Abstract
Thiamethoxam (TMX), a second-generation neonicotinoid, is extensively used to control numerous pests that infest crops. We investigated the effects of TMX (10, 20, 30, 40, and 50 μg/mL for 24, 48, 72, and 96 h) on biomarkers such as antioxidant enzymes (superoxide dismutase (SOD) and catalase (CAT)); malondialdehyde (MDA), protein, lipid, and carbohydrate levels; micronucleus formation; and total hemocyte count in a model organism, Galleria mellonella L. SOD and CAT activities significantly decreased after 72 and 96 h of treatment at all TMX concentrations compared with control. MDA level increased following treatment with all TMX doses, with the exception of that following treatment with the lowest dose (10 μg/mL) at all tested treatment durations. Lipid and carbohydrate levels significantly decreased following treatment with high doses of TMX (40 and 50 μg/mL) after 48, 72, and 96 h. Micronucleated cell number significantly increased following treatment with all TMX doses at all tested treatment durations, except with 10 μg/mL of TMX for 24 h, when compared with control. During the first 72 h, total hemocyte count significantly decreased following treatment with 20-, 30-, 40-, and 50-μg/mL TMX; however, it was significantly reduced at all doses of TMX after 96 h. These results suggest that TMX can induce immunotoxicity, oxidative stress, and genotoxicity in a potential target and also in the model organism, G. mellonella. In addition, our study provides additional information regarding the prospective toxic effects of TMX.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of neonicotinoids such as thiamethoxam (TMX), imidacloprid, and clothianidin has rapidly increased owing to the broad-spectrum insecticidal activity, relatively selective toxicity to insect pests, and low toxicological effects on non-target organisms of these neonicotinoids (Tomizawa and Casida 2003; Yan et al. 2016). Nevertheless, the use of neonicotinoid insecticides is suspected to be associated with negative effects on bees, which is why these insecticides have been banned by the European Union since 2013 (European Commission 2013).
TMX is a second-generation neonicotinoid that has an agonistic effect on nicotinic acetylcholine receptors (nAChRs) and blocks neurotransmission in both insects and mammals (Tomizawa et al. 2000; Casida and Durkin 2013). Owing to the structural differences in nAChR receptors between mammals and insects, it has been suggested that the toxic effect of TMX on mammals is limited (Tomizawa and Casida 2003; Zhang et al. 2018).
Several studies on TMX have demonstrated that it induces changes in cell structures and apoptosis in the midgut of honeybees (Gregorc and Ellis 2011; Catae et al. 2018); in antioxidant responses in zebrafish (Yan et al. 2016) and rats (Keshta et al. 2016); in immune components in honeybee queens (Brandt et al. 2017); in hemato-biochemical parameters in cockerels (Gul et al. 2017); and in genotoxic biomarkers in snails and humans (Gad et al. 2016; Guo et al. 2018).
Biomarkers are powerful indicators for demonstrating the toxic effects of insecticides. Oxidative stress parameters such as superoxide dismutase (SOD), which scavenges superoxide radicals, and catalase (CAT), which removes H2O2, protect cells from oxidative stress. Malondialdehyde (MDA) is an important metabolite resulting from lipid peroxidation; thus, any increase in MDA level is a certain indicator of damage to the cell membrane. Changes in the activities of these enzymes and an increase in MDA level are reliable indicators to determine the toxic effects of insecticides in insects. Changes in antioxidant enzyme activities and increases in MDA level, resulting from insecticide exposure, have been demonstrated in insects (Buyukguzel 2006; Buyukguzel 2009; Yucel and Kayis 2019).
Hemocytes play various roles in phagocytosis, nodule and capsule formation in cellular defense, the transport of hormones and nutrients, and detoxification of metabolites (Parakash 2008). Therefore, they are of great importance in insect physiology studies; changes in the number and structure of hemocytes have been used to demonstrate the effects of insecticides (Juhel et al. 2017). For example, imidacloprid, thiacloprid, and clothianidin change the total hemocyte number in insects (Kurt and Kayis 2015; Brandt et al. 2017; Yucel and Kayis 2019).
The micronucleus (MN) is an extra-nuclear body that forms during mitosis and is often used to show the genotoxic effects of toxic substances on DNA (Guo et al. 2018). It has been proven that pesticides can increase MN formation in certain cells of different organisms, including insect hemocytes (Karabay and Oguz 2005; Bolognesi et al. 2011; Kataria et al. 2016; Yucel and Kayis 2019).
Proteins, lipids, and carbohydrates are structural molecules playing significant roles in the reproduction and expansion of insect populations. However, they are also crucial energy sources (Olson et al. 2000; Hogervorst et al. 2007); specifically, these molecules are used as energy sources in detoxification processes. Therefore, changes in their quantities are considered important biomarkers of toxic stress (Maryanski et al. 2002; Emre et al. 2013; Yucel and Kayis 2019). For example, insecticides cause depletions in the lipid levels of Pimpla turionellae (Sak et al. 2006) and Spodoptera littoralis (Rashwan 2013); and in the carbohydrate level of Drosophila melanogaster (Kissoum and Soltani 2016).
Galleria mellonella L. is a pest in the apiculture industry; however, it has also long been used as a host for the production of parasitoid and predator species in biological control programs (Ellis et al. 2013). Because insect and mammalian models of the innate immune response are positively correlated in many ways, many studies have been conducted on G. mellonella as an alternative model organism in the past two decades (Kavanagh and Reeves 2007; Tsai et al. 2016; Yucel and Kayis 2019). G. mellonella larvae offer a number of advantages over their mammalian counterparts: their production is labor and cost-effective; they do not require special laboratory conditions; and they require neither expensive equipment nor ethics committee permits (Tsai et al. 2016).
