Abstract
Polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethane (DDT), and dichlorodiphenyldichloroethylene (DDE) are suspected to be associated with breast cancer risk, but the results are controversial. This study was performed to evaluate the associations between adipose tissue PCB, DDT, and DDE concentrations and breast cancer risk. Two hundred and nine pathologically diagnosed breast cancer cases and 165 controls were recruited from three local hospitals in Shantou city, China, from 2014 to 2016. Concentrations of 7 PCB congeners, p,p′-DDT, and p,p′-DDE were measured in adipose tissues obtained from the breast for cases and the breast/abdomen for controls during surgery. Clinicopathologic information and demographic characteristics were collected from medical records. PCBs, p,p′-DDT, and p,p′-DDE concentrations in adipose tissues were compared between cases and controls. Multivariate logistic regression model was used to analyze the risk of breast cancer by PCBs, p,p′-DDT, and p,p′-DDE concentrations in adipose tissues. Breast cancer cases have relatively higher menarche age, higher breastfeeding and postmenopausal proportion than controls. Levels of PCB-52, PCB-101, PCB-118, PCB-138, PCB-153, PCB-180, total PCBs (∑PCBs), and p,p′-DDE were relatively higher in breast cancer cases than controls. Breast cancer risk was increased in the third tertile of PCB-101, PCB-118, PCB-138, PCB-153, PCB-180, ∑PCBs, and p,p′-DDE as compared with the first tertile in both adjusted and unadjusted logistic regression models (odds ratios [ORs] were from 1.58 to 7.88); and increased linearly across categories of PCB-118 and p,p′-DDE in unadjusted model, and PCB-118 and PCB-153 in the adjusted model with trend (all P < 0.01). While breast cancer risk was declined in the second tertile of PCB-28, PCB-52, and PCB-101 in both unadjusted and adjusted models, also second tertile of p,p′-DDT and third tertile of PCB-28 in the adjusted models. This study suggests associations between the exposure of PCBs, p,p′-DDT, and p,p′-DDE and breast cancer risk. Based on adjusted models, PCB-118, PCB-138, PCB-153, PCB-180, ∑PCBs, and p,p′-DDE exposures increase breast cancer risk at current exposure levels, despite existing inconsistent even inverse results in PCB-28, PCB-52, PCB-101, and p,p′-DDT. More epidemiological studies are still needed to verify these findings in different populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer with a high incidence was commonly diagnosed among women, which has become one of the most common female cancers (Ferlay et al. 2015). According to GLOBOCAN report, the incidence of breast cancer has been increasing globally in recent years. In China, the incidence of breast cancer was relatively lower than that in European and American countries (Dubey et al. 2015), but the increasing incidence has classed as the first among female malignant tumors in some economically developed regions and cities (Chen et al. 2016; Li et al. 2015). Although much attention has been paid to breast cancer, the etiology is not explicit. Existing literatures supported that causes of breast cancer mainly come from familial genetics or genetic damage, hormonal or reproductive factors, unhealthy lifestyle, and environmental or medical harmful exposure (Salehi et al. 2008). Genetic inheritance made 5–10% of contribution to breast cancer patients (Campeau et al. 2008; Martin and Weber 2000), other factors may play a significant role in breast cancer development. Especially, endocrine disrupting chemicals (EDCs) were suspected to play an important role through estrogen-related pathways (Lee et al. 2014; Sifakis et al. 2017).
Polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDT) were two kinds of EDCs and they were suspected of associating with breast cancer risk. Dichlorodiphenyldichloroethylene (DDE) is the main degradation metabolites of DDT. PCBs were industrially synthetic chemicals with 209 kinds of congeners, which were widely used in industry since 1929. DDT was synthetic insecticide and was originally used in the 1940s. Then DDT was widely used in military, agriculture, and daily life, and especially showed a vital function for combating malaria. PCBs and DDT were both banned in the 1970s in Western Europe and America and in 1983 in China (ATSDR 2000; ATSDR 2002). After being banned, DDT was produced as a raw material until 2003 in China (Wang et al. 2007). Until now, PCBs and DDT are still existing in the environment, the organisms, and human body, because of their persistence, resistance to decomposition, and bioaccumulation (Floehr et al. 2013). Because of the long half-life of PCBs and DDT in the human body (respectively about 10 to 15 years and 10 years) (Ritter et al. 2011), human beings will be exposed to them in a period of the future. Therefore, it is meaningful to explore the associations of PCBs and DDT with breast cancer.
