compositional patterns of HCHs in both lindane and technical HCH, the relatively higher contribution of β-HCH implied that historical application was the main source of HCHs in the HLW. To explain further, the ratio of α-HCH/γ-HCH was calculated to distinguish HCH source caused by technical HCH (4–7) from that of lindane (~ 0) (Li et al., 2016). In this study, values of α-HCH/γ-HCH in 87.5% of samples were below 4 (0.28–2.46), suggesting mixed usage of lindane and technical HCH. In line with the present result reported in bird samples, our recent analyses of water, sediment and plant samples from the core zone of HLW (Fig. S2) also demonstrated that β-HCH was predominant in 85% of samples and the ratios of α-HCH/γ-HCH were 0.78–8.99 (two samples were excluded because γ-HCH was not detected).
Regardless of bird species, heptachlor predominated the HEPT profiles in the muscle samples (Fig. 3), with a contribution of more than 74% to ∑HEPTs. Heptachlor has a shorter half-life compared to other OCPs (Yadav et al., 2017), and can be quickly metabolized to heptachlor epoxide in the environment (Espín et al., 2010). The high ratios (> 1) between heptachlor and its epoxide indicated recent inputs of this insecticide in the HLW region. Heptachlor was banned in 1980s, and it was not produced or used any more in China (Zhang et al., 2014). However, heptachlor is one component of technical chlordane (~ 5%), which was widely applied to control termites in buildings and was completely banned until 2009 (Niu et al., 2016). Given that cis-chlordane averagely accounted for 70.4% of ∑CHLs here, and the ratios of cis-/trans-chlordane in 90.4% of samples were above one. The heptachlor occurred in this area could not be the degradation of technical chlordane. Hence, further research on possible sources of heptachlor should be carried out. In the case of HCB, it was detected in all of the muscle samples making about 8.2% of total OCPs (Fig. S1). HCB was used as an impurity in the production of chlorothalonil (~ 0.05%), pentachloronitrobenzene (PCNB) (1 ~ 6%), pentachlorophenol (PCP) and its sodium salt (Na-PCP) (~ 13%) in China (Zhou et al., 2011). For this reason, it was very likely the usage of these HCB-containing chlorinated chemicals in agriculture and schistosome control in the HLW, one typical agricultural base but also an epidemic area of schistosomiasis prevalence. Moreover, HCB can be produced during the metallurgy, incineration and combustion processes (Lv et al., 2020). The endosulfan sulfate was the main contributor among endosulfan compounds (74.6% as average), suggesting that endosulfan in the HLW was mainly from historical usage. Aldrin and dieldrin in China had never been produced and used in agriculture (Lu et al., 2015). They were still detected in over 65% of bird samples despite of their lower levels in this study, perhaps due to the long-range transportation and illegal usage from some regions (Niu et al., 2016). It was supported by the report about their residues in liver samples of both resident and migratory waterbirds from HLW (Hu et al., 2014).
Exposure risk assessment
With regard to HCH isomers, the residue concentrations of γ-HCH (5500 ng/g ww) and β-HCH (10,000 ng/g ww) in ring-necked pheasants could not affect their hatching (Wiemeyer, 1996). Connell et al. (2003) reported that the limit value of 1000 ng/g ww DDTs in eggs was related to a reduction of survival for Ardeid species. Obviously, the highest contents of HCHs and DDTs in the muscle of birds studied here were all below the toxicological thresholds described above, meaning safe levels for birds. Nevertheless, using threshold values to assess toxic hazards in birds failed to reveal their realistic exposure and the results varied with species and bird tissues sampled. Most notably, concentration data used in this method were obtained by sampling bird tissues, which is a destructive way for birds. In this case, an integrated exposure model was conducted to characterize potential risks to birds of the present work based on OCP levels in bird-relevant matries (e.g. food, water, sediment).