Previous studies on TMX have measured its effects on different parameters within different organisms. For example, Yan et al. (2016) focused on antioxidant enzyme activities in Danio rerio; Catae et al. (2014) looked at the cytotoxic effects of TMX on midgut and Malpighian tubules in Apis mellifera; and Calderón-Segura et al. (2012) investigated the effects of TMX on human micronucleus formation. Therefore, we focused on studying the effects of TMX on multiple biomarkers using a single-model organism, G. mellonella.
Materials and methods
Thiamethoxam
TMX (Actara 240 SC, Syngenta) was obtained from a local market in Adiyaman, Turkey.
Insect rearing
G. mellonella larvae were reared under laboratory conditions (30 °C ± 2 °C, 70% ± 5% RH, and all-day dark period) with Bronskill’s (1961) diet.
Experimental design
In all experiments, the last instar larvae (250–300 mg) of G. mellonella were used. After determining the LD50 value of TMX according to Finney (1971), 10 μL of phosphate buffer solution (PBS) was used as a control and equal volumes of PBS containing 10, 20, 30, 40, and 50 μg/mL TMX were injected into the hemocoel of larvae using a Hamilton syringe. The larvae were kept on a synthetic diet for 24, 48, 72, and 96 h. At the end of each time duration, the larvae were weighed and kept at − 80 °C until the subsequent experiments were performed. Each experiment was performed with five replicates.
Homogenization
For the determination of protein, SOD and CAT activities, and MDA levels, four larvae (250–300 mg) were pooled together and homogenized at the rate of 1:20 (w/v) in phosphate-buffered saline (50 mM, pH = 7.4). Each replicate contained 0.001 g of phenylthiourea to prevent the melanization. The homogenate was centrifuged at 10,000×g and 4 °C, and the supernatant was used for the protein, antioxidant enzyme activities, and MDA analyses.
For the determination of total carbohydrate and lipid levels, one larva was homogenized in 2 mL of sodium sulfate (2%) with phenylthiourea (0.001 g) at 24000 rpm for 5 min. Eight milliliters of a chloroform/methanol mixture (1:2) was added into each tube, and then, the tubes were centrifuged at 9000×g for 10 min. The supernatant was used for subsequent analyses.
SOD activity
SOD activity was determined according to Sun et al. (1988). This method is based on the inhibition of the reaction between nitroblue tetrazolium and superoxide radicals by SOD. Fifty microliters of sample and 25 μL of xanthine oxidase were mixed with 1.425 mL of SOD reagent. The mixture was incubated for 20 min at room temperature; then, the reaction was stopped by adding copper chloride (50 μL). The SOD activity was measured at 560 nm spectrophotometrically and expressed as U/mg protein.
CAT activity
Catalase activity was determined according to the method of Aebi (1984). Two hundred microliters of sample was mixed with 3 mL of hydrogen peroxide (30 mM) and then shaken rapidly. The decreasing absorbance value was measured kinetically at 240 nm at 30-s intervals for 1 min. Catalase activity was expressed as U/mg protein.
MDA levels
MDA levels were measured by the thiobarbituric acid (TBA) assay following the method of Bar-Or et al. (2001). A 250-μL aliquot of the sample was mixed with 125 μL trichloroacetic acid (TCA) (25%) in a microcentrifuge tube, and the mixture was centrifuged at 15000 rpm for 10 min at 4 °C. After removing the supernatant, 200 μL of TBA (0.8%) was added to each tube. The mixture was incubated for 60 min in a water bath at 90 °C, and the absorbance of samples was measured at 535 nm against a blank sample. The level of MDA was expressed as nmol/mg protein.
Total protein levels
Total proteins were determined according to the method of Lowry et al. (1951). Three-hundred-microliter aliquots of supernatant were placed into test tubes, and 3 mL of a reactive solution that were prepared according to the method of Lowry et al. (1951) was added to the tubes. Samples were incubated for 15 min at room temperature. Finally, 300 μL of the Folin–Ciocalteu reagent was added to the tubes. After 30 min, the absorbance value of each mixture was measured spectrophotometrically at 750 nm. Bovine serum albumin (0.1%) was used as a standard and the amount of total protein was expressed as mg/100 mg.
Total lipid and carbohydrate levels
Total lipid and carbohydrate levels were determined according to Van Handel’s (1985a, 1985b) methods.
For lipid analysis, 200 μL of supernatant was kept in a water bath at 90 °C until the chloroform/methanol solution has been completely evaporated. Forty microliters of concentrated sulfuric acid was added to the dried supernatant, and then, the mixture was kept in a water bath for 2 min at 90 °C. After cooling, 960 μL of vanillin phosphoric acid solution was added and mixed. The mixtures were incubated at room temperature for 30 min; then, the absorbances were measured spectrophotometrically at 525 nm. Soy oil (0.1%) was used as a standard and the total amount of lipid was expressed as mg/100 mg.
For carbohydrate analysis, 200 μL of supernatant was kept in a water bath at 90 °C until the chloroform/methanol solution had completely evaporated. After cooling, 950 μL of anthrone solution was added. The sample was kept in a water bath for 15 min at 90 °C. The absorbances of samples were measured spectrophotometrically at 625 nm. Glucose (0.1%) was used as a standard and the total amount of carbohydrate was expressed as mg/100 mg.
Micronucleus assay
The method of Venier et al. (1997) was used to identify micronuclei. Briefly, three larvae were pierced with a sterile needle and the hemolymph was spread on a glass slide. After air-drying for 15 min, the smear was fixed with methanol for 5 min; then, the slides were stained with Giemsa (10%) for 10 min. Subsequently, the slides were rinsed in distilled water. A total of 1000 hemocytes were scored per slide in every replicate. This assay was performed in five replicates.
Total hemocyte counts
The total hemocyte count was calculated using the method of Jones (1962). Four G. mellonella larvae were anesthetized by cold treatment and pierced with a sterile needle. The resulting hemolymph was mixed with the Tauber–Yeager solution (Tauber and Yeager 1936) at a rate of 1:10; then, 10 μL of the dilution was placed in a Neubauer hemocytometer and hemocytes were counted using a light microscope (Olympus CX21). Total hemocyte counts were calculated according to Jones (1962), with five replicates.