But the associations of PCBs and DDT with breast cancer were still ambiguous, and the epidemiological information is insufficient. PCBs and DDT are both persistent organochlorines with similar structure, so they had similar property in some respects. Experiments in vitro showed PCBs, DDT, and DDE have estrogenic property and can stimulate breast cancer cell proliferation through estrogenic pathway (Aube et al. 2011); PCB-126 can elevate estradiol level in H295R human adenocarcinoma cell line through upregulating the mRNA level of CYP19 which can regulate the synthesis of estradiol (Kraugerud et al. 2010); PCBs also can induce oxidative stress leading to DNA damage in human MDAMB-231 (MDA) breast cancer cells (Lin et al. 2009). As genetic damage may induce cancerization of cells, PCB exposure may increase breast cancer risk. Population-based studies showed inconsistent conclusions. Some studies found PCB/DDT exposure to be associated with increased breast cancer risk (Aronson et al. 2000; Arrebola et al. 2015), but some found no significant even negative results (Gatto et al. 2007; Itoh et al. 2009; Rubin et al. 2006). Dichlorodiphenyldichloroethylene (p, p′-DDE) was the main metabolites of DDT and studies also showed p,p′-DDE exposure was associated with breast cancer (Arrebola et al. 2015; Iwasaki et al. 2008).
In order to explore the associations between PCB and organochlorine exposures and breast cancer risk, further investigations and experiments are needed. In addition, most of the epidemiological studies usually employed blood specimens to evaluate the associations between organochlorine pesticides and PCB body burden and breast cancer risk, other more appropriated kinds of specimen are also encouraged. PCB and organochlorine levels in breast adipose tissue are higher than in serum and represent cumulative internal exposure at the target site for breast cancer. Therefore in the present study, we used PCB and organochlorine levels of breast adipose tissues in cases and their levels of abdominal adipose tissues in controls to explore the associations between PCB and organochlorine exposures and breast cancer risk in Chinese Chaoshan women.
Methods
Study population
The study population consisted of 374 women residents from Chaoshan area, located in the southeast coastal area of China. This place includes Shantou (Swatow), Chaozhou (Teochew), Jieyang, and other neighboring areas. These subjects were recruited from January 2014 to May 2016 at three local hospitals. Among them, 209 participants were undergoing surgery for newly diagnosed as invasive breast cancer who were histopathologically confirmed as cases. A total of 165 controls who were histopathology confirmed as benign breast disease or non-breast-related disease would also provide breast or abdominal adipose tissue after undergoing surgery. All cases and controls were recruited from Chaoshan area where they had to be born and lived, and without any preceding cancer diagnoses as well as receiving radiotherapy. The cases and controls have similar lifestyles and dietary habits, same ethnic and religious practices. Ethical approval was obtained from the Human Ethical Committee of Shantou University Medical College. All participants gave their informed written consent before enrollment.
Demographic data collection
Demography information and basically clinical data for subjects were acquired from medical records. All clinical and pathological documents, including history of disease, cancer in the family and menstrual, reproductive history, pathological diagnose of breast lumps, were carefully extracted. And information on demographic characteristics, such as age, marriage, parity, breastfeeding, type of job, lifestyle, and residential history, were also collected at the same time.
Sample collection
The collection of human breast tissue samples and abdominal adipose tissues was previously described (Hernandez et al. 2009; He et al. 2017). Approximately 2 g and more adipose tissues were obtained from biopsies taken during surgery. These samples were placed in hexane-washed polyethylene tubes, marking identity and disease classification, then immediately frozen, stored at − 80 °C.
Measurement of PCB, DDT, and DDE levels in adipose tissues
The procedure for specimen preparation, extraction, and purification of the compounds was as described (Covaci et al. 2008; Hernandez et al. 2009) with minor modification, and was introduced detailed in our previous studies (He et al. 2017; He et al. 2018). The instrument measurement and quantification were also described in previous study (He et al. 2017). The detailed information was also provided in Supplementary Information files.
Statistical analysis
All statistical analyses were conducted using SPSS 23.0 (SPSS Inc., Chicago, IL, USA). Concentrations below the limit of detection (LOD) were calculated as the value of the LOD divided by the square root of 2 (Holmes et al. 2014). ∑PCBs was defined as the sum concentrations of the 7 congeners in adipose tissues. Continuous data are described according to their mean ± SD and non-normally distributed data with median (interquartile range [IQR], P25–P75). The difference was compared between case group and control group by Student’s t test or nonparametric test. The numeration data was analyzed by chi-square test, or Fisher’s exact test was used when there are data of certain theoretical frequency less than 5. Mann–Whitney U test was used for comparing between groups because PCBs/p,p′-DDT/p,p′-DDE concentrations in adipose tissue were not distributed normally. Levels of p,p′-DDT, p,p′-DDE, and total and individual PCB congeners were divided into tertiles by the concentrations of these compounds in controls. Bivariate logistic regressions were performed to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) for total and individual PCBs/p,p′-DDT/p,p′-DDE and breast cancer risk, with adjustment for some possible confounders. Because age, menarche age, menopause status, and ever breasting data were not balanced in cases and controls, these variables were adjusted in the logistic regression models. We calculated P values for trend from the Cochrane-Mantel-Haenszel chi-square test. Two-tailed with P < 0.05 was considered statistically significant.