For risk assessment, OCP residues in water, sediment and plants from the main bird habitat of HLW were investigated. Due to limited toxicity data, seven OCPs were chosen, their concentrations and toxicity parameters are listed in Table 2. Similar to bird profiles, DDTs existed as greater concentrations in both sediment and plant samples. However, HCH contamination was dominant in water samples because of their less lipophilic and higher water solubility than other OCPs (Grung et al., 2015). The NOAEL and LOAEL values of γ-HCH, HCHs, DDTs, heptachlor and chlordane were obtained from toxicological benchmarks for wildlife (Sample et al., 1996). For endosulfan and dieldrin, their LOAEL values were estimated by multiplying the NOAEL by a factor of 5.6 (Su et al., 2014). According to Eq. 5, the calculated TDIs of individual OCPs were decreased in the following order: endosulfan > γ-HCH > chlordane > HCHs > heptachlor > dieldrin > DDTs.
On this basis, the exposure doses of studied OCPs to bird species via food uptake, water drinking and soil ingestion were calculated, and the results are presented in Fig. 4. As the OCPs are concerned, the highest total exposure dose through three exposure routes was 1.55 × 10–3 ± 8.50 × 10–4 mg/kg/d for CT and 8.11 × 10–4 ± 8.98 × 10–4 mg/kg/d for BG. From Fig. 4 and Table 2, values of γ-HCH, HCHs, heptachlor, chlordane, endosulfan and dieldrin through each exposure route were far below their corresponding TDIs. It demonstrated that these OCP levels were safe to the target birds. Regarding DDTs, both water and soil exposure doses to all birds were less than TDI value. However, due to high exposure from food ingestion, total exposure doses were greater than TDI, indicating that DDT contamination in food might be unsafe for birds living here. For all OCPs, exposure doses via food intake were one to four orders of magnitude greater than soil ingestion and water drinking. Food intake was evidently significant, and it was the main route for birds exposed to OCPs. By contrast, the contributions of soil ingestion and water drinking to total oral intake of OCPs were very small or even negligible. It was mostly attributed to the relatively low OCP concentrations in sediment and water. This result was different from previous studies that considered soil exposure as an important route when estimating bird exposure to heavy metals (Liang et al., 2016; Zarrintab & Mirzaei, 2018).
Food, water, soil and total exposure doses of selected organochlorine pesticides (OCPs) to waterbirds in the HLW: A for Common teal, B for Bean goose. Ed: exposure dose via food intake; Ew: exposure dose via water drinking; Es: exposure dose via soil ingestion; Etotal: exposure dose via food, water-drinking and soil pathways
The HQs of OCPs to common teals and bean geese are summarized in Fig. 5. Generally, small animals show less water and food ingestion but higher metabolic rate, as compared to large ones (Liu et al., 2015). Common teals thus presented higher HQ values than bean geese, suggesting that birds with lighter weight had greater risks. The HQs caused by DDTs in 60% of samples were above the safety level of 1, with the highest HQ of 3.27, revealing that DDTs could induce adverse risks to birds. The other OCPs appeared to be no risk to birds as their HQs varied from 3.22 × 10–6 to 4.32 × 10–2, which were much less than 1. In terms of individual OCPs, the exposure risks for CT followed the trend of: DDTs > heptachlor > HCHs > dieldrin > endosulfan > chlordane > γ-HCH. Meanwhile, the highest HQ value in BG was also observed for DDTs, followed by heptachlor. The DDTs were surely considered to be the priority pollutants due to their higher concentrations and lower TDI. Particularly, heptachlor exhibited the second highest HQ, although it had lower total exposure dose than HCHs. Likewise, the total exposure dose of dieldrin was less than that of endosulfan, but the exposure risk of dieldrin was higher. These findings could be explained mainly by their much lower TDI values. Heptachlor and dieldrin are well-known endocrine disrupters, which are associated with decreased reproduction and survival rates of birds (Hu et al., 2018; Walker & Newton, 1998). Therefore, attentions should be paid to these OCPs despite of their trace levels in the environment.
