Abstract
A wide variety of contaminants in the aquatic environment causes several deleterious effects on aquatic organism traits ranging from molecular to individual and population levels. This in situ study investigated morphometry, growth performance, and reproduction success of a teleost fish, Diplodus annularis, from a polluted site “Sayada” and a reference site “Salakta” in the eastern Tunisian coastline. Morphometric indexes, generalized Procruste analysis, thin plate, and otolith contour methods were used to assess the pollution effects on morphological traits. The growth performance of fish from contaminated and control sites were studied using the Von Bertalanffy equation. Gonadosomatic (GSI) and Hepatosomatic (HSI) Indexes and absolute and relative fecundity were used to assess the impact of pollution on the reproduction success of this species. The obtained results showed that the fish of the contaminated zone had significant alteration of the morphology, slower growth, significant increase in the HSI, significant decrease in female GSI, and a decrease in absolute and relative fecundity compared to specimens from the unpolluted zone. Based on these results, the biological traits of Diplodus annularis can be used as biological biomarkers in the monitoring and protection programs of the marine contamination in the Mediterranean Sea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal littoral ecosystems may receive inputs from terrestrial contaminant sources, leading animals to be exposed to higher concentrations of chemicals (Ben-Khedher et al. 2014). This situation endangers the health of organisms inhabiting this ecosystem (e.g., Jebali et al. 2013a; 2014). Exposure of marine fishes to mixtures of xenobiotics may have different mechanisms of action on the animal health. Several molecular, biochemical, physiological, and morphological variations have been reported in field-contaminated fishes (Sanchez et al. 2011; Jebali et al. 2013a). Disturbance of fish growth and reproduction may be related to physiological changes caused by chemical contamination and natural environmental factors, including temperature and food availability which may also be altered by anthropogenic actions (Pajuelo and Lorenzo 2002; Van-Poorten and Walters 2016).
Monastir Bay is located in the center of the East Tunisian coastline and is considered an economically important body of water due to a variety of fishing and aquaculture activities in the Bay. Many pollutants have been detected in recent years in Monastir Bay emanating from treated and non-treated municipal wastewater, limitroph agglomerations, and the fishing harbor (Jebali et al. 2011; Nouira et al. 2012 and 2013; Zrafi et al. 2013). In addition, the bay is subject to eutrophication during the summer season. Although many studies have investigated the infield effect of mixtures of pollutants on biochemical dysfunctions in the indigenous species of Monastir Bay, including Mediterranean crab (Carcinus maenas) and common sole (Solea solea) (Jebali et al. 2011, 2013a; Ben-Khedher et al. 2013), few studies have looked at the effect of pollutants on reproductive success in wild fish (Mills and Chichester 2005; Prado et al. 2014). This study showed how multiple pollution could affect the reproductive cycle and growth of the annular sea bream, Diplodus annularis, a major species of fishing on the Tunisian coast. As a control site, we chose the coastline of Salakta which is formed by a protected cape free from any discharge of wastewater and characterized by constant agitation and coastal currents favoring good water renewal. Annualar sea bream, Diplodus annularis L., is widespread in the Mediterranean Sea including the Tunisian coastline, where it is abundant throughout the year. It constitutes an important fishery resource in the region. Different aspects of the biology of the species have been studied, e.g., settlement (Garcia-Rubies and Macpherson 1995), feeding (Bell and Harmelin-Vivien 1983), age, and growth (Gordoa and Molí 1997; Pajuello and Lorenzo 2001). There is, however, little information available about the possible effects of environmental contamination on the morphology, growth, and reproduction of the species. An assessment of the impact of contamination on this species and other important fishery species is critical to manage and conserve resources in the region.
In this work, we investigated the effect of anthropogenic chemical inputs on the biological traits of Diplodus annularis across heavily contaminated by chronic urban discharges and relatively uncontaminated sites in eastern Tunisia. Traits measured were morphometry by means of the meristic and metric characters and otolith shape characteristics, age, and growth based on the Von Bertalanffy model and reproduction by tracking body indexes including gonadosomatic index (GSI) and hepatosomatic index (HSI) and fecundity estimation.
Materials and methods
Study areas and fish sampling
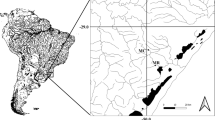
To investigate the effects of anthropogenic chemical inputs on the morphology, growth, and reproduction in D. annularis fish, two sites of the eastern Tunisian coasts (Fig. 1) were selected: As a reference site, we selected Salacta (35°24′N 11°03′E) which is a relatively clean area, without industrial or urban influences and has been considered as reference in our previous study (Gharred et al. 2019). Sayada site (35°40′N 10°54′E) is located in the Monastir bay and considered as contaminated site. It is an important industrial and urban runoff site and has been classified as a high-contamination area. Sediments at this site contain high concentrations of metals (iron 2706.68 ppm, zinc 15.18 ppm, lead 5.29 ppm, chromium 3.64 ppm) (Damak et al. 2019) and polyaromatic hydrocarbons (PAHs). Total PAH concentrations were in the range of 25.6 to 576.8 ng/g d.wt in winter and 44.9–395.8 ng/g d.wt in summer (Nouira et al. 2012).
Sampling was performed during a full year from April 2014 to March 2015. At each sampling site, fish were collected monthly with the help of local fishermen using gill nets, at depths that do not exceed 10 m. The size of each sample differs according to analyzed biological parameter.