Results
General characteristics of breast cancer cases and controls
The general characteristics of breast cancer cases and controls are summarized in Table 1. Age, menarche age, age at first birth, family history of breast cancer, marriage, ever breastfeeding, parity, menopausal status, and type of employment and residence were compared between cases and controls. A total of 209 cases with mean age of 52.00 ± 9.89 years and 165 controls with mean age of 48.64 ± 10.88 years were recruited in this study. The control samples included 14 benign breast adipose tissues and 151 abdominal adipose tissues (from 28 patients with uterine fibroids, 67 patients with cesarean section, 32 patients with gallstone, 25 patients with lipoma, and 13 patients with abdominal hernia). Women in cases had older mean menarche age than that in controls (14.63 vs. 13.95 years, P < 0.001). The proportion of ever breastfeeding among women in cases was 90.0% which was larger than that in controls (82.4%). None of the participants’ marriage, age at first birth, parity, family history of breast cancer, type of employment, and residence was significantly different between cases and controls (all P > 0.05).
Levels of PCBs, p, p′-DDT, and p, p′-DDE in breast cancer cases and controls
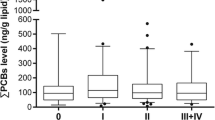
Seven PCB congener, p, p′-DDT, and p, p′-DDE levels were measured in adipose tissue samples. Most of the participants were found to have PCB, p, p′-DDT, and/or p, p′-DDE exposures, the detection rate was from 57.9~100%. Median levels of PCB-52, PCB-101, PCB-118, PCB-138, PCB-153, PCB-180, ∑PCBs, and p, p′-DDE were significantly higher in cases than in controls (Table 2). No significant differences of p, p′-DDT and PCB-28 were found between the cases and controls.
Levels of PCBs, p, p′-DDT, and p, p′-DDE and breast cancer risk
Unconditional logistic regression models were used to estimate the unadjusted and adjusted ORs and 95%CIs for breast cancer risk associated with adipose tissue levels of DDT, DDE, and PCBs. Breast cancer risk was increased in the 3rd tertile of PCB-101, PCB-118, PCB-138, PCB-153, PCB-180, ∑PCBs, and DDE as compared with the 1st tertile in both unadjusted and adjusted logistic regression models (Table 3). The risk was increased in both 2nd and 3rd tertiles of PCB-118 and DDE in unadjusted model, and PCB-118 and PCB-153 in the adjusted model with trend (all P < 0.01). The risk was also increased in 3rd tertile of PCB-52 as compared with the 1st tertile, while breast cancer risk was declined in the 2nd tertile of PCB-28, PCB-52, and PCB-101 in both unadjusted and adjusted models, also 2nd tertile of DDT and 3rd tertile of PCB-28 in the adjusted model (Table 3).
Discussions
This study was a case-control study based on hospitals. We aimed to explore the associations between adipose tissue PCB, p,p′-DDT, and p,p′-DDE exposures and breast cancer risk among women in Chaoshan area. Levels of 7 PCB congeners, p,p′-DDT, and p,p′-DDE in breast or abdominal adipose tissues were measured, and their associations with breast cancer risk were evaluated. The results suggested that most of the PCB individual congeners and total PCBs as well as p,p′-DDE were significantly higher in breast cancer cases. Multiple logistic regressions also suggested positive associations between these compound exposures and breast cancer risk.
In this study, PCB-52, PCB-101, PCB-118, PCB-138, PCB-153, PCB-180, and total PCBs showed significantly higher median levels in breast cancer cases than in controls. Similarly, previous studies also have found that female breast cancer cases have higher serum concentration of PCBs than controls (Arrebola et al. 2015; Cohn et al. 2012; Holmes et al. 2014). Other studies also have found that breast cancer cases have higher breast adipose concentration of PCBs than controls (Aronson et al. 2000; Muscat et al. 2003; Stellman et al. 2000; Zheng et al. 2000). In this study, p,p′-DDE was relatively high in our participants when compared with other population, both in cases and controls. This may be attributed by the environmental pollution and the residuals in vegetables, meat, and fish. p,p′-DDE was also found to be higher in cases than in controls, which was consistent with other study (300.1 vs. 167.7 ng/g lipid, P < 0.012) (Ramos et al. 2017). While in this study, p,p′-DDT had lower median level in cases than in controls. This result was agreement with one study in Japanese population, in which women of cases were found to have lower median levels of p,p′-DDT than women in controls (9.3 vs. 9.9 ng/g lipid, P = 0.03) (Itoh et al. 2009). Another study also found the similar result (153.0 vs. 217.0 ng/g lipid, P < 0.001) (Ramos et al. 2017).