Statistical analysis
The LD50 value of TMX at 96 h was determined using Finney’s probit analysis (Finney 1971). Means, standard errors, and significance levels were calculated from five independent replicates. The results are presented as means ± SE.
Homogeneity of variance, checked using Levene’s test, showed homogeneous data subsets. Therefore, treatment effects were tested using one-way ANOVA and the Student–Newman–Keuls (SNK) test was conducted to evaluate the differences between means using SPSS 13.0. The significance level was set at 0.05. The relationships between TMX and the amounts of proteins, lipids, and carbohydrates; SOD and CAT activities; MDA levels; micronucleus formation; and total hemocyte counts were assessed using Pearson’s correlation.
Results
Antioxidant enzyme activities and MDA level
Antioxidant enzyme activities and MDA levels of the different treatments are shown in Table 1. After 24 h, only the 50-μg TMX dose resulted in a decrease in SOD activity (df = 5; F = 15.923; P < 0.05). At higher doses of TMX (40 and 50 μg), SOD activity significantly decreased compared with control after 48 h (df = 5; F = 33.157; P < 0.05). All TMX doses caused a decrease in SOD activity when compared with control after 72 h (df = 5; F = 26.848; P < 0.05) and 96 h (df = 5; F = 36.459; P < 0.05). Similar to SOD, CAT enzyme activity was significantly reduced in response to increased concentrations of TMX. Except for the lowest dose of TMX (10 μg) after 24 h (0.063 ± 0.001) (df = 5; F = 244.594; P > 0.05), all doses of TMX caused a reduction in CAT activity when compared with control at all tested treatment durations (Table 1).
MDA levels increased significantly in all of the tested doses of TMX in all of the tested treatment durations when compared with control, with the exception of the 10-μg dose after 24, 48, and 72 h (Table 1). The negative correlation between MDA levels and antioxidant enzyme activities was observed at all tested treatment durations (Table 3).
Protein, lipid, and carbohydrate levels
There were no significant differences between the total protein levels of the control and TMX-treated larvae after 24, 48, 72, and 96 h (2.194, 2.342, 2.511, and 2.680 mg/100 mg, respectively) (P > 0.05) The primary energy sources (lipids and carbohydrates) were significantly affected by TMX. The amounts of lipids and carbohydrates decreased significantly at the highest dose of TMX (50 μg) after 24 h of treatment (df = 5, F = 9.959, P < 0.05; and df = 5, F = 50.863, P < 0.05, respectively). At 48 h, total lipid levels decreased significantly at all doses of TMX, except for the dose of 30 μg compared with control (df = 5; F = 16.145; P < 0.05). Total carbohydrate levels also decreased significantly at TMX doses of 20, 40, and 50 μg compared with control (df = 5; F = 92.871; P < 0.05). Furthermore, total lipid and carbohydrate levels also decreased significantly at TMX doses of 20, 30, 40, and 50 μg compared with control after 72 h (df = 5; F = 49.225; P < 0.05 and df = 5; F = 152.193; P < 0.05, respectively) and after 96 h (df = 5; F = 88.050; P < 0.05 and df = 5; F = 127.881; P < 0.05, respectively) (Table 2).
Micronucleus formation
TMX caused an increase in micronucleus formation in hemocytes. All tested doses of TMX significantly increased the number of micronucleated cells at all tested treatment durations except for 10 μg TMX after 24 h (1.00 ± 0.32) when compared with the control (0.20 ± 0.20) (df = 5; F = 30.678; P > 0.05) (Fig. 1). There was a high positive correlation between MDA level and micronucleus formation at all tested treatment durations (Table 3).
Total hemocyte count
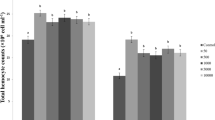
Compared with control, TMX caused a decline in THC after 24, 48, and 72 h, except for the 10-μg dose of TMX; meanwhile, all doses of TMX caused a significant reduction in THC compared with control after 96 h (df = 5, F = 12.957; P < 0.05) (Fig. 2). THC was negatively correlated with MDA level (Table 3).
Discussion
G. mellonella antioxidant enzyme activities, energy source levels, and THCs decreased significantly; while lipid peroxidation levels and micronucleus formation increased significantly in response to TMX, in a dose-dependent manner (Table 1).
Previous studies have shown significant changes in antioxidant enzyme activities in insects exposed to various pesticide formulations (Buyukguzel 2009; Aslanturk et al. 2011; Kayis et al. 2015). The increase in antioxidant enzyme activities is an important reaction to xenobiotic-mediated toxicity that occurs in response to oxidative stress due to exposure to insecticides (Al-Barty 2014; Muthusamy and Rajakumar 2016). Nevertheless, Cheung et al. (2001) and Yonar (2013) stated that this cannot be assumed to be a general rule because enzyme activities may increase or decrease, depending on the concentration of the stress factor or the duration of administration.
Pesticides may enter the redox cycle directly and may cause oxidative stress by increasing the amount of reactive oxygen species (ROS). In addition, they can cause oxidative stress without entering the redox cycle by inhibiting the antioxidant enzymes (Lushchak 2016). In our study, only high doses of TMX caused a decrease in SOD activity after 24 and 48 h; meanwhile, all TMX doses decreased SOD activity significantly after 72 and 96 h. CAT activity was positively correlated with SOD activity (Table 3). It was reduced in all TMX doses at all treatment durations, except for the lowest dose (10 μg) of TMX for 24 h.
SOD is the first line of defense against ROS, and it is induced as the main cellular response to superoxide anion production (Ighodaro and Akinloye 2018). The superoxide radical is formed by the addition of an electron to molecular oxygen and is dismutated by the SOD enzyme, leading to the formation of hydrogen peroxide. The hydrogen peroxide formed by SOD enzyme activity is converted to water and molecular oxygen by catalase (Nordberg and Arnér 2001; Wickens 2001). However, any excess amount of ROS can cause direct inhibition of antioxidant enzyme activities (Yonar 2013; Han et al. 2016; Yan et al. 2016). Such decreases in antioxidant enzyme activities are caused by the oxidation of the cysteine residues in antioxidant enzymes by ROS (Dimitrova et al. 1994; Bagnyukova et al. 2006).