Morphometric analysis of fish
Traditional morphometry
The sample used for the study of meristic features includes 80 randomly selected fish per site. After sexing animals, eight meristic characters were determined by the use of binocular microscope. The measurement includes the rays (R) and spines (S) of the dorsal (D), pelvic (P), and anal (A) fins and the rays of the pectoral fin (PR). For the vertebrae enumeration, we determined particularly the number of thoracic vertebrae (TV) from the first cervical vertebrae to the last vertebra with a fully opened haemal arc and the number of caudal vertebrae (CV) having a closed haemal arc including urostyle.
The metric variables taken on each individual of the 169 fish from each study site are shown in Fig. 2. All measurements were determined with a digital caliper to the nearest 0.01 mm.
Different measurements performed on D.annularis: total length (TL), fork length (FL), standard length (SL), head length (HL), pectoral length (PecL), dorsal length (DL), pelvic length (PelL), anal length (AL), Body Height (BH), predorsal distance (PD), prepectoral distance (Ppec), preanal distance (PA), prepelvic distance (PPel), preorbital distance (PO), eye diameter (Ø), interorbital distance (IO)
From these metric measurements, several morphometric ratios were calculated to assess the chronic effect of multiple pollutions on the morphometry of fish at Sayada contaminated site with comparison to Salacta as reference site. We specifically determined the TL/SL, FL/LS, HL/SL, BH/SL, PD/SL, PA/SL, PPec/SL, PPel/SL, PecL/SL, DL/SL, AL/SL, PelL/SL, IO/HL, Ø/HL, and PO/HL.
The analysis of variance (ANOVA) was performed to test the statistical significance of mean variations among contaminated and control fish population and for each measurement. Principal component analysis (PCA) was used to elucidate pattern of the morphological variation between these two populations.
Geometric morphometry
Generalized Procruste analysis
The generalized Procrustes Analysis approach was used to investigate the fish body shape variability of control and contaminated sites populations (Straüss and Bookstein 1982; Montaño-Campaz et al. 2019). The sample used to study the body shape is composed of 50 specimens taken at random and irrespective of sex from each site, the size of these fish varies between 105 and 140 mm, and the weight between 30 and 45 g. These fish were sampled during the sexual rest period (July to December) to avoid the influence of reproduction on morphometry. The left side of each fish, with the fins in the extended position, was photographed with a high-quality digital camera (Samsung ST72 with 16 MP resolution) mounted on a tripod. All images were digitized in TPS Dig 2 using 16 homolog landmarks to perform geometric morphometrics analysis (Fig. 3).
Landmarks used in geometric morphometry: 1: anterior extremity of the upper jaw, 2: the anterior base of the spines of the dorsal fin, 3: the posterior base of the rays of the dorsal fin, 4: the dorsal attachment point of the caudal fin, 5: the point of intersection of the lateral line with the caudal fin, 6: the ventral attachment point of the caudal fin, 7: the posterior base of the rays of the anal fin, 8: the anterior base of the spines of the anal fin, 9: insertion of the pelvic fin, 10: the ventral end of the head, 11: the tip of the mouth, 12: the center of the orbit, 13: the tip of the opening of the operculum, 14 the dorsal end of the head, 15: the dorsal base of the pectoral fin and 16: the ventral base of the pectoral fin
In order to pass from a configuration of simple landmarks to shape variables, the generalized Procuste analysis was performed using Morpho J 1.03d (Klingenberg 2011) on the Cartesian coordinates X and Y of the homologous points digitized by the TPS software series. This transformation consists in standardizing measures to control for differences in individual body size, by translating and rotating the configuration of landmarks to minimize the sum of squared distances between homolog landmarks (Zelditch et al. 2012). The principal component analysis (PCA) was used to assess the pattern of morphological variation between control and contaminated fish populations to reveal the degree of similarities or differences in fish body between the studied populations.
Otolith shape analysis
A sample of 60 left and right otoliths were carefully removed and mounted on the glass slides and photographed with a high-quality digital camera (Samsung ST72 with 16 MP resolution). All images were analyzed by the Shape software 1.3 (Iwata and Ukai 2002). The Fourier Elliptic Analysis (F.E.A.) was carried out to determine the shape of the otolith. The shape outline of the otolith is characterized by several components, named harmonics. Each harmonic is characterized by four coefficients, resulting from the projection of each point of the outline on axes (x) and (y) (Kuhl and Giardiana 1982). The minimum number of harmonics needed to explain 99.99% of otolith shape variation was determined with a power spectrum analysis. The four coefficients of these seven harmonics are then used in principal component and discriminant analyses to better characterize the differences between fish populations of Salakta and Sayada through otolith shape.
Age and growth
Samples of 60 fish from Salakta and 50 fish from Sayada were used for age and growth studies. Total length (mm) and whole mass (g) were recorded. Five to eight scales, more legible than the otolith, were extracted from each individual from the body area between the lateral line and the dorsal fin. Scales were cleaned with water and immersed in 10% formalin solution. Growth was estimated by scale reading and using Bertalanffy equation: TL (t) = TL∞ (1 − e–K(t–to)) where TL is the total length at age t, TL∞ is the theoretical asymptotic length, k is the growth-rate coefficient, and t0 is the theoretical age at length zero. The linear Ford–Walford plot (Gulland 1969) was first used to provide initial or starting values for the non-linear or iterative methods. For non-linear estimation of the growth parameters, Levenberg-Marquardt method in Statistica.10 was used with the individual size and age data.
