Abstract
Silver nanoparticles (AgNPs) have been widely produced for different industrial purposes. Recently, biogenic synthesis of AgNPs has emerged although the extent of effects from exposure, oral exposure in particular, to nanomaterials synthesized in such a manner remains elusive. The main objective of this study was to evaluate the effects of oral administration of a dose of 50 mg/Kg body weight AgNPs biosynthesized in baker’s yeast (Saccharomyces cerevisiae) over a period of eight weeks on the reproductive performance and the possibility of a protective effect through co-administration of morin. Forty-eight male Sprague-Dawley rats were used in four experimental groups (control, morin-treated group, AgNP-treated, and AgNP + morin co-treatment). AgNPs produced no significant alteration in daily food intake or body weight. Both the absolute and relative testicular weights were significantly reduced but not the epididymal weight. Also, serum levels of urea, creatinine, uric acid, and liver enzymes were significantly elevated. Furthermore, AgNPs significantly downregulated the hypothalamic–pituitary–gonadal axis. This corresponds to lower motility and viability percent, reduced sperm concentration, and a higher abnormality ratio as well as a prominent alteration in the blood–testis barrier (BTB) and testicular histology and induction of testicular apoptosis and oxidative stress. The supplementation of morin evidently restored most of the reproductive characters to its physiological range. We can conclude that exposure to the biologically synthesized AgNPs for an extended period of time has proven to be a health risk that can be ameliorated via oral administration of some bioactive agents including morin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have many innovative applications in the different areas of daily life, and medicine in particular (Rai et al. 2016). However, the widespread nature of their uses and the extremely small size of these NPs can have a potential important impact on health. Products containing silver and most recently silver nanoparticles (AgNPs) are commonly used in commercialized products including cosmetics, deodorants, and contraceptives due to the antibacterial properties (Ahamed et al. 2010; Chen and Schluesener 2008; Franci et al. 2015), thus increasing the risk of exposure. Recently, the development of innovative synthesis protocols and characterization techniques for nanoparticles including AgNPs have emerged (Sharma et al. 2009). While chemical and physical synthesis methods often result in nanoparticles with poor morphology and more toxic potential to the environment (Birla et al. 2009; Rai et al. 2008), the biological synthesis of nanoparticles provide a less toxic environmentally friendly synthetic procedures. NPs can gain access to our body systems via several routes such as oral, inhalation, and cutaneous routes (Wijnhoven et al. 2009). Oral exposure can occur via contamination of food, water, or both. Once inside the body, NPs, AgNPs in particular, can be diffused through systemic circulation to the different vital organs producing several health effects that can adversely affect several bodily functions (Ahamed et al. 2010; Hadrup and Lam 2014). AgNPs by different routes have significant biochemical and pathological effects in treated animals (Al Gurabi et al. 2015; Dziendzikowska et al. 2012). In vitro studies have reported a relation between AgNP toxicity and oxidative stress induction (Hussain et al. 2005; Schrand et al. 2008), and induction of lipid peroxidation (Arora et al. 2008). Furthermore, AgNPs have been reported to negatively affect the major organs of the reproductive system including the testes and eventually some reproductive parameters (Greco et al. 2015; Hutz et al. 2014; Thakur et al. 2014). Those studies have proposed several mechanisms through which AgNPs can produce a potential cytotoxic effect, oxidative damage, and, eventually, apoptosis or cell death (de Lima et al. 2012; Zhang et al. 2014b). However, to date, only few available studies have addressed the impact of AgNPs, especially the biologically synthesized, on the different reproductive and fertility parameters and sex hormones. AgNP treatment has cytotoxic effects on both Leydig and Sertoli cells in a way hindering spermatogenesis (Braydich-Stolle et al. 2010; Zhang et al. 2014b; Zhang et al. 2015). The effects of AgNPs on the various parameters of rat sperm are size and dose dependent (Gromadzka-Ostrowska et al. 2012; Miresmaeili et al. 2013). The blood-testis barrier (BTB) is a tight blood-tissue barrier formed mainly of junctional and cytoskeletal structures (Cheng and Mruk 2012; Franca et al. 2012). The primary functions of the BTB are mainly to allow a proper microenvironment for germ cell development and maturation and to shield haploid germ cells from harmful cytotoxic molecules (Mital et al. 2011). The tight junction, an important structural component of the Sertoli cell junctional complex, comprise a transmembrane region of molecules that mechanically confer adhesiveness to cells such as occludin, the claudin multigene family, and tight junction protein 1 (Tjp1) (González-Mariscal et al. 2000; Krause et al. 2008; Moroi et al. 1998). Signaling molecules and several transcription factors have also been identified in association with tight junctions (Mruk Dolores and Cheng 2010). TGF beta (TGF-β)and testosterone can both modulate the junction dynamics and subsequently BTB function (Cheng et al. 2010). The use of different natural antioxidants to alleviate the impact of oxidative stress and peroxidative damage in male infertility has been widely reported. These natural antioxidants can serve as alternatives to synthetic drugs thus preventing their various drawbacks, treatment failure, and drug resistance. Bioflavonoids such as morin (3,5,7,2′,4′-pentahydroxyflavone) are found in a wide array of herbs and fruits. Previous studies have shown that morin possesses a potential anti-inflammatory antioxidant, both in vivo and in vitro (Kapoor and Kakkar 2012; Ma et al. 2016; Ola et al. 2014; Zhang et al. 2011). To the best of our knowledge, the possibility of protective effects for morin on reproductive dysfunction and injury via exposure to AgNPs has not yet been fully explored. Therefore, the aim of the present study was to assess the potential effects of the biologically synthesized AgNPs at a dose of 50 mg/kg body weight for an 8-week period on the reproductive performance, blood–testis barrier function, modulation of the testicular expression of the different apoptotic genes, and the extent of morin-induced protective effect of the reproductive function of male rats.
Methods
Synthesis and characterization of AgNPs
Silver nitrate (AgNO3) and morin hydrate were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). AgNPs were prepared using the baker’s yeast (Saccharomyces cerevisiae) extract under visible light following the protocol employed in our previous reports (Attia et al. 2016a, b). Briefly, active baker’s yeast (1 g) was dissolved in 100 mL of distilled water, heated to 90 °C for 2 h, and then filtered with 50-μm-pore filter paper. The resulting aqueous extract was maintained at 5 °C. Equal volumes of the yeast extract and AgNO3 solution (1 mM) were mixed in a quartz vessel. The vessel reaction was irradiated with a halogen lamp (HALOPAR 20 75 W 230 V 301 GU10, Italy) for 30 min. Continuous visual inspection of the test tubes for a change in the color of the culture medium was carried out. Formation of a brownish-yellow color indicated the successful formation of AgNPs.
AgNPs solution (50 mg/mL) was prepared at the National Institute of Laser Enhanced Sciences, Cairo University. AgNPs were characterized as previously described (Attia et al. 2016b). Ultraviolet–visible (UV–Vis) spectra were recorded with a PerkinElmer lambda 40 UV–visible spectrophotometer using 1-cm path length Hellma quartz cuvettes. X-ray diffraction (XRD) measurement was performed using a Philips PW1710 X-ray diffractometer using Cu Ka radiation (k = 1.54186 A°). The XRD patterns were