In our study, the SOD activity may have been inhibited by excess ROS, when it was present at high concentrations. Given the positive correlation between SOD and CAT enzyme activities, CAT activity may have also been directly inhibited by ROS; it is also possible that decreased CAT activity may involve the reduction of H2O2 production as a result of inhibition of SOD activity. Catalase activity is directly regulated by H2O2 concentration, and because of the high Km values of CAT for H2O2, the enzyme is inefficient at low concentrations of H2O2. In insects, low concentrations of H2O2 are scavenged by ascorbate peroxidase (APOX) and dehydro-ascorbate reductase (DHAR) (Summers and Felton 1993; Mathews et al. 1997). Zhang et al. (2018) reported that high doses of fluoxastrobin cause the formation of excess amounts of reactive oxygen derivatives, resulting in lipid peroxidation due to the inhibition of SOD and CAT enzymes by their substrates. Gultekin et al. (2000) showed that chlorpyrifos-ethyl administered in vitro increased ROS production and therefore decreased SOD and CAT enzyme activities. Yan et al. (2016) and Keshta et al. (2016) demonstrated that TMX reduces both SOD and CAT activities in fish and rats, respectively. Similarly, findings that antioxidant enzyme activities decrease in response to different pesticides in different organisms (Ge et al. 2015; Lushchak 2016; Noshy et al. 2017; Velisek and Stara 2018) agree with the results of our study.
If an organism’s antioxidant defense system is insufficient, then, ROS may cause lipid peroxidation. MDA, a product of lipid peroxidation, may cause a loss of membrane permeability and function, apoptosis, and necrosis in the cell (Toroser et al. 2007). Moreover, MDA levels can also reflect the level of lipid peroxidation indirectly (Kanbur et al. 2008; Zhang et al. 2014). In the present study, all doses of TMX (except for 10 μg), administered for up to 72 h, caused a dose-dependent increase in MDA levels in G. mellonella. Given that antioxidant enzyme activities (SOD and CAT) are negatively correlated to MDA levels (Table 3), then, increased MDA levels may be related to increased levels of ROS, causing a decrease in antioxidant enzyme activities, which are ultimately induced by TMX. Yucel and Kayis (2019) demonstrated that MDA levels are significantly increased in G. mellonella exposed to imidacloprid. A similar increase in MDA level was observed in Danio rerio (Ge et al. 2015; Shuklaa et al. 2017; Zhang et al. 2018) after imidacloprid, TMX, and fluoxastrobin treatments.
Energy molecules, such as proteins, lipids, and carbohydrates, are affected by ROS arising from toxic substances; the synthesis and utilization of these molecules may alter under toxic stress (Saleem et al. 1998). These molecules play central roles in growth, development, and reproduction; however, they are also used as sources of energy during the biotransformation of toxic substances (Olson et al. 2000; Hogervorst et al. 2007). For example, the energy derived from these molecules is used to produce enzymes and heat shock proteins involved in detoxification and removal of toxic substances (Maryanski et al. 2002).
In the present study, all doses of TMX (except for 10 μg) caused dose-dependent decreases in lipid and carbohydrate levels after 48, 72, and 96 h of treatment in G. mellonella. Similar decreases in lipid and carbohydrate levels were observed in Oreochromis mossambicus, Spodoptera littoralis, P. turionellae, and Leptinotarsa decemlineata by Raj and Joseph (2015), Rashwan (2013), Sak et al. (2006), and Fotouhi et al. (2015), respectively, after insecticide stress. In this study, lipid and carbohydrate levels were positively correlated with SOD and CAT activities, while they were all negatively correlated with MDA. Given the observed correlations between lipid and carbohydrate levels and oxidative stress biomarkers, we find it likely that the decreased levels of lipids and carbohydrates may be related to the synthesis of detoxification enzymes or to the damage of lipids and carbohydrates by ROS (Birben et al. 2012).
Micronucleus formation reflects damage to the genetic material; thus, it has been used as a biomarker for the genotoxic effects induced by environmental contaminants (Shimizu 2011; Guo et al. 2018).
There are very few studies on MN formation in insects in response to insecticides (Uckan and Sak 2010; Kurt and Kayis 2015; Kalita et al. 2016; Yucel and Kayis 2019). Studies have been shown that neonicotinoids such as imidacloprid (Stivaktakis et al. 2010; Ansoar-Rodriguez et al. 2015; Kataria et al. 2016; Yucel and Kayis 2019), acetamiprid (Kocaman and Topaktas 2007; Cavas et al. 2014), and clothianidin (Calderón-Segura et al. 2015) cause an increase in MN formation in different organisms.
Although there may not be enough evidence to conclude confidently that TMX has a genotoxic effect (Hertner 1995; Sinha and Thaker 2013), studies conducted in recent years have shown that TMX induces DNA damage and MN formation in human neuroblastoma cells, in mice, and in the white garden snail (Salema et al. 2014; Gad et al. 2016; Senyildiz et al. 2018). In the present study, a significant increase in the abundance of micronucleated cells was observed at all tested doses (except for 10 μg for 24 h) of TMX. Yan et al. (2016) stated that increased ROS levels and excess MDA levels are the main agents of DNA damage and there is a positive correlation between MDA levels and micronucleus formation. We found that MDA levels are positively correlated with micronucleus formation; and antioxidant enzyme activities are negatively correlated to micronucleus formation. We suggest that the increase in abundance of micronucleated cells is due to DNA damage caused by increased levels of ROS that cannot be eliminated due to decreased activities of antioxidant enzymes and increased MDA level.