Reproduction and fecundity
Sample of 30–40 fish per site was monthly collected between April 2014 and March 2015 to assess the physiological effects of multiple pollution on reproduction and fecundity of fish in control and contaminated areas from Tunisian coast. Sexes were distinguished by macroscopic observation of gonads. The reproductive period was established by monthly determination of the gonadosomatic index (GSI) and the hepatosomatic index (HSI).
For the study of fecundity, the spawning nature and the last modal distribution of fish oocytes was used to estimate the absolute fecundity by spawning act. For the analysis of the frequency distribution of oocyte diameters, we randomly measured under an optical microscope equipped with an ocular micrometer, the diameters of 1000 oocytes per female. The results were compiled in frequency polygons.
The absolute fecundity by spawning act is defined as the number of oocytes whose diameters constitute the last modal distribution (Fantana and Le Guen 1969). The absolute fecundity (AF) was calculated using the following equation: AF = (n / OFW) × OW. Where AF: absolute fecundity, n: number of oocytes of the last spawning act contained in a fraction of the ovary, OW: ovary weight (g), OFW: weight of ovary fraction (1 g).
The relative fecundity (RF) was estimated by counting the number of oocytes per body mass unit. The equation used was: RF = AF / EM, where RF: relative fecundity, AF: absolute fecundity, EM: eviscerated body mass.
Statistical analysis
An analysis of variance (ANOVA) was performed to compare means of meristic and metric characters and fecundity between the two populations using SPSS software (version 17.0). Multivariate analyses were used to elucidate pattern of the morphological variation between samples. Particularly, principal component analysis (PCA) and discriminating function procedure, in XLSTAT 2014 software, was used to analyze morphometric variability and examine separation between the two fish populations collected from control and polluted sites. Multivariate one-way-PERMANOVA test was used to compare morphometric ratios of the annular sea bream collected from the studied sites. Significant difference level between sites was obtained by using Bonferroni test. Mann Whitney test was used to compare monthly GSI and HSI of the contaminated and control populations.
Results
Morphometry of Diplodus annularis
The incidence of the multiple pollution on the morphometry of D. annularis by the traditional morphometry showed no significant differences between meristic variables of control and contaminated fish (Table 1). However, we note significant differences in the following five metric ratios: BH/SL; PD/SL; PA/SL; IO/HL, and Ø HL (Table 1).
The principal component analysis (PCA) of the 15 studied metric ratios showed that the first two components explained 36.36% of the overall variation. These components absorb respectively 23.13% and 13.23% of the total inertia. The graphical representation of variable points shows that component 1 has a strong positive covariance with LF/LS, TL/LS, and BH/SL ratios, while component 2 is positively covariate with PO/BH, Ø/BH, and PD/SL ratios. The graphic representation of the “observation points” shows that there is no tendency to isolate the two populations (Fig. 4). This distribution does not allow a clear discrimination between the individuals of the two environments. But the multivariate one-way-PERMANOVA test of the annular sea bream morphometric ratios showed a significant difference between sites (Table 2). The geometric morphometric analysis using the landmarks was applied to better discriminate between the two studied populations.
PCA obtained from the residual multivariate regression of the 16 studied landmarks showed that the first two components explain 41.27% of the total variation. The graphical representation of the observation points shows that there is a tendency to isolate the two studied samples (Fig. 5). The generalized Procuste method seems to be more efficient in detecting variation than the traditional method. The PCA based on the landmarks allows separation between the two studied populations, but it does not show the changes between the landmarks on the general morphology of fish body. Hence, the use of the thin-plate method, which gives information on the nature of morphological deformations, is attributed to environmental stress. The main displacements of the landmarks between the specimens of D. annularis of Salakta and Sayada affect mainly the anterior, ventral, and dorsal parts of the body (Fig. 6). These deformations affect particularly the height of the body, which is due to the displacement of the landmarks 2 and 9; the head and the cephalic part due to the displacement of landmarks 1, 10, 11, 13, and 14; the pectoral fin, which is caused by the displacement of landmarks 15 and 16; and the anal fin, which is caused by the displacement of the landmark 8. Other smaller deformations affect the caudal region.
Concerning the study of otolith shape, the principal component analysis of the right and left otoliths shapes shows a tendency of discrimination between control and contaminated fish populations. The left otolith shape shows a higher variation than the right one (Fig. 7a, b). Thus, a deep analysis of this otolith by the discriminant function analysis showed a distinct discrimination between the two D. annularis fish populations, confirming the utility of using the left otoliths shape as effective tool for the assessment of multiple and chronic contamination of marine ecosystems. (Fig. 8). In fact, the Mahalanobis distance calculated between the two groups (Salakta and Sayada) was 0.48 (P < 0.001), confirming the significance of the separation according to the form of otolith left between the two samples.
Age and growth
The maximum observed age in specimens collected from both areas was 8 years and the maximum observed length was 170.5 mm for fishes from Salakta and 169 mm for those from Sayada (Table 3).
The theoretical growth parameters of the Von Bertalanffy equation showed that LT∞ of Sayada specimens (202.94 mm) are slightly higher than those of Salakta (200.4 mm). However, the growth rates K of Sayada specimens show a lower value (0.16) than those of Salakta (0.18) (Table 4).
Reproduction
The monthly variation of gonadosomatic index (GSI) of D. annularis from Salakta site between April 2014 and March 2015 indicates similar pattern of the reproductive cycle for both sexes. The spawning period was from April to June with a peak of gonadosomatic index (GSI) (indicating the maximum maturation of the gonads) in April (5.80 and 9.33% for males and females respectively). A significant decrease of this index was observed from April to June corresponding to the gametic emission phase. This decrease is followed by a sexual rest phase since June (Fig. 9a).