Serum levels of environmental pollutants are suggested as a risk factor for breast cancer (Wielsoe et al. 2017). PCBs, p,p′-DDT, and p,p′-DDE were endocrine disrupting chemicals (EDCs), which can be stored in the body fat tissue and interfere with the process of the synthesis and release, transport, metabolism, and combination of normal hormone in the body. PCBs, p,p′-DDT, and p,p′-DDE were reported to be associated with breast cancer in previous studies with controversy (Arrebola et al. 2015; Arrebola et al. 2016; Dorgan et al. 1999). The mechanisms of EDCs associated with breast cancer are still uncertain. And the mechanisms had been proposed as following: affecting the level of estrogen exposure in body or mimicking estrogen function and disrupting the function of the endogenous estrogen (Dickerson and Gore 2007); destroying the epigenomic landscape (Knower et al. 2014); study has shown that some known EDCs do cause cellular toxicity related to DNA damage, protein damage, oxidative damage, and membrane damage (Gu et al. 2002). In vitro experiments found that PCBs, p,p′-DDT, and p, p′-DDE can induce and promote the proliferation of breast cancer cells (Aube et al. 2011). There have existed a certain amount of researches which explored the associations of PCBs, p,p′-DDT, and p,p′-DDE with breast cancer risk, but the results were controversial (Dorgan et al. 1999; Laden et al. 2001). The controversial results may be caused by different design of experiments, different measurement methods for organochlorine compounds, objects of study from different countries, and race with different eating habits, and different beginning time and quantity of use of organochlorine pesticides.
Further unconditional logistic regression models were used to evaluated the associations between PCB, p, p′-DDT, and p,p′-DDE exposures and breast cancer risks. Our results suggested some individual PCB congeners including PCB-101, PCB-118, PCB-138, PCB-153, PCB-180, total PCBs, and p,p′-DDE were positively associated with breast cancer risk while PCB-28 and p,p′-DDT were negatively associated with breast cancer risk. PCB-118 is a kind of dioxin-like PCB congener and is similar to dioxin with toxic effect, and PCB-118 was evaluated relatively more in epidemiologic studies. In vitro studies suggested PCB-118 may be associated with breast cancer risk, can stimulate MCF-7 cell proliferation, and improve the secretion of 17 β-estradiol (Radice et al. 2008). For other individual congeners, one study suggests the possibility that PCB-138 and PCB-153 contribute to the action of endogenous 17 β-estradiol on cell proliferation and apoptosis in MCF-7 cells (Ptak et al. 2011). Dioxin-like PCB congeners such as PCB-126 and PCB-169 concentrations dependently induced 2-MeOE(1/2) formation and ethoxyresorufin-O-deethylation (EROD) activity through induced CYP1A1 expression in MCF-7 and MCF-10A cells (van Duursen et al. 2003). The highest PCB-174 tertile was associated with an increase in all-cause (HR = 2.22) and breast cancer–specific (HR = 3.15) mortalities within 5 years of diagnosis and remained associated with breast cancer–specific mortality (HR = 1.88) at 15 years (Parada et al. 2016). Our study and some previous epidemiological studies also found the specific individual congener PCB-105, PCB-118, PCB-156, or PCB-183 exposure can increase the risk of breast cancer (Demers et al. 2002; Stellman et al. 2000). Especially some results suggest that exposure to dioxin-like PCBs increases breast cancer risk (Demers et al. 2002). Further and deeper studies were needed to explain the association between dioxin-like PCB-118 exposure and breast cancer risk.