In addition to being an important component of the immune system, hemocytes play important roles in the detoxification of toxic substances and the transport of hormones and metabolites (Parakash 2008).
TMX caused a significant reduction in THCs of G. mellonella larvae, especially at doses higher than 10 μg. Similar decreases in THCs were observed in Apis mellifera exposed to imidacloprid, thiacloprid, and clothianidin (Brandt et al. 2017); and in Apis dorsata and G. mellonella exposed to imidacloprid (Perveen and Ahmad 2017; Yucel and Kayis 2019).
Pesticides can inhibit hematopoietic function (Zhu et al. 2012) and mitotic activity (Rajak et al. 2015) and promote apoptosis (Gregorc and Ellis 2011; Wu et al. 2015) in insect cells. In addition, studies have shown that TMX may cause structural deformation of insect cells (Oliveira et al. 2013; Catae et al. 2014). Because insects have an open circulatory system, circulating hemocytes can be targeted by toxic substances entering their body. The decrease in THCs may be due to either necrosis resulting from cellular damage or the induction of apoptosis, resulting from elevated levels of ROS due to TMX exposure. In addition, Salema et al. (2014) reported that the cytotoxic effect of TMX on mice is due to high levels of MDA. This agrees with the negative correlation between THC and MDA levels in our study; thus, the decrease in THC may be due to elevated levels of MDA.
Conclusions
According to present data, TMX causes significant alterations on oxidative, biochemical, genotoxic, and immunotoxic biomarkers in G. mellonella. These effects may be related to the disruptive effect of TMX on balance between the production of free radicals and antioxidant defense system. All of the changes in the biomarkers could also be used for further inspection of toxic effects of TMX in various organisms.
In the light of present findings, it can be concluded that TMX has a potential risk to non-target organisms such as parasitoid and predator insects, and honeybees. Even the observed findings within the model organism, G. mellonella, may reflect the potential toxic effects of TMX on mammalian organisms. Further investigations on the genetic effects of TMX at the molecular level should be conducted to determine the role of specific genes in the synthesis of antioxidant enzymes and the effects of TMX on cellular pathways leading to apoptosis and necrosis, thus leading to a better understanding in the toxicity mechanism of TMX.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Al-Barty AMF (2014) Laboratory evaluation of superoxide dismutase (SOD) of methylamine avermectin control of the rice red weevil (Sitophilus oryzae L.) in stored wheat grains. J Chem Pharm Res 6(4):979–983
Ansoar-Rodriguez Y, Christofoletti C, Marcato A, Correia J, Bueno O, Malaspina O, Fontanetti C (2015) Genotoxic potential of the insecticide imidacloprid in a non-target organism (Oreochromis niloticus- Pisces). J Environ Protect 6(12):1360–1367. https://doi.org/10.4236/jep.2015.612118
Aslanturk A, Kalender S, Uzunhisarcikli M, Kalender Y (2011) Effects of methidathion on antioxidant enzyme activities and malondialdehyde level in midgut tissues of Lymantria dispar (Lepidoptera) larvae. J Entomol Res Soc 13(3):27–38
Bagnyukova TV, Chahrak OL, Lushchak VI (2006) Coordinated response of goldfish antioxidant defenses to environmental stress. Aquatic Toxicol 78(4):325–331. https://doi.org/10.1016/j.aquatox.2006.04.005
Bar-Or D, Rael LT, Lau EP, Rao NKR, Thomas GW, Winkler JV, Yukl RL, Kingston RG, Curtis CG (2001) An analog of the human albumin N-terminus (Asp-Ala-His-Lys) prevents formation of copper induced reactive oxygen species. Biochem Biophys Res Commun 284(3):856–862. https://doi.org/10.1006/bbrc.2001.5042
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. WAO J 5(1):9–19
Bolognesi C, Creus A, Ostrosky-Wegman P, Marcos R (2011) Micronuclei and pesticide exposure. Mutagenesis 26(1):19–26. https://doi.org/10.1093/mutage/geq070
Brandt A, Goreflo A, Siede R, Meixner M, Büchler R (2017) The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J Insect Physiol 86:40–47. https://doi.org/10.1016/j.jinsphys.2016.01.001
Bronskill JK (1961) A cage to simplify the rearing of greater wax moth, Galleria mellonella (Pyralidae). J Lepid Soc 15(2):102–104
Buyukguzel K (2006) Malathion-induced oxidative stress in a parasitoid wasp: effect on adult emergence, longevity and oxidative and antioxidative response of Pimpla turionellae (Hymenoptera: Ichneumonidae). J Econ Entomol 99(4):1225–1234. https://doi.org/10.1093/jee/99.4.1225
Buyukguzel E (2009) Evidence of oxidative and antioxidative responses by Galleria mellonella larvae to malathion. J Econ Entomol 120(1):152–159. https://doi.org/10.1603/029.102.0122
Calderón-Segura ME, Gómez-Arroyo S, Villalobos-Pietrini R, Martínez-Valenzuela C, Carbajal-Carbajal-López Y, Calderón-Ezquerro MC, Cortés-Eslava J, García-Martínez R, Flores-Ramírez D, Rodríguez-Romero MI, Méndez-Pérez P, Bañuelos-Ruíz E (2012) Evaluation of genotoxic and cytotoxic effects in human peripheral blood lymphocytes exposed in vitro to neonicotinoid insecticides news. J Toxicol 1(11). https://doi.org/10.1155/2012/612647