Interestingly, the comparison study of the GSI variations of the control and contaminated fish highlights two essential phenomena: the first is a shift of 1 month in the full maturation period and gametic emission phase for both sexes and the second is a lower maximum value of GSI in contaminated female fish (Fig. 9a; Table 5).
The monthly fluctuation of hepatosomatic index (HSI) of fishes from Sallakta and Sayada shows similar pattern during the studied period. (Fig. 9b). The HSI reached a maximum in April, then decreased gradually to a minimum in June, and then increased significantly in the following months. Interestingly, we noted significant higher HSI in contaminated fish from sayada than those from sallakta (Table 5).
The distribution frequency of oocyte diameter in both studied sites is tri-modal pattern (Fig. 10a, b). The three observed cohorts correspond to three spawning acts suggesting that spawning phenomena of D. annularis is fractionated. The first spawning act includes oocytes with diameters between 20 and 220 μm, the second one is between 200 and 380 μm, and the third is superior to 340 μm. For the fecundity study, we considered only the last spawning act (oocytes diameters Ø ≥ 340 μm) corresponding to the most advanced modal distribution. The absolute (AF) and relative (RF) fecundity by spawning act in fish from Sallakta and Sayada sites are illustrated in Table 6. The comparison of absolute and relative fecundity per spawning act indicates a significant difference between the two studied populations. These fecundities are significantly higher in reference area (Sallakta site) than those of contaminated zone (Sayada site) (ANOVA, Fa = 7.7, Fr = 12.9, P < 0.0005) (Table 6). We noted also that the highest oocyte diameter is found in reference site (Salakta) and was about 720 μm, while it does not exceed 580 μm in contaminated site (Sayada).
Discussion
Morphometry
Several in-field studies highlighted the use of morphometric characters for fish species characterization (Silva 2003; Kaouèche et al. 2017; Deepa et al. 2019) and to assess the impact of the environment stress on the morphology variability of fish (Amin et al. 2014). It is well known that the annular sea bream, Diplodus annularis, is a littoral fish, common in the bottoms covered by seagrass beds from 0 to 50 m depth (Bauchot 1987). Its reduced mobility, sedentary, and high site fidelity (March et al. 2011) are also related to the suitable utility as bioindicators to evaluate the local exposure to human impacts. Several studies have used D. annularis to assess the impacts of chemicals in the Tunisian (Khenfech and Boumaiza 2011) and other Mediterranean marine ecosytems (Gonul and Kucuksezgin 2007; Darilmaz and Kucuksezgin 2012).
The analysis of meristic variables of D. annularis specimens from Salakta and Sayada showed no significant difference. It is well accepted that the meristic characteristics are considered as discrete quantitative variables with weak sensibility to environmental stressors (Gharred et al. 2019), independent of individual size and genetically fixed since the embryonic development (Turki et al. 2009).
To better assess the body deformations associated with marine pollution, the geometric morphometry of the D. annularis specimens from reference (Salakta) and contaminated (Sayada) sites was applied. It showed clear discrimination between the two studied fish populations. This inter-population morphometry variation could be attributed to the chronic and continuous urban discharge effect on the population of Sayada. Recent and old chemical and ecological available data have shown higher metallic and hydrocarbon concentrations in Monastir bay littoral (Sassi et al. 1998; Zrafi et al. 2013; Damak et al. 2018, 2019). Morphological changes of fish population integrate the multiple effects of several stress factors including endogen factors such as genetic variability or exogenous factors like chemical exposure and natural environment degradation. In in-field, biomonitoring studies, the endogen and environmental factors affect simultaneously all the biological responses of an animal under environmental stress including molecular, biochemical, physiological tissue and morphological responses (Shinn et al. 2015). Morphological changes have been used as biological markers of chronic exposure of various organisms to pollutants as well as when facing habitat degradation (Shinn et al. 2015; Abdelhady 2016).
It has been shown that the variation of morphometric characters is attributed not only to a genetic component but also to the environmental one. In fact, fish exhibit high phenotypic plasticity in response to environmental changes and can adapt rapidly through appropriate morphological changes (Vasil’eva and Vasil’ev 2010; Hossain et al. 2010). The previous work on the gastropod Patella caerulea collected from reference zone and three polluted areas of the Tunisian coastline suggest that the anthropogenic impacts induce alterations in the shape of the limpet shell. These deformities were attributed to multiple stress factors, among which we quote the chemical pollutants metabolized by the organisms under contaminated environments (Gharred et al. 2019).
Otoliths are calcified structures whose form has been widely used to characterize a given species or populations of fish species. They keep a continuous record of the life cycle of fish from birth to adulthood. The shape and chemical composition of otoliths can be modified depending on the interaction between fish and its environment during the growth (Morat et al. 2008). However, few studies interested in the otolith shape variability as a biological tool of marine contamination assessment. The otolith shape variability is influenced by chronic and mixture of anthropogenic pollutants. It has been shown that Neogobius melanostomus fish captured from high-contaminated sites of Hamilton Harbor (Canada) with metals (e.g., cadmium, zinc, lead), polyaromatic hydrocarbons (PAH) and polychlorinated biphenyls (PCB), displayed a high amount of variation degrees otolith shape (Bose et al. 2018). Many environmental stressors, such as pollutants, temperature, food availability, and even fish physiology, may affect the metabolism of these organisms and have the potential to alter the growth and morphology of teleost otoliths. For example, the exposure of African catfish Clarias gariepinus, to textile factory effluent, resulted in fish with smaller sagittal otoliths than those unexposed (Adeogun and Chukwuka 2011).