There are some limitations related to exposure can result in uncertainty in such risk assessment studies. For example, our risk analysis did not consider the bird population density, age and sex differences, daily movement, food composition, length of exposure, combined effects among pollutants. Small number of samples and short duration of sampling were also the reasons for uncertainty. In addition, only seven OCPs were studied in our study due to limited toxicity data. These factors could underestimate overall risks and lead to unrealistic expectations for control. Anyway, this study has provided a non-invasive method for understanding exposure risks of OCPs to birds especially for endangered or protected species.
Conclusions
Of the 18 analyzed OCPs, HCHs, DDTs, HEPTs and HCB were measured at relatively higher levels in two waterbird species living in the HLW, Central China. Common teals exhibited significantly higher OCP concentrations than bean geese (p = 0.02, F = 6.41), mainly due to their food habits. The OCP concentrations in waterbirds studied here were comparable or even higher than the reported results worldwide. The compositional patterns suggested that p,p′-DDE and β-HCH dominated among their two compound classes, respectively. Most of OCPs were mainly from historical application, while new inputs of heptachlor probably existed. The risks caused by OCPs to birds indicated that food intake was a significant exposure route. Among all studied OCPs, DDT pollution for bird exposure was not at a safe level. However, the current levels and risks of other pesticides (e.g. heptachlor, dieldrin) are also issues of great concern due to their high persistence and ecotoxicity. Thus, continuous investigation and comprehensive risk assessment of these pollutants are required to better protect birds and other biotic resources in wetlands.
Data availability
The data used in this study is available with the corresponding author upon a reasonable request.
References
Abbasi, N. A., Malik, R. N., Frantz, A., & Jaspers, V. L. B. (2016). A review on current knowledge and future prospects of organohalogen contaminants (OHCs) in Asian birds. Science of Total Environment, 542, 411–426.
Bai, J., Lu, Q., Zhao, Q., Wang, J., Gao, Z., & Zhang, G. (2015). Organochlorine pesticides (OCPs) in wetland soils under different land uses along a 100-year chronosequence of reclamation in a Chinese estuary. Scientific Reports, 5, 17624.
Barghi, M., Jin, X., Lee, S., Jeong, Y., Yu, J., Paek, W., & Moon, H. (2018). Accumulation and exposure assessment of persistent chlorinated and fluorinated contaminants in Korean birds. Science of Total Environment, 645, 220–228.
Bozlaker, A., Muezzinoglu, A., & Odabasi, M. (2009). Processes affecting the movement of organochlorine pesticides (OCPs) between soil and air in an industrial site in Turkey. Chemosphere, 77, 1168–1176.
Bustnes, J. O., Bakken, V., Skaare, J. U., & Erikstad, K. E. (2003). Age and accumulation of persistent organochlorines: A study of Arctic-breeding glaucous gulls (Larus hyperboreus). Environmental Toxicology and Chemistry, 22, 2173–2179.
CCME (Canadian Council of Ministers of the Environment), (1998). Protocol for the derivation of Canadian tissue residue guidelines for the protection of wildlife that consume aquatic biota. Report 1299. Canadian Council of Ministers of the Environment, Winnipeg, MB.
Connell, D. W., Fung, C. N., Minh, T. B., Tanabe, S., Lam, P. K. S., Wong, B. S. F., Lam, M. H. W., Wong, L. C., Wu, R. S. S., & Richardson, B. (2003). Risk to breeding success of fish-eating Ardeids due to persistent organic contaminants in Hong Kong: Evidence from organochlorine compounds in eggs. Water Research, 37, 459–467.
Daley, J. M., Paterson, G., & Drouillard, K. G. (2014). Bioamplification as a bioaccumulation mechanism for persistent organic pollutants (POPs) in wildlife. Review of Environmental Contamination and Toxicology, 227, 107–155.
Devi, N. L., Yadav, I. C., Raha, P., Shihua, Q., & Dan, Y. (2015). Spatial distribution, source apportionment and ecological risk assessment of residual organochlorine pesticides (OCPs) in the Himalayas. Environmental Science and Pollution Research, 22, 20154–20166.