Calderón-Segura ME, Rojas JAM, Brito MGM, TecCab M, Calderón-Ezquerro MC, Gómez-Arroyo S (2015) Genotoxicity of the neonicotinoid insecticide poncho (clothianidin) on CD1 mice based on alkaline comet and micronucleus assays. In: Larramendy ML, Soloneski S (eds) Toxicity and hazard of agrochemicals, Croatia, pp 113–125
Casida JE, Durkin HA (2013) Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Ann Rev Entomol 58:99–117. https://doi.org/. https://doi.org/10.1146/annurev-ento-120811-153645
Catae AF, Roat TC, Oliveira RA, Nocelli RE, Malaspina O (2014) Cytotoxic effects of thiamethoxam in the midgut and malpighian tubules of Africanized Apis mellifera (Hymenoptera: Apidae). Microsc Res Tech 77(4):274–281. https://doi.org/10.1002/jemt.22339
Catae AF, Roat TC, Pratavieira M, Silva Menegasso ARD, Palma MS, Malaspina O (2018) Exposure to a sublethal concentration of imidacloprid and the side effects on target and nontarget organs of Apis mellifera (Hymenoptera, Apidae). Ecotoxicology 27(2):109–121. https://doi.org/10.1007/s10646-017-1874-4
Cavas T, Cinkilic N, Vatan O, Yılmaz D (2014) Effects of fullerenol nanoparticles on acetamiprid induced cytoxicity and genotoxicity in cultured human lung fibroblasts. Pest Biochem Physiol 114:1–7. https://doi.org/10.1016/j.pestbp.2014.07.008
Cheung CCC, Zheng GJ, Li AMY, Richardson BJ, Lam PKS (2001) Relationship between tissue concentrations of polycylic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquatic Toxicol 52(3-4):189–203. https://doi.org/10.1016/S0166-445X(00)00145-4
Dimitrova MST, Tsinova V, Velcheva V (1994) Combined effect of zinc and lead on the hepatic superoxide dismutase-catalase system in carp (Cyprinus carpio). Comp Biochem Physiol C Toxicol Pharmacol 108(1):43–46. https://doi.org/10.1016/1367-8280(94)90087-6
Ellis JD, Graham JR, Mortensen A (2013) Standard methods for wax moth research. J Apic Res 52(1):1–17. https://doi.org/10.3896/IBRA.1.52.1.10
Emre I, Kayis T, Coskun M, Dursun O, Cogun HY (2013) Changes in antioxidative enzyme activity, glycogen, lipid, protein, and malondialdehyde content in cadmium-treated Galleria mellonella larvae. Ann Entomol Soc Am 106(3):371–377. https://doi.org/10.1603/AN12137
European Commission (2013) Bee health: EU-wide restrictions on pesticide use to enter into force. European Commission, Brussels
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Fotouhi K, Fazel MM, Kavaousi A (2015) Effects of pyriproxyfen on bioenergetic resources of Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Turk Entomol Derg 39(1):11–22. https://doi.org/10.16970/ted.14717
Gad AF, Radwan MA, EL-Gendy KS, Eshra EH, Seehy MA, Khamis A (2016) Genotoxic potential of some pollutants in Theba pisana snails using the micronucleus test. IJZI 2(2):197–205
Ge WL, Yan SH, Wang JH, Zhu LS, Chen AM, Wang J (2015) Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J Agric Food Chem 63(6):1856–1862. https://doi.org/10.1021/jf504895h
Gregorc A, Ellis JD (2011) Cell death localization in situ in laboratory reared honey bee (Apis mellifera L.) larvae treated with pesticides. Pest Biochem Physiol 99(2):200–207. https://doi.org/10.1016/j.pestbp.2010.12.005
Gul ST, Khan A, Farooq M, Niaz S, Ahmad M, Khatoon A, Hussain R, Saleemi MK, Hassan MF (2017) Effect of sub lethal doses of thiamethoxam (a pesticide) on hemato-biochemical values in cockerels. Pak Vet J 37(2):135–138
Gultekin F, Ozturk M, Akdogan M (2000) The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro). Arch Toxicol 74(9):533–538. https://doi.org/10.1007/s002040000167
Guo J, Shi R, Cao Y, Luan Y, Zhou Y, Gao Y, Tian Y (2018) Genotoxic effects of imidacloprid in human lymphoblastoid TK6 cells. Drug Chem Toxicol 13:1–5. https://doi.org/10.1080/01480545.2018.1497048
Han YN, Liu T, Wang JH, Wang J, Zhang C, Zhu LS (2016) Genotoxicity and oxidative stress induced by the fungicide azoxystrobin in zebrafish (Danio rerio) livers. Pest Biochem Physiol 133:13–19. https://doi.org/10.1016/j.pestbp.2016.03.011
Hertner T (1995) CGA 293343 tech.—Micronucleus test, mouse (OECD conform). Ciba-Geigy Ltd, Genetic Toxicology, Basel, Switzerland. Unpublished report No. 952018, 15 December 1995. Submitted to WHO by Syngenta Crop Protection AG
Hogervorst PAM, Wäckers FL, Romeis J (2007) Effect of honeydew sugar composition on the longevity of Aphidius ervi. Entomol Exp Appl 122(3):223–232. https://doi.org/10.1111/j.1570-7458.2006.00505.x
Ighodaro OM, Akinloye OA (2018) First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defense grid. Alexandria J Med 54(4):287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Jones JC (1962) Current concepts concerning insect hemocytes. Amer Zool 2:209–246
Juhel G, Bayen S, Goh C, Lee WK, Kelly BC (2017) Use of a suite of biomarkers to assess the effects of carbamazepine, bisphenol A, atrazine and their mixture on green mussels, Perna viridis. Environ Toxicol Chem 36(2):429–441. https://doi.org/10.1002/etc.3556
Kalita MK, Haloi K, Devi D (2016) Larval exposure to Chlorpyrifos affects nutritional physiology and induces genotoxicity in silkworm Philosamia ricini (Lepidoptera: Saturniidae). Front Physiol 7:535. https://doi.org/10.3389/fphys.2016.00535