Age and growth
D. annularis fish from contaminated site seems to have lower somatic growth rates than those of reference site. Many environmental contaminants and stressors are known to affect metabolism through molecular and biochemical toxicity (Jebali et al. 2012). Environmental pollutants may also increase ATP demand for detoxification and cellular homeostasis, potentially altering somatic growth (Toro et al. 2003). In this study, D. annularis from high-contaminated site have been chronically exposed to PAHs, PCBs, and metals such as lead, zinc, and cadmium in their environments (Sassi et al. 1998; Nouira et al. 2012 and 2013; Zrafi et al. 2013) leading to a lower growth rate. These results are consistent with a previous study of Bradai et al. (2001) that noted a slow growth rate of D. annularis fish from a contaminated area: Golf of Gabes. The authors attributed the delay in somatic growth of contaminated fish to lack of sufficient energy for growth.
Reproduction
Despite the fact that D. annularis is one of the most common and abundant sparid species of the Mediterranean littoral and is considered as a species of high commercial value in Tunisia, few studies have been published on the reproductive biology of this fish.
In this work, the gonad activity of D. annularis from (Sallakta site) reached a maximum in April. These results are in agreement with those found in the central-western Mediterranean seas (Donnaloia et al. 2017). Fish from contaminated region have a shift in the spawning period 1 month later when compared to those of control region. Indeed, the maximum gonad activity is recorded in May. These results are consistent with those found in contaminated areas of Tunisia such as Golf of Gabes (Chaouch et al. 2013) and Golf of Tunis (Mouine et al. 2012). It is well known that the timing of Diplodus annularis spawning appears to be correlated with food availability and sea temperature (Matic-Skoko et al. 2007). However, other environmental factors such as chemical pollution for example endocrine disruptor compounds (EDCs) and the alteration of physicochemical proprieties of seawater (pH, salinity, dissolved oxygen, turbidity…etc.) affect the timing of spawning of fishes.
The GSI in females of the sea bream decreased considerably in the contaminated site (Sayada) compared to the reference site (Salakta). These findings may be related to impaired ovarian development, decreased oocyte diameter, and decreased vitellogenin stores (Johnson et al. 2008; Bugel et al. 2009; Gerbron et al. 2014). Several endocrine disrupting compounds (EDCs) detected in the aquatic environment have been shown to be responsible for the inhibition of vitellogenin biosynthesis via binding to estrogenic receptors and negative feedback on the hypothalamic pituitary axis (Solé et al. 2003). Therefore, it is important to note that female fish from polluted areas produce fewer oocytes than control fish. This can be considered as the adaptive aspect of these fish to pollution. In fact, environmental stressors have significant effects on fish performance due to the reorientation of energy resources in response to stress (Schreck 2010). We note that the GSI and HSI reports vary in the same way over time for the two studied populations, indicating that D. annularis is a fatty fish for which the accumulation of lipid reserves is carried out in the muscles. The liver is only involved in the transformation of these lipid reserves.
Interestingly, we noted higher HSI in fish from polluted area (Sayada site) than those of reference area (Salakta site). It is obvious that the liver is the main crossroads of the metabolism of endogenous and chemical compounds. The chronic exposure of fish of Sayada to mixture of contaminants increase the metabolic activity of the liver in response to chemical contamination (Van der Oost et al. 2003; Ghedira et al. 2010). The higher HSI can be explained by the hypertrophy and to the hyperplasia of the hepatocyte cells linked to increased detoxification metabolism caused by the high pollution mixture in the polluted site (Dragun et al. 2013; Agbohessi et al. 2015). The chronic treated wastewater discharges in Sayada site increased the metals and hydrocarbon concentrations in sediments and seawater and could be related to the increase of fish hepatosomatic index in this site. Indeed, the north-south sea currents can disperse chemical pollutants by geochemical processes from other points of treated wastewater from limitroph agglomerations such as Khenis city and thus harm the health of the fish and their environment (Jebali et al. 2013b).
Other studies have shown a decrease of the HSI following the exposure of fish to pollutants (Hauser-Davis et al. 2012; Bervoets et al. 2013). The authors suggest that chronic exposure to pollutants causes histopathological lesions of liver tissue and depletion of energy stores (glycogen and lipid content) in fish living at sublethal pollution levels in polluted areas. Nevertheless, it is important to note that high HSI in contaminated fish is correlated with decreased reproductive performance and morphological alteration caused by exposure to multiple pollution.
Conclusion
This study investigated in situ morphometry, growth performance, and reproduction success of D. annularis fish from reference and contaminated area of the Tunisian coastline. Several different biological alterations were characterized in fish living in polluted area. The analysis of the meristic characteristics revealed that these traits are insensitive to marine pollution exposure. However, the use of the geometric morphometry approach based on the generalized Procuste superposition and the thin-plate approaches show a distinct body and otolith shape variations of fish living in the contaminated area. The contaminated fish morphometry alteration was associated with the decrease of the somatic growth and reproduction performance. The results concerning gonadosomatic and hepatosomatic indexes and the fecundity success demonstrate clearly the alteration of the reproductive cycle of D. annularis from polluted site. Interestingly, we noted for the first time pollution induced a reduction in the number and diameter of oocytes in contaminated fish. Our results recommended the usefulness of D. annularis body and otolith shapes, gonadosomatic and hepatosomatic indexes, and fecundity success responses as biological biomarkers in the monitoring and protection programs of the marine contamination in the Mediterranean sea.