Espín, S., Martínez-López, E., Gómez-Ramírez, P., María-Mojica, P., & García-Fernández, A. J. (2010). Assessment of organochlorine pesticide exposure in a wintering population of razorbills (Alca torda) from the southwestern Mediterranean. Chemosphere, 80, 1190–1198.
Glaser, D., & Connolly, J. P. (2002). A model of p, p′-DDE and total PCB bioaccumulation in birds from the Southern California Bight. Continental Shelf Research, 22, 1079–1100.
Goutner, V., Becker, P. H., & Liordos, V. (2011). Organochlorines and mercury in livers of great cormorants (Phalacrocorax carbo sinensis) wintering in northeastern Mediterranean wetlands in relation to area, bird age, and gender. Science of Total Environment, 409, 710–718.
Grung, M., Lin, Y., Zhang, H., Steen, A. O., Huang, J., Zhang, G., & Larssen, T. (2015). Pesticide levels and environmental risk in aquatic environments in China—a review. Environment International, 81, 87–97.
Hu, G. C., Xu, Z. C., Dai, J. Y., Xu, M. Q., & Shi, Z. M. (2010). Distribution characteristic of organochlorine pesticides and polybrominated diphenyl ethers in tissues of ducks from Baiyangdian Lake, North China. China Environmental Science, 31, 3081–3087. (in Chinese).
Hu, Y., Qi, S., Yuan, L., Liu, H., & Xing, X. (2018). Assessment of organochlorine pesticide contamination in waterbirds from an agricultural region, Central China. Environmental Geochemistry and Health, 40, 175–187.
Hu, Y., Qi, S. H., & Yuan, L. X. (2014). Distribution of organochlorine pesticides in the liver of waterbirds from Honghu wetland, Central China. China Environmental Science, 34, 2140–2147. (in Chinese).
Jiang, Y. F., Wang, X. T., Jia, Y., Wang, F., Wu, M. H., Sheng, G. Y., & Fu, J. M. (2009). Occurrence, distribution and possible sources of organochlorine pesticides in agricultural soil of Shanghai, China. Journal of Hazardous Materials, 170, 989–997.
Jin, M., Fu, J., Xue, B., Zhou, S., Zhang, L., & Li, A. (2017). Distribution and enantiomeric profiles of organochlorine pesticides in surface sediments from the Bering Sea, Chukchi Sea and adjacent Arctic areas. Environmental Pollution, 222, 109–117.
Kocagöz, R., Onmuş, O., Onat, İ, Çağdaş, B., Sıkı, M., & Orhan, H. (2014). Environmental and biological monitoring of persistent organic pollutants in waterbirds by non-invasive versus invasive sampling. Toxicology Letters, 230, 208–217.
Kuang, L., Hou, Y., Huang, F., Guo, A., Deng, W., Sun, H., Shen, L., Lin, H., & Hong, H. (2020). Pesticides in human milk collected from Jinhua, China: Levels, influencing factors and health risk assessment. Ecotoxicology and Environmental Safety, 205, 111331.
Li, K. L. (1983). An investigation on the fishing fishery in Honghu Lake. Issues in Agricultural Economy, 2, 56–58. (in Chinese).
Li, Y., Cai, D., Shan, Z., & Zhu, Z. (2001). Gridded usage inventories of technical hexachlorocyclohexane and lindane for China with 1/6 latitude by 1/4 longitude resolution. Archives of Environmental Contamination and Toxicology., 41, 261–266.
Li, Y., Zhang, H., Li, Q., Zhou, Q., Chen, X., Tu, C., Luo, Y., Christie, P., Hu, X., & Li, L. (2016). Characteristics of residual organochlorine pesticides in soils under different land-use types on a coastal plain of the Yellow River Delta. Environmental Geochemistry and Health, 38, 535–547.