Kanbur M, Liman BC, Eraslan G, Altinordulu S (2008) Effects of cypermethrin, propetamphos, and combination involving cypermethrin and propetamphos on lipid peroxidation in mice. Environ Toxicol 23(4):473–479. https://doi.org/10.1002/tox.20360
Karabay NU, Oguz MN (2005) Cytogenetic and genotoxic effects of the insecticides, imidacloprid and methamidophos. Genet Mol Res 4(4):653–662
Kataria SK, Chhillar AK, Kumar A, Tomar M, Malik V (2016) Cytogenetic and hematological alterations induced by acute oral exposure of imidacloprid in female mice. Drug Chem Toxicol 39(1):59–65. https://doi.org/10.3109/01480545.2015.1026972
Kavanagh K, Reeves PE (2007) Insects and mammalian innate immune responses are much alike. Microbe 2(12):596–599
Kayis T, Coskun M, Dursun O, Emre I (2015) Alterations in antioxidant enzyme activity, lipid peroxidation and ion balance induced by Dichlorvos in Galleria mellonella L. Ann Entomol Soc Am 108(4):570–574. https://doi.org/10.1093/aesa/sav038
Keshta AT, Hataba AA, Mead HMI, El-Shafey NM (2016) Oxidative stress and biochemical changes induced by thiamethoxam and acetamiprid insecticides in rats. Pharm Sci Pharmacol 5(6):44–60. https://doi.org/10.20959/wjpps20166-6837
Kissoum N, Soltani N (2016) Spiromesifen, an insecticide inhibitor of lipid synthesis, affects the amounts of carbohydrates, glycogen and the activity of lactate dehydrogenase in Drosophila melanogaster. J Entomol Zool Stud 4(1):452–456
Kocaman AY, Topaktas M (2007) In vitro evaluation of the genotoxicity of acetamiprid in human peripheral blood lymphocytes. Environ Mol Mutagen 48(6):483–490. https://doi.org/10.1002/em.20309
Kurt D, Kayis T (2015) Effects of the pyrethroid insecticide deltamethrin on the hemocytes of Galleria mellonella. Turk J Zool 39:452–457. https://doi.org/10.3906/zoo-1405-66
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Lushchak VI (2016) Contaminant-induced oxidative stress in fish: a mechanistic approach. Fish Physiol Biochem 42(2):711–747. https://doi.org/10.1007/s10695-015-0171-5
Maryanski M, Kramarz P, Laskowski R, Niklinska M (2002) Decreased energetic reserves, morphological changes and accumulation of metals in Carabid Beetles (Poecilus cupreus L.) exposed to zinc or cadmium contaminated food. Ecotoxicology 11(2):127–139. https://doi.org/10.1023/A:1014425113481
Mathews CM, Summers CB, Felton GW (1997) Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch Insect Biochem Physiol 34:57–68. https://doi.org/10.1002/(SICI)1520-6327(1997)34:1<57::AID-ARCH5>3.0.CO;2-T
Muthusamy R, Rajakumar S (2016) Antioxidative response in a silkworm, Bombyx mori larvae to dichlorvos insecticide. Free Rad Biol Antioxid 6(1):58–63. https://doi.org/10.5530/fra.2016.1.7
Nordberg J, Arnér ESJ (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Rad Biol Med 31(11):1287–1312. https://doi.org/10.1016/S0891-5849(01)00724-9
Noshy MM, Saad-Hussein A, Shahy EM, El-Shorbagy HM, Taha MM, Abdel-Shafy EA (2017) Assessment of anticholinesterase toxicity, oxidative stress and antioxidant status in carbamate and organophosphorus pesticides-exposed agricultural workers. Int J Pharm Clin Res 9(3):205–209. https://doi.org/10.25258/ijpcr.v9i3.8319
Oliveira RA, Roat TC, Carvalho SM, Malaspina O (2013) Side-effects of thiamethoxam on the brain and midgut of the Africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environ Toxicol 29(10):1122–1133. https://doi.org/10.1002/tox.21842
Olson DM, Fadamiro H, Lundgren JG, Heimpel GE (2000) Effects of sugar feeding on carbohydrate and lipid metabolism in a parasitoid wasp. Physiol Entomol 25(1):17–25. https://doi.org/10.1046/j.1365-3032.2000.00155.x
Parakash M (2008) Insect physiology. In: Encyclopedia of Entomology, 3nd edn. Discovery Pub. House Pvt. Ltd., New Delhi, pp 216–257
Perveen N, Ahmad M (2017) Toxicity of some insecticides to the haemocytes of giant honeybee, Apis dorsata F. under laboratory conditions. Saudi J Biol Sci 24(5):1016–1022. https://doi.org/10.1016/j.sjbs.2016.12.011
Raj SJ, Joseph B (2015) Impact of acetamiprid toxicity on biochemical biomarkers (protein and carbohydrate) in some tissues of the fish Oreochromis mossambicus. Int J Zool Res 11(5):222–227. https://doi.org/10.3923/ijzr.2015.222.227
Rajak P, Dutta M, Roy S (2015) Altered differential hemocyte count in 3rd instar larvae of Drosophila melanogaster as a response to chronic exposure of Acephate. Interdiscip Toxicol 8(2):84–88. https://doi.org/10.1515/intox-2015-0013
Rashwan M (2013) Biochemical impacts of rynaxypyr (Coragen) and spinetoram (Radiant) on Spodoptera littoralis (Boisd.). J Nat Sci 11(8):40–47
Sak O, Uckan F, Ergin E (2006) Effects of cypermetrin on total body weight, glycogen, protein, and lipid contents of Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Belgian J Zool 136(1):53–58
Saleem MA, Shakoori AR, Mantle D (1998) Macromolecular and enzymatic abnormalities induced by a synthetic pyrethroid, Ripcord (cypermethrin) in adult beetles of stored grain pests, Tribolium castaneum (Herbst.) (Col. Tenebrionidae). Arch Insect Biochem Physiol 39:144–154. https://doi.org/10.1002/(SICI)1520-6327(1998)39:4<144::AID-ARCH2>3.0.CO;2-6
Salema LH, Alwan MJ, Afaf AY (2014) Antioxidative and antigenotoxic effects against cytotoxicity of thiamethoxam on mice. Int J Adv Res 2(10):507–511