References
Abdelhady AA (2016) Phenotypic differentiation of the Red Sea gastropods in response to the environmental deterioration: geometric morphometric approach. J Afr Earth Sci 115:191–202
Adeogun AO, Chukwuka AV (2011) Effect of textile factory effluent on otolith and somatic growth parameters in Clarias gariepinus. Zoologist 9:70–77
Agbohessi PT, Imorou Toko I, Ouédraogo A, Jauniaux T, Mandiki SN, Kestemont P (2015) Assessment of the health status of wild fish inhabiting a cotton basin heavily impacted by pesticides in Benin (West Africa). Sci Total Enviran 506-507:567–584
Amin MR, Mollah MF, Taslima K, Muhammadullah (2014) Morphological observation and length-weight relationship of critically endangered riverine catfish Rita rita (Hamilton). Pak J Biol Sci 17(2):234–240
Bauchot ML (1987) Sparidae. In: Fischer W, Bauchot ML, Schneider M (Eds.), Fiches FAO d’Indentification des espèces pour les Besoins de la Pêche (Revison 1), Méditerranée et Mer Noire. Zones de pechê 37, vol. II (Vertébrés). FAO-CEE, Rome, pp. 761-1530
Bell JD, Harmelin-Vivien ML (1983) Fish fauna of French Mediterranean Posidonia oceanica seagrass meadows. 2. Feeding habits. Tethys 11:1–14
Ben-Khedher S, Jebali J, Kamel N, Banni M, Rameh M, Jrad A, Boussetta H (2013) Biochemical effects in crabs (Carcinus maenas) and contamination levels in the Bizerta lagoon: an integrated approach in biomonitoring of marine complex pollution. Environ Sci Pollut Res 20(4):2616–2631
Ben-Khedher S, Jamel J, Zohra H, Amel NHJ, Hamadi BMB (2014) Metals bioaccumulation and histopathological biomarkers in Carcinus maenas crab from Bizerta lagoon. Environ Sci Pollut Res 21(6):4343–4357
Bervoets L, Knapen D, De Jonge M, Van Campenhout K, Blust R (2013) Differential hepatic metal and metallothionein levels in three feral fish species along a metal pollution gradient. PLoS One 8(3):e60805
Bose APH, McCallum ES, Raymond K, Marentette JR, Balshine S (2018) Growth and otolith morphology vary with alternative reproductive tactics and contaminant exposure in the round goby Neogobius melanostomus. J Fish Biol 93:674–684
Bradai MN, Jarboui O, Ghorbel M, Bouain A, El Abed A (2001) Age et croissance du sparaillon D. annularis (Teleosteens, Sparidae) du Golfe de Gabes. Rapp Comm Int Mer Medit. 36: 246
Bugel SM, White LA, Cooper KR (2009) Impaired reproductive health of killifish (Fundulus heteroclitus) inhabiting Newark Bay NJ, a chronically contaminated estuary. Aquat Toxicol 96(3):182–193
Chaouch, H.,Ben Abdallah, O.,Ghorbel, M., & Jarboui,O. (2013). Reproductive biology of the annular seabream,Diplodus annularis(Linnaeus, 1758), in the Gulf of Gabes( centel Mediterranean). Jour of App Ichthyol 29:796–800
Damak M, Frontalini F, Elleuch B, Kallel M (2018) Benthic foraminiferal assemblages as pollution proxies along the coastal fringe of the Monastir Bay (Tunisia). J Afr Earth Sci. https://doi.org/10.1016/j.jafrearsci.2018.11.013
Damak M, Fourati R, Ellech B, Kallel M (2019) Assessment of organic and metallic contamination in the surface sediment of Monastir Bay (eastern Tunisia): spatial distribution, potential sources, and ecological risk assessment. Mar Pollut Bull 149:110500
Darilmaz E, Kucuksezgin F (2012) Distribution of aliphatic and aromatic hydrocarbons in red mullet (Mullus barbatus) and annular sea bream (Diplodus annularis) from the Izmir Bay (eastern Aegean). Bull Environ Contam Toxicol 88:283–289
Deepa KPA, Kumar KV, Kottnis O, Nikkia R, Bineesh KK, Hashim M, Saravanane N, Sudhakar M (2019) Population variations of opal fish, Bembrops caudimacula Steindachner, 1876 from Arabian Sea and Andaman Sea: evidence from otolith morphometry. Reg Stud Mar Sci 25:100466
Donnaloia M, Zupa W, Arnesano M, Neglia C, Facchini MT, Carbonara P (2017) Reproductive biology of Diplodus annularis (linnaeus, 1758) in the central-western mediterranean seas. Biol Mar Mediterr 24(1):182–183
Dragun Z, Filipović Marijić V, Kapetanović D, Valić D, Vardić Smrzlić I, Krasnići N, Strižak Ž, Kurtović B, Teskeredžić E, Raspor B (2013) Assessment of general condition of fish inhabiting a moderately contaminated aquatic environment. Environ Sci Pollut Res Int 20(7):4954–4968
Fontana A, Le Guen JC (1969) Étude de la maturité sexuelle et de la fécondité de Pseudotolithus (fonticulus) elongatus. ORSTOM, série Océanographique, 7(3):9–19.
García-Rubies A, Macpherson E, (1995) Substrate use and temporal pattern of recruitment in juvenile fishes of the Mediterranean littoral. Mar Biol 124:35–42. https://doi.org/10.1007/BF00349144.