DDT was classified as possible cancerogen to humans in 1991 (Goldsmith 2000). DDT was probably carcinogenic to non-Hodgkin lymphoma and testicular cancer (Pahwa et al. 2012) and the carcinogenicity of DDT is based on a lot of animal experiments. But, the association between DDT and breast cancer was indeterminate, and the results of abundant studies were controversial. Several studies of meta-analysis on epidemiologic studies were found no association between DDT exposure and breast cancer risk (Ingber et al. 2013; Lopez-Cervantes et al. 2004; Park et al. 2014). However, previous several cohort studies found DDT to be associated with breast cancer risk (Dorgan et al. 1999; Helzlsouer et al. 1999; Laden et al. 2001) and some studies suggested DDT exposure in windows of breast susceptibility can increase breast cancer risk. Such as, a study showed women with a higher level of DDT exposure in early life had bigger risk of breast cancer (Cohn et al. 2007); a study found that fetus with DDT exposure in utero had increased risk of breast cancer in later life (Cohn et al. 2015); a study in Taiwan found the greatest mortality of breast cancer in population were born in 1951 when DDT was largely used to malaria control (Ho et al. 2015). Moreover, an animal experiment also found that prepubertal female mice with DDT exposure at level of environmental exposure in human can accelerate the occurrence of breast tumor (Johnson et al. 2012). These studies suggested that DDT exposure in windows of breast susceptibility associated with increased risk of breast cancer. Inversely, our study found that p,p′-DDT was negatively associated with breast cancer, consistent with the result of a study which found o,p′-DDT exposure negatively associated with breast cancer risk (Cohn et al. 2015). The relationships between DDT and breast cancer may be not linearly, and different concentrations of these compounds may present different results. This discrepancy may be also caused by p,p′-DDE. In this study, women in cases had higher levels of p,p′-DDE but lower levels of p,p′-DDT than the ones in controls. As p,p′-DDE is the main metabolites of p,p′-DDT, the present levels of DDT did not represent the early p,p′-DDT exposure of the body. And we found association between p,p′-DDE exposure and breast cancer risk. Further and deeper studies were needed to explore the clear association between p,p′-DDT exposure as well as p,p′-DDE exposure and breast cancer risk.
Besides environmental pollutants with estrogen potential, lifestyle, obesity, and reproductive factors, genetic inheritance and clinical radiation are also risk factors for breast cancer. Explicit expressions of BRCA1, BRCA2, and CHEK2 are found to increase the risk of breast cancer (Hall and Easton 2013; Rudolph et al. 2016). Diet is the main way of harmful liposoluble organics bioaccumulating in the human body (Arrebola et al. 2009; Brauner et al. 2012), and regarded as a risk factor for breast cancer. Smoking, alcohol, reproductive factors, hormone replacement therapies, and clinical radiation are also risk factor for breast cancer (Barnard et al. 2015; Salehi et al. 2008; Trichopoulos et al. 2008). Older age, early menarche age, older age at first birth, family history of breast cancer, no lactation, and no parity were established to be risk factors for breast cancer, so these factors were usually regarded as confounding factors to estimating the ORs in multiple logistic regression models. In the present study, cases have older menarche age than controls, which differed from some other studies which found that breast cancer patients have earlier menarche age than healthy population and earlier menarche age was a risk factor for breast cancer (Gaudet et al. 2011; Xing et al. 2010). Breast cancer cases have a bigger proportion of lactation than controls in this study. This result also contradicted with previous results of some studies showing that breastfeeding can reduce breast cancer risk (Barnard et al. 2015). These two contradictions may reflect that reproductive factors (such as menarche age and lactation) were not the decisive factors in the process of breast cancerization. Further studies are needed to explain the contradictions. No association was found in this study for age at first birth, family history, marriage, parity, and type of employee with breast cancer.
There exist several strengths in this study. Firstly, few studies assessing associations between PCB and organochlorine pesticide exposures and breast cancer risk used adipose tissues in China, a developing country with rapid development these decades accompanied by severe pollution. We have investigated the concentrations of PCBs as well as organochlorine pesticides in adipose tissues in women from Chaoshan area with relatively high burden in the environment and their exposures associated with breast cancer risk. Secondly, only newly diagnosed breast cancer cases with surgery were included in our study to avoid Neyman bias or prevalence-incidence bias. Because for the newly diagnosed cases, the lifestyles and dietary styles are not changed with the disease diagnosis, the exposure information is relatively credibly.
However, some limitations were also existed in this study. Firstly, as a case-control study, we measured PCB and organochlorine pesticide levels after the diagnosis of breast cancer, the levels may not represent the concentrations of PCB and organochlorine pesticide exposures in special periods of early exposure. It cannot very well evaluate the influence of PCBs and organochlorine pesticides in process of breast cancerization. Further prospective studies from all over the world are needed to confirm the associations. Secondly, our controls were women with benign breast disease or non-breast-related disease, and the collected adipose tissues included normal breast and abdominal adipose tissues, which may lead to the unbalance between the cases and controls. But previous study suggested that measurements of PCBs and organochlorine pesticides in breast and abdominal adipose tissues were correlated well and concentrations of target chemicals in one tissue could be derived from measurements in the other tissue (Petreas et al. 2004). Actually, it is difficult to obtained breast adipose tissue from healthy woman. Thirdly, breast cancer susceptibility genes were not analyzed in this study. Some breast cancer susceptibility genes are reported as important risk factors for the breast cancer risk, the interactions between environment pollutants and gene susceptibility could be evaluated if the information can be obtained, and more comprehensive explanation would be generated for associations between PCB exposure as well as organochlorine pesticide exposure and breast cancer risk.