Liang, J., Liu, J., Yuan, X., Zeng, G., Yuan, Y., Wu, H., & Li, F. (2016). A method for heavy metal exposure risk assessment to migratory herbivorous birds and identification of priority pollutants/areas in wetlands. Environmental Science and Pollution Research, 23, 11806–11813.
Liu, J., Liang, J., Yuan, X., Zeng, G., Yuan, Y., Wu, H., Huang, X., Liu, J., Hua, S., Li, F., & Li, X. (2015). An integrated model for assessing heavy metal exposure risk to migratory birds in wetland ecosystem: A case study in Dongting Lake Wetland, China. Chemosphere, 135, 14–19.
Lu, D., Wang, D., Ni, R., Lin, Y., Feng, C., Xu, Q., Jia, X., Wang, G., & Zhou, Z. (2015). Organochlorine pesticides and their metabolites in human breast milk from Shanghai, China. Environmental Science and Pollution Research, 22, 9293–9306.
Luo, X. J., Zhang, X. L., Liu, J., Wu, J. P., Luo, Y., Chen, S. J., Mai, B. X., & Yang, Z. Y. (2009). Persistent Halogenated Compounds in Waterbirds from an e-Waste Recycling Region in South China. Environmental Science and Technology, 43, 306–311.
Luo, Y., Yang, R., Li, Y., Wang, P., Zhu, Y., Yuan, G., Zhang, Q., & Jiang, G. (2019). Accumulation and fate processes of organochlorine pesticides (OCPs) in soil profiles in Mt. Shergyla, Tibetan Plateau: A comparison on different forest types. Chemosphere, 231, 571–578.
Luzardo, O. P., Ruiz-Suárez, N., Henríquez-Hernández, L. A., Valerón, P. F., Camacho, M., Zumbado, M., & Boada, L. D. (2014). Assessment of the exposure to organochlorine pesticides, PCBs and PAHs in six species of predatory birds of the Canary Islands, Spain. Science of Total Environment, 472, 146–153.
Lv, M., Luan, X. L., Guo, X. T., Liao, C. Y., Guo, D. F., Miao, L., Wu, X. Q., Zhou, R. C., Liu, D. Y., Wang, D. Q., Zhao, Y. C., & Chen, L. X. (2020). A national-scale characterization of organochlorine pesticides (OCPs) in intertidal sediment of China: Occurrence, fate and influential factors. Environmental Pollution, 257, 113634.
Maul, J. D., Blackstock, C., & Brain, R. A. (2018). Derivation of avian dermal LD50 values for dermal exposure models using in vitro percutaneous absorption of [14C]-atrazine through rat, mallard, and northern bobwhite full thickness skin. Science of Total Environment, 630, 517–525.
Niu, L., Xu, C., Zhu, S., & Liu, W. (2016). Residue patterns of currently, historically and never-used organochlorine pesticides in agricultural soils across China and associated health risks. Environmental Pollution, 219, 315–322.
Nordstad, T., Moe, B., Bustnes, J. O., Bech, C., Chastel, O., Goutte, A., Sagerup, K., Trouvé, C., Herzke, D., & Gabrielsen, G. W. (2012). Relationships between POPs and baseline corticosterone levels in black-legged kittiwakes (Rissa tridactyla) across their breeding cycle. Environmental Pollution, 164, 219–226.
Norstrom, R. J., Clark, T. P., Enright, M., Leung, B., Drouillard, K. G., & Macdonald, C. R. (2007). ABAM, a model for bioaccumulation of POPs in Birds: Validation for adult herring gulls and their eggs in lake Ontario. Environmental Science and Technology, 41, 4339–4347.
Pan, H., Geng, J., Qin, Y., Tou, F., Zhou, J., Liu, M., & Yang, Y. (2016). PCBs and OCPs in fish along coastal fisheries in China: Distribution and health risk assessment. Marine Pollution Bulletin, 111, 483–487.