Gerbron M, Geraudie P, Fernandes D, Rotchell JM, Porte C, Minier C (2014) Evidence of altered fertility in female roach (Rutilus rutilus) from the River Seine (France). Environ Pollut 191:85–61
Gharred T, Helaoui A, Mannai R, Jebali J (2019) Effect of multiple pollution on the cholinesterase activity, morphometry and reproduction performance of Patella caerulea collected from eastern Tunisian coasts. Cah Biol Mar 60:11–20
Ghedira J, Jebali J, Bouraoui Z, Banni M, Guerbej H, Boussetta H (2010) Metallothionein and metal levels in liver, gills and kidney of Sparus aurata exposed to sublethal doses of cadmium and copper. Fish Physiol Biochem 36:101–107
Gonul LT, Kucuksezgin F (2007) Mercury accumulation and speciation in the muscle of red mullet (Mullus barbatus) and annular sea bream (Diplodus annularis) from Izmir Bay (eastern Aegean). Mar Pollut Bull 54:1962–1989
Gordoa A, Molí B (1997) Age and growth of the sparids Diplodus vulgaris D. sargus and D. annularis in adult populations and the differences in their juvenile growth patterns in the north-western Mediterranean Sea. Fish Res 33(1–3):123–129
Gulland JA (1969) Manual methods for fish stock assessment. Part I. Fish population analysis. FAO Fish sci 4:154
Hauser-Davis RA, Lavandier RC, Bastos FF, Oliveira TF, Ribeiro CA, Ziolli RL, de Campos RC (2012) Alterations in morphometric and organosomatic indices and histopathological analyses indicative of environmental contamination in millet, Mugil liza, from southeastern. Bull Environ Contam Toxicol 89(6):1154–1160
Hossain M, Nahiduzzaman MD, Saha D, Khanam H, Alam M (2010) Landmark-based morphometric and meristic variations of the endangered carp, Kalibaus Labeo calbasu, from stocks of two isolated Rivers, the Jamuna and Halda, and a hatchery. Zoological Stadies 49(4):556–563
Iwata H, Ukai Y (2002) SHAPE: a computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J Hered 93:384–385
Jebali J, Ben-Khedher S, Ghedira J, Kamel N, Boussetta H (2011) Integrated assessment of biochemical responses in Mediterranean crab (Carcinus maenas) collected from Monastir Bay Tunisia. J Environ Sci 23(10):1714–1720
Jebali J, Banni M, Boussetta H (2012). Biochemical biomarkers in aquatic ecotoxicology: fundamental mechanisms, application, and perspectives. NOVA Science Publishers, Inc. advances in Environmental Research, 23: 359 pp.
Jebali J, Chicano-Gálvez E, Banni M, Guerbej H, Boussetta H, López-Barea J, Alhama J (2013a) Biochemical responses in seabream (Sparus aurata) caged in-field or exposed to benzo(a)pyrene and paraquat. Characterization of glutathione S-transferases. Ecotoxicol Environ Saf 88:169–177
Jebali J, Sabbagh M, Banni M, Kamel N, Ben-Khedher S, M'hamdi N, Boussetta H (2013b) Multiple biomarkers of pollution effects in Solea solea fish on the Tunisia coastline. Environ Sci Pollut Res 20(6):3812–3821
Jebali J, Chicano-Gálvez E, Fernandez-Cinal R, Banni M, Chouba L, Boussetta H, López-Barea J, Alhama J (2014) Proteomic analysis in caged Mediterranean crab (Carcinus maenas) and chemical contaminant exposure in Téboulba harbour, Tunisia. Ecotoxicol Environ Saf 100:15–26
Johnson LL, Lomax DP, Myers MS, Olson OP, Sol SY, O'Neill SM, West J, Collier TK (2008) Xenoestrogen exposure and effects in English sole (Parophrys vetulus) from Puget Sound, WA. Aquat Toxicol 88(1):29–38
Kaouèche M, Bahri-Sfar L, Hammami I, Ben Hassine OK (2017) Morphometric variations in white seabream Diplodus sargus (Linneus, 1758) populations along the Tunisian coast. Oceanologia 59:129–138
Khenfech NH, Boumaiza M (2011) Morphological abnormalities in the annular sea bream M Diplodus annularis (Osteichthyes: Sparidae) from the lagoon of Bizerte (Notheastern Tunisia, Central Mediterranean). Annales, Series Historia Naturalis 21:161–166
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11:353–357
Kuhl FP, Giardina CR (1982) Elliptic Fourier features of a closed contour. Com G Ima Proc 18:236–258
March D, Alós J, Grau A, Palmer M (2011) Short-term residence and movement patterns of the annular seabream Diplodus annularis in a temperate marine reserve. Estuar Coast Shelf Sci 92:581–587
Matic-Skoko S, Kraljevisc M, Dulcic J, Jardas I (2007) Age, growth, mortality and yield-per-recruit for annular seabrean (Diplodus annularis L.) from the eastern middle Adriatic Sea. J Appel Ichthyol 23:152–157
Mills LJ, Chichester C (2005) Review of evidence: are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci Total Environ 343(1–3):1–34
Montaño-Campaz ML, Gomes-Dias L, Toro Restrepo BE, Garcıa-Merchan VH (2019) Incidence of deformities and variation in shape of mentum and wing of Chironomus columbiensis (Diptera, Chironomidae) as tools to assess aquatic contamination. PLoS One 14(1):e0210348
Morat F, Betoulle S, Robert M, Thailly AF, Biagianti-Risbourg S, Lecomte-Finiger R (2008) What can otolith examination tell us about the level of perturbations of salmonid fish from the Kerguelen Islands? Ecol Freshw Fish 17:617–627