Conclusions
In conclusion, our study demonstrated that some individual PCB congeners (PCB-101, PCB-118, PCB-138, PCB-153, and PCB-180), total PCBs, and p,p′-DDE exposures in adipose tissues increased breast cancer risk, especially PCB-118, PCB-138, PCB-153, PCB-180, ∑PCBs, and p,p’-DDE with a linear trend. Further and deeper experimental studies in vitro and in vivo are needed to verify the findings and underlying mechanisms.
References
Aronson KJ, Miller AB, Woolcott CG, Sterns EE, McCready DR, Lickley LA et al (2000) Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomark Prev 9:55–63
Arrebola JP, Martin-Olmedo P, Fernandez MF, Sanchez-Cantalejo E, Jimenez-Rios JA, Torne P et al (2009) Predictors of concentrations of hexachlorobenzene in human adipose tissue: a multivariate analysis by gender in southern Spain. Environ Int 35:27–32
Arrebola JP, Belhassen H, Artacho-Cordon F, Ghali R, Ghorbel H, Boussen H et al (2015) Risk of female breast cancer and serum concentrations of organochlorine pesticides and polychlorinated biphenyls: a case-control study in Tunisia. Sci Total Environ 520:106–113
Arrebola JP, Fernandez-Rodriguez M, Artacho-Cordon F, Garde C, Perez-Carrascosa F, Linares I et al (2016) Associations of persistent organic pollutants in serum and adipose tissue with breast cancer prognostic markers. Sci Total Environ 566-567:41–49
ATSDR (2000) Toxicological profile for polychlorinated biphenyls. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta
ATSDR (2002) Toxicological profile for DDT, DDE, and DDD. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta
Aube M, Larochelle C, Ayotte P (2011) Differential effects of a complex organochlorine mixture on the proliferation of breast cancer cell lines. Environ Res 111:337–347
Barnard ME, Boeke CE, Tamimi RM (2015) Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta 1856:73–85
Brauner EV, Raaschou-Nielsen O, Gaudreau E, Leblanc A, Tjonneland A, Overvad K et al (2012) Predictors of adipose tissue concentrations of organochlorine pesticides in a general Danish population. J Expo Sci Environ Epidemiol 22:52–59
Campeau PM, Foulkes WD, Tischkowitz MD (2008) Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet 124:31–42
Chen W, Zheng R, Zeng H, Zhang S (2016) The incidence and mortality of major cancers in China, 2012. Chin J Cancer 35:73
Cohn BA, Wolff MS, Cirillo PM, Sholtz RI (2007) DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect 115:1406–1414
Cohn BA, Terry MB, Plumb M, Cirillo PM (2012) Exposure to polychlorinated biphenyl (PCB) congeners measured shortly after giving birth and subsequent risk of maternal breast cancer before age 50. Breast Cancer Res Treat 136:267–275
Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L et al (2015) DDT exposure in utero and breast cancer. J Clin Endocrinol Metab 100:2865–2872
Covaci A, Voorspoels S, Roosens L, Jacobs W, Blust R, Neels H (2008) Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human liver and adipose tissue samples from Belgium. Chemosphere 73:170–175
Demers A, Ayotte P, Brisson J, Dodin S, Robert J, Dewailly E (2002) Plasma concentrations of polychlorinated biphenyls and the risk of breast cancer: a congener-specific analysis. Am J Epidemiol 155:629–635
Dickerson SM, Gore AC (2007) Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord 8:143–159
Dorgan JF, Brock JW, Rothman N, Needham LL, Miller R, Stephenson HE Jr et al (1999) Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA). Cancer Causes Control 10:1–11
Dubey AK, Gupta U, Jain S (2015) Breast cancer statistics and prediction methodology: a systematic review and analysis. Asian Pac J Cancer Prev 16:4237–4245
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Floehr T, Xiao H, Scholz-Starke B, Wu L, Hou J, Yin D, Zhang X, Ji R, Yuan X, Ottermanns R, Roß-Nickoll M, Schäffer A, Hollert H (2013) Solution by dilution?--a review on the pollution status of the Yangtze River. Environ Sci Pollut Res Int 20:6934–6971
Gatto NM, Longnecker MP, Press MF, Sullivan-Halley J, McKean-Cowdin R, Bernstein L (2007) Serum organochlorines and breast cancer: a case-control study among African-American women. Cancer Causes Control 18:29–39
Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, Gammon MD, Douglas Thompson W, Bernstein JL (2011) Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat 130:587–597