Senyildiz M, Kilinc A, Ozden S (2018) Investigation of the genotoxic and cytotoxic effects of widely used neonicotinoid insecticides in HepG2 and SH-SY5Y cells. Toxicol Ind Health 34(6):375–383. https://doi.org/10.1177/0748233718762609
Shimizu N (2011) Molecular mechanisms of the origin of micronuclei from extrachromosomal elements. Mutagenesis 26(1):119–123. https://doi.org/10.1093/mutage/geq053
Shuklaa S, Jhamtania RC, Dahiyab MS, Agarwal R (2017) Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol Rep 4:240–244. https://doi.org/10.1016/j.toxrep.2017.05.002
Sinha S, Thaker AM (2013) Sub-acute genotoxicity studies of thiamethoxam in mice. Indian Vet J 90(9):42–44
Stivaktakis P, Vlastos D, Giannakopoulos E, Matthopoulos DP (2010) Differential micronuclei induction in human lymphocyte cultures by imidacloprid in the presence of potassium nitrate. Sci World J 10:80–89. https://doi.org/10.1100/tsw.2010.9
Summers CB, Felton GW (1993) Antioxidant role of dehydroascorbic acid reductase in insects. Biochim Biophys Acta 1156(2):235–238. https://doi.org/10.1016/0304-4165(93)90142-U
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Tauber OE, Yeager JF (1936) On the total hemolymph (blood) cell counts of insects II. Neuroptera, Coleoptera, Lepidoptera, and Hymenoptera. Ann Entomol Soc Am 29(1):112–118
Tomizawa M, Casida JE (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 48:339–364. https://doi.org/10.1146/annurev.ento.48.091801.112731
Tomizawa M, Lee DL, Casida JE (2000) Neonicotinoid insecticides: molecular features conferring selectivity for insect versus mammalian nicotinic receptors. J Agric Food Chem 48(12):6016–6024. https://doi.org/10.1021/jf000873c
Toroser D, Orr WC, Sohal RS (2007) Carbonylation of mitochondrial proteins in Drosophila melanogaster during aging. Biochem Biophys Res Commun 363(2):418–524. https://doi.org/10.1016/j.bbrc.2007.08.193
Tsai CJ, Loh JM, Proft T (2016) Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 7(3):214–229. https://doi.org/10.1080/21505594.2015.1135289
Uckan F, Sak O (2010) Cytotoxic effect of cypermethrin on Pimpla turionellae (Hymenoptera: Ichneumonidae) larval hemocytes. Ekoloji 19(75):20–26. https://doi.org/10.5053/ekoloji.2010.753
Van Handel E (1985a) Rapid determination of glycogen and sugar in mosquitoes. J Am Mosq Control Assoc 1(3):299–301
Van Handel E (1985b) Rapid determination of total lipids in mosquitoes. J Am Mosq Control Assoc 1(3):302–304
Velisek J, Stara A (2018) Effect of thiacloprid on early life stages of common carp (Cyprinus carpio). Chemosphere 194:481–487. https://doi.org/10.1016/j.chemosphere.2017.11.176
Venier P, Maron S, Canova S (1997) Detection of micronuclei in gill cells and haemocytes of mussels exposed to benzo(a)pyrene. Mutat Res 390(1–2):33–44. https://doi.org/10.1016/S0165-1218(96)00162-0
Wickens PA (2001) Ageing and the free radical theory. Respir Physiol 128(3):379–391. https://doi.org/10.1016/S0034-5687(01)00313-9
Wu YY, Zhou T, Wang Q, Dai PL, Xu SF, Jia HR, Wang X (2015) Programmed cell death in the honey bee (Apis mellifera) (Hymenoptera: Apidae) worker brain induced by imidacloprid. J Econ Entomol 108(4):1486–1494. https://doi.org/10.1093/jee/tov146
Yan SH, Wang JH, Zhu LS, Chen AM, Wang J (2016) Thiamethoxam induces oxidative stress and antioxidant response in zebrafish (Danio Rerio) livers. Environ Toxicol 31(12):2006–2015. https://doi.org/10.1002/tox.22201
Yonar ME (2013) Protective effect of lycopene on oxidative stress and antioxidant status in Cyprinus carpio during cypermethrin exposure. Environ Toxicol 28(11):609–616. https://doi.org/10.1002/tox.20757
Yucel MS, Kayis T (2019) Imidacloprid induced alterations in oxidative stress, biochemical, genotoxic, and immunotoxic biomarkers in non-mammalian model organism Galleria mellonella L. (Lepidoptera: Pyralidae). J Environ Sci Health B 54(1):27–34. https://doi.org/10.1080/03601234.2018.1530545
Zhang Q, Zhan B, Wang C (2014) Ecotoxicological effects on the earthworm Eisenia fetida following exposure to contaminated with imidacloprid. Environ Sci Pollut Res 21(21):12345–12353. https://doi.org/10.1007/s11356-014-3178-z
Zhang P, Sun H, Ren C, Min L, Zhang H (2018) Sorption mechanisms of neonicotinoids on biochars and the impact of deashing treatments on biochar structure and neonicotinoids sorption. Environ Pollut 234:812–820. https://doi.org/10.1016/j.envpol.2017.12.013
Zhu Q, He Y, Yao J, Liu Y, Tao L, Huang Q (2012) Effects of sublethal concentrations of the chitin synthesis inhibitor, hexaflumuron, on the development and hemolymph physiology of the cutworm, Spodoptera litura. J Insect Sci 12(27):1–13. https://doi.org/10.1673/031.012.2701
Acknowledgments
We thank Dr. Yusuf Sevgiler and Dr. Muhsin Aydin for helpful comments and edits of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Giovanni Benelli
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kayis, T., Altun, M. & Coskun, M. Thiamethoxam-mediated alteration in multi-biomarkers of a model organism, Galleria mellonella L. (Lepidoptera: Pyralidae). Environ Sci Pollut Res 26, 36623–36633 (2019). https://doi.org/10.1007/s11356-019-06810-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06810-7