Rajaei, F., Bahramifar, N., Sari, A. E., & Ghasempouri, S. M. (2010). PCBs and organochlorine pesticides in ducks of Fereydoon-kenar wildlife refuge in Iran. Bulletin of Environmental Contamination and Toxicology, 84, 577–581.
Sakellarides, T. M., Konstantinou, I. K., Hela, D. G., Lambropoulou, D., Dimou, A., & Albanis, T. A. (2006). Accumulation profiles of persistent organochlorines in liver and fat tissues of various waterbird species from Greece. Chemosphere, 63, 1392–1409.
Sample, B.E., Opresko, D.M., Suter, I.G.W., (1996). Toxicological benchmarks for wildlife: 1996 Revision. United States.
Su, H., Wu, F., Zhang, R., Zhao, X., Mu, Y., Feng, C., & Giesy, J. P. (2014). Toxicity reference values for protecting aquatic birds in china from the effects of polychlorinated biphenyls. In D. M. Whitacre (Ed.), Reviews of environmental contamination and toxicology volume: with cumulative and comprehensive index subjects covered (pp. 59–82)
Torre, Adl, Sanz, P., Navarro, I., & Martínez, M. Á. (2016). Time trends of persistent organic pollutants in spanish air. Environmental Pollution, 217, 26–32.
Vizuete, J., Hernández-Moreno, D., Fidalgo, L. E., Bertini, S., Andreini, R., Soler, F., Míguez-Santiyán, M. P., López-Beceiro, A., & Pérez-López, M. (2018). Concentrations of chlorinated pollutants in adipose tissue of yellow-legged gulls (Larus michahellis) from Spain: Role of gender and age. Ecotoxicology and Environmental Safety, 164, 493–499.
Walker, C. H., & Newton, I. (1998). Effects of cyclodiene insecticides on the Sparrowhawk (Accipiter Nisus) in Britain—a Reappraisal of the Evidence. Ecotoxicology, 7, 185–189.
Wang, H. S., Zhao, Y. G., Man, Y. B., Wong, C. K. C., & Wong, M. H. (2011). Oral bioaccessibility and human risk assessment of organochlorine pesticides (OCPs) via fish consumption, using an in vitro gastrointestinal model. Food Chemistry, 127, 1673–1679.
Wang, W., Wang, Y., Zhang, R., Wang, S., Wei, C., Chaemfa, C., Li, J., Zhang, G., & Yu, K. (2016). Seasonal characteristics and current sources of OCPs and PCBs and enantiomeric signatures of chiral OCPs in the atmosphere of Vietnam. Science of Total Environment, 542, 777–786.
Wiemeyer, S.N., (1996). Other organochlorine pesticides in birds. In: Beyer, W., Heinz, N., Redmon-Norwood, G.H., A.W. (Eds.), Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations. SETAC Special Publication Series. CRC Press, Boca Raton, FL, pp. 99–115.
Wu, J. P., Peng, Y., Zhi, H., Chen, X. Y., Wu, S. K., Tao, L., Zeng, Y. H., Luo, X. J., & Mai, B. X. (2019). Contamination of organohalogen chemicals and hepatic steatosis in common kingfisher (Alcedo atthis) breeding at a nature reserve near e-waste recycling sites in South China. Science of Total Environment, 659, 561–567.
Xu, C., Yin, S., Tang, M., Liu, K., Yang, F., & Liu, W. (2017). Environmental exposure to DDT and its metabolites in cord serum: Distribution, enantiomeric patterns, and effects on infant birth outcomes. Science of Total Environment, 580, 491–498.
Yadav, I. C., Devi, N. L., Li, J., Zhang, G., & Breivik, K. (2017). Possible emissions of POPs in plain and hilly areas of Nepal: Implications for source apportionment and health risk assessment. Environmental Pollution, 220, 1289–1300.
Yang, Y., Deng, Y., & Cao, L. (2016). Characterizing the interspecific variations and convergence of gut microbiota in Anseriformes herbivores at wintering areas. Scientific Reports, 6, 32655.