Goldsmith DF (2000) Linking environmental cancer with occupational epidemiology research: the role of the International Agency for Research on Cancer (IARC). J Environ Pathol Toxicol Oncol 19:171–175
Gu MB, Min J, Kim EJ (2002) Toxicity monitoring and classification of endocrine disrupting chemicals (EDCs) using recombinant bioluminescent bacteria. Chemosphere 46:289–294
Hall P, Easton D (2013) Breast cancer screening: time to target women at risk. Br J Cancer 108:2202–2204
He Y, Peng L, Huang Y, Peng X, Zheng S, Liu C, Wu K (2017) Association of breast adipose tissue levels of polychlorinated biphenyls and breast cancer development in women from Chaoshan. China Environ Sci Pollut Res Int 24:4778–4790
He Y, Peng L, Zhang W, Liu C, Yang Q, Zheng S, Bao M, Huang Y, Wu K (2018) Adipose tissue levels of polybrominated diphenyl ethers and breast cancer risk in Chinese women: a case–control study. Environ Res 167:160–168
Helzlsouer KJ, Alberg AJ, Huang HY, Hoffman SC, Strickland PT, Brock JW et al (1999) Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiol Biomark Prev 8:525–532
Hernandez F, Portoles T, Pitarch E, Lopez FJ (2009) Searching for anthropogenic contaminants in human breast adipose tissues using gas chromatography-time-of-flight mass spectrometry. J Mass Spectrom 44:1–11
Ho ML, Hsiao YH, Su SY, Chou MC, Liaw YP (2015) Mortality of breast cancer in Taiwan, 1971-2010: temporal changes and an age-period-cohort analysis. J Obstet Gynaecol 35:60–63
Holmes AK, Koller KR, Kieszak SM, Sjodin A, Calafat AM, Sacco FD, Varner DW, Lanier AP, Rubin CH (2014) Case-control study of breast cancer and exposure to synthetic environmental chemicals among Alaska native women. Int J Circumpolar Health 73:25760
Ingber SZ, Buser MC, Pohl HR, Abadin HG, Murray HE, Scinicariello F (2013) DDT/DDE and breast cancer: a meta-analysis. Regul Toxicol Pharmacol 67:421–433
Itoh H, Iwasaki M, Hanaoka T, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, Kusama R, Tsugane S (2009) Serum organochlorines and breast cancer risk in Japanese women: a case-control study. Cancer Causes Control 20:567–580
Iwasaki M, Inoue M, Sasazuki S, Kurahashi N, Itoh H, Usuda M, Tsugane S (2008) Plasma organochlorine levels and subsequent risk of breast cancer among Japanese women: a nested case-control study. Sci Total Environ 402:176–183
Johnson NA, Ho A, Cline JM, Hughes CL, Foster WG, Davis VL (2012) Accelerated mammary tumor onset in a HER2/Neu mouse model exposed to DDT metabolites locally delivered to the mammary gland. Environ Health Perspect 120:1170–1176
Knower KC, To SQ, Leung YK, Ho SM, Clyne CD (2014) Endocrine disruption of the epigenome: a breast cancer link. Endocr Relat Cancer 21:T33–T55
Kraugerud M, Zimmer KE, Dahl E, Berg V, Olsaker I, Farstad W, Ropstad E, Verhaegen S (2010) Three structurally different polychlorinated biphenyl congeners (Pcb 118, 153, and 126) affect hormone production and gene expression in the human H295R in vitro model. J Toxicol Environ Health A 73:1122–1132
Laden F, Hankinson SE, Wolff MS, Colditz GA, Willett WC, Speizer FE, Hunter DJ (2001) Plasma organochlorine levels and the risk of breast cancer: an extended follow-up in the Nurses’ Health Study. Int J Cancer 91:568–574
Lee HR, Hwang KA, Nam KH, Kim HC, Choi KC (2014) Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol 27:834–842
Li C, Yu C, Wang P (2015) An age-period-cohort analysis of female breast cancer mortality from 1990-2009 in China. Int J Equity Health 14:76
Lin CH, Huang CL, Chuang MC, Wang YJ, Chen DR, Chen ST, Lin PH (2009) Protective role of estrogen receptor-alpha on lower chlorinated PCB congener-induced DNA damage and repair in human tumoral breast cells. Toxicol Lett 188:11–19
Lopez-Cervantes M, Torres-Sanchez L, Tobias A, Lopez-Carrillo L (2004) Dichlorodiphenyldichloroethane burden and breast cancer risk: a meta-analysis of the epidemiologic evidence. Environ Health Perspect 112:207–214
Martin AM, Weber BL (2000) Genetic and hormonal risk factors in breast cancer. J Natl Cancer Inst 92:1126–1135
Muscat JE, Britton JA, Djordjevic MV, Citron ML, Kemeny M, Busch-Devereaux E et al (2003) Adipose concentrations of organochlorine compounds and breast cancer recurrence in Long Island, New York. Cancer Epidemiol Biomark Prev 12:1474–1478
Pahwa M, Harris SA, Hohenadel K, McLaughlin JR, Spinelli JJ, Pahwa P et al (2012) Pesticide use, immunologic conditions, and risk of non-Hodgkin lymphoma in Canadian men in six provinces. Int J Cancer 131:2650–2659