Mouine N, Ktari M, Chakroun-Marzouk N (2012) Reproductive biology of four Diplodus species Diplodus vulgaris, D. annularis, D. sargus sargus and D.puntazzo (Sparidae) in the gulf of Tunis (Central Mediterranean). J Mar Biol Assoc UK 92(3):623–631
Nouira T, Tagorti MA, Budzinski H, Etchebert H, Boussetta H (2012) Polycyclic aromatic hydrocarbons (PAHs) in surface sediments of Monastir Bay (Tunisia, Central Mediterranean): distribution, origin and seasonal variations. Int J Environ Anal Chem 93:1470–1483. https://doi.org/10.1080/03067319.2012.746325
Nouira T, Risso C, Chouba L, Budzinski H, and Boussetta H (2013) Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in surface sediments from Monastir Bay (Tunisia, Central Mediterranean): occurrence, distribution and seasonal variations,” Chemosphere 93(3)487–493, 2013. https://doi.org/10.1016/j.chemosphere.2013.06.017
Pajuello J, Lorenzo J (2001) Biology of the annular seabream, Diplodus annularis(Sparidae), in the coastal waters of the canary islands. J Appl Ichihyol 17:121–125
Pajuelo JG, Lorenzo JM (2002) Growth and age estimation of Diplodus sargus cadenati (Sparidae) off the Canary Islands. Fish Res 59:93–100
Prado PS, Pinheiro APB, Bazzoli N, Rizzo E (2014) Reproductive biomarkers responses induced by xenoestrogens in the characid fish Astyanax fasciatus inhabiting a South American reservoir: an integrated field and laboratory approach. Environ Res 131:165–173
Sanchez W, Sremski W, Piccini B, Palluel O, Maillot-Maréchal E, Betoulle S, Jaffal A, Aït-Aïssa S, Brion F, Thybaud E, Hinfray N, Porcher JM (2011) Adverse effects in wild fish living downstream from pharmaceutical manufacture discharges. Environ Int 2011 37(8):1342–1348
Sassi R, Souissi F, Soussi N, Boukaaba M, Belayouni H (1998) Organic matter geochemistry to analyse the degradation of the Monastir-Ksibet el Mediouni littoral (eastern Tunisia). Earth Planet Sci 327:303–308
Schreck CB (2010) Stress and fish reproduction: the roles of allostasis and hormesis. Gen Comp Endocrinol 165:549–556
Shinn C, Blanchet S, Loot G, Lek S, Grenouillet G (2015) Phenotypic variation as an indicator of pesticide stress in gudgeon: accounting for confounding factors in the wild. Sci Total Environ 538:733–742
Silva A (2003) Morphometric variation among sardine (Sardina pilchardus) populations from the northeastern Atlantic and the western Mediterranean. ICES J Marine Sci 60:1352–1360
Solé M, Raldua D, Piferrer F, Barceló D, Porte C (2003) Feminization of wild carp, Cyprinus carpio, in a polluted environment: plasma steroid hormones, gonadal morphology and xenobiotic metabolizing system. Comp Biochem Physiol Toxicol Pharmacol 136(2):145–156
Straüss RE, Bookstein FL (1982) The truss: body from reconstructions in morphometrics. Syst Zool 31:113–135
Toro B, Navarro JM, Palma-flemming H (2003) Relationship between bioenergetics responses and organic pollutants in the giant mussel, Chromitilus chorus (Molusca mytilidae). Aquat Toxicol 63:257–269
Turki O, M’hetli M, Chriki A, Kraem MM (2009) Characterization and phenotypic and biological variation in the two sexes of pikeperch Sander lucioperca (L., 1758) (Teleostei, Percidae) in the Nebhana reservoir (Central Tunisia). Bull. Inst. Natn. Scien. Tech. Mer de Salammbô, Vol. 36:75–86
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Van Poorten BT, Walters CJ, (2016) How can bioenergetics help us predict changes in fish growth patterns? Fish. Res. 180: 23–30. https://doi.org/10.1016/j.fishres.2015.07.031.
Vasil’eva ED, Vasil’ev VP (2010) Genetic and environmental variations in quantitative characters in fishes: a comparative analysis of monoclonal triploid and bisexual tetraploid spined loaches (Cobitis, Cobitidae). J Ichthyol 50:960–968
Zelditch ML, Swiderski DL, David Sheets H (2012) Geometric morphometrics for biologists: aprimer. Academic Press, 488p
Zrafi I, Bakhrouf A, Rouabhia M, Saidane-Mosbahi D (2013) Aliphatic and aromatic biomarkers for petroleum hydrocarbon monitoring in Khniss Tunisian-coast, (Mediterranean Sea) Procedia Environmental Sciences, 18: 211–220
Funding
This work was supported by a fund from the Ministry of Higher Education and Scientific Research, University of Monastir, Tunisia (Research Laboratory Bioresources: Integrative Biology and Valorisation, High Institute of Biotechnology of Monastir, Tunisia).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gharred, T., Mannai, R., Belgacem, M. et al. Incidence of morphometry variation, growth alteration, and reproduction performance of the annular sea bream (Diplodus annularis) as effective tools to assess marine contamination: how useful is a multi-biotimarkers approach?. Environ Sci Pollut Res 27, 4075–4088 (2020). https://doi.org/10.1007/s11356-019-07014-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07014-9