Yin, W., Zhang, Y., Wang, P., Zheng, S., Zhu, C., Han, X., Zhang, Q., Liang, Y., & Jiang, G. (2018). Distribution of polybrominated diphenyl ethers (PBDEs) in feather and muscle of the birds of prey from Beijing, China. Ecotoxicology and Environmental Safety, 165, 343–348.
Yuan, L., Qi, S., Wu, X., Wu, C., Xing, X., & Gong, X. (2013). Spatial and Temporal Variations of Organochlorine Pesticides (OCPs) in Water and Sediments from Honghu Lake, China. Journal of Geochemical Exploration, 132, 181–187.
Zapata, P., Ballesteros-Cano, R., Colomer, P., Bertolero, A., Viana, P., Lacorte, S., & Santos, F. J. (2018). Presence and impact of Stockholm Convention POPs in gull eggs from Spanish and Portuguese natural and national parks. Science of Total Environment, 633, 704–715.
Zarrintab, M., & Mirzaei, R. (2018). Tissue distribution and oral exposure risk assessment of heavy metals in an urban bird: Magpie from Central Iran. Environmental Science and Pollution Research, 25, 1–10.
Zhang, G., Pan, Z., Bai, A., Li, J., & Li, X. (2014). Distribution and bioaccumulation of organochlorine pesticides (OCPs) in food web of Nansi Lake, China. Environmental Monitoring and Assessment, 186, 2039–2051.
Zhang, X. L., Luo, X. J., Liu, J., Luo, Y., Chen, S. J., & Mai, B. X. (2011). Polychlorinated biphenyls and organochlorinated pesticides in birds from a contaminated region in South China: Association with trophic level, tissue distribution and risk assessment. Environmental Science and Pollution Research, 18, 556–565.
Zheng, S., Wang, P., Sun, H., Matsiko, J., Hao, Y., Meng, D., Li, Y., Zhang, G., Zhang, Q., & Jiang, G. (2018). Tissue distribution and maternal transfer of persistent organic pollutants in Kentish Plovers (Charadrius alexandrines) from Cangzhou Wetland, Bohai Bay, China. Science of Total Environment, 612, 1105–1113.
Zhi, H., Zhao, Z., & Zhang, L. (2015). The fate of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in water from Poyang Lake, the largest freshwater lake in China. Chemosphere, 119, 1134–1140.
Zhou, P., Wu, Y., Yin, S., Li, J., Zhao, Y., Zhang, L., Chen, H., Liu, Y., Yang, X., & Li, X. (2011). National survey of the levels of persistent organochlorine pesticides in the breast milk of mothers in China. Environmental Pollution, 159, 524–531.
Zhou, W. C., Pang, H. D., Yang, G. X., Zheng, L., & Zhu, Z. Q. (2018). Species and quantify of waterbirds in Hubei Province. Wetland Science, 16, 9–16. (in Chinese).
Acknowledgements
The study was funded by China Postdoctoral Foundation (Y71I321K02), Talent Introduction Project of Yangtze University (No. 8021002902), and Project of Hubei Provincial Department of Education (No. B2020034). We gratefully acknowledge the Hubei Wildlife Protection Centre.
Funding
The study was funded by China Postdoctoral Foundation (Y71I321K02), Talent Introduction Project of Yangtze University (No. 8021002902), and Project of Hubei Provincial Department of Education (No. B2020034).
Author information
Authors and Affiliations
Contributions
YH: contributed to methodology, sampling, formal analysis and writing—original draft. HL: contributed to sampling, instrument analysis and data curation. XX: contributed to writing—review & editing and resources. JL: contributed to writing—review & editing. FL: contributed to data curation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
This manuscript was approved for publication by all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, Y., Liu, H., Xing, X. et al. Occurrence and exposure risk assessment of organochlorine pesticides in two waterbird species from Honghu Lake Wetland, Central China. Environ Geochem Health 45, 1919–1931 (2023). https://doi.org/10.1007/s10653-022-01316-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01316-7