Parada H Jr, Wolff MS, Engel LS, Eng SM, Khankari NK, Neugut AI, Teitelbaum SL, Gammon MD (2016) Polychlorinated biphenyls and their association with survival following breast cancer. Eur J Cancer 56:21–30
Park JH, Cha ES, Ko Y, Hwang MS, Hong JH, Lee WJ (2014) Exposure to dichlorodiphenyltrichloroethane and the risk of breast cancer: a systematic review and meta-analysis. Osong Public Health Res Perspect 5:77–84
Petreas M, Smith D, Hurley S, Jeffrey SS, Gilliss D, Reynolds P (2004) Distribution of persistent, lipid-soluble chemicals in breast and abdominal adipose tissues: lessons learned from a breast cancer study. Cancer Epidemiol Biomark Prev 13:416–424
Ptak A, Mazur K, Gregoraszczuk EL (2011) Comparison of combinatory effects of PCBs (118, 138, 153 and 180) with 17 beta-estradiol on proliferation and apoptosis in MCF-7 breast cancer cells. Toxicol Ind Health 27:315–321
Radice S, Chiesara E, Fucile S, Marabini L (2008) Different effects of PCB101, PCB118, PCB138 and PCB153 alone or mixed in MCF-7 breast cancer cells. Food Chem Toxicol 46:2561–2567
Ramos JJ, Huetos O, Gonzalez S, Esteban M, Calvo E, Perez-Gomez B et al (2017) Organochlorinated pesticides levels in a representative sample of the Spanish adult population: the Bioambient.es project. Int J Hyg Environ Health 220:217–226
Ritter R, Scheringer M, MacLeod M, Hungerbuhler K (2011) Assessment of nonoccupational exposure to DDT in the tropics and the north: relevance of uptake via inhalation from indoor residual spraying. Environ Health Perspect 119:707–712
Rubin CH, Lanier A, Kieszak S, Brock JW, Koller KR, Strosnider H, Needham L, Zahm S, Harpster A (2006) Breast cancer among Alaska native women potentially exposed to environmental organochlorine chemicals. Int J Circumpolar Health 65:18–27
Rudolph A, Chang-Claude J, Schmidt MK (2016) Gene-environment interaction and risk of breast cancer. Br J Cancer 114:125–133
Salehi F, Turner MC, Phillips KP, Wigle DT, Krewski D, Aronson KJ (2008) Review of the etiology of breast cancer with special attention to organochlorines as potential endocrine disruptors. J Toxicol Environ Health B Crit Rev 11:276–300
Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA (2017) Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol 51:56–70
Stellman SD, Djordjevic MV, Britton JA, Muscat JE, Citron ML, Kemeny M et al (2000) Breast cancer risk in relation to adipose concentrations of organochlorine pesticides and polychlorinated biphenyls in Long Island, New York. Cancer Epidemiol Biomark Prev 9:1241–1249
Trichopoulos D, Adami HO, Ekbom A, Hsieh CC, Lagiou P (2008) Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer 122:481–485
van Duursen MB, Sanderson JT, van der Bruggen M, van der Linden J, van den Berg M (2003) Effects of several dioxin-like compounds on estrogen metabolism in the malignant MCF-7 and nontumorigenic MCF-10A human mammary epithelial cell lines. Toxicol Appl Pharmacol 190:241–250
Wang H, He M, Lin C, Quan X, Guo W, Yang Z (2007) Monitoring and assessment of persistent organochlorine residues in sediments from the Daliaohe River watershed, northeast of China. Environ Monit Assess 133:231–242
Wielsoe M, Kern P, Bonefeld-Jorgensen EC (2017) Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: a case control study. Environ Health 16:56
Xing P, Li J, Jin F (2010) A case-control study of reproductive factors associated with subtypes of breast cancer in Northeast China. Med Oncol 27:926–931
Zheng T, Holford TR, Tessari J, Mayne ST, Owens PH, Ward B, Carter D, Boyle P, Dubrow R, Archibeque-Engle S, Zahm SH (2000) Breast cancer risk associated with congeners of polychlorinated biphenyls. Am J Epidemiol 152:50–58
Acknowledgments
We thank all the volunteers for participating in the present study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81470152).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval was obtained from the Human Ethical Committee of Shantou University Medical College. All participants gave their informed written consent before enrollment.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Huang, W., He, Y., Xiao, J. et al. Risk of breast cancer and adipose tissue concentrations of polychlorinated biphenyls and organochlorine pesticides: a hospital-based case-control study in Chinese women. Environ Sci Pollut Res 26, 32128–32136 (2019). https://doi.org/10.1007/s11356-019-06404-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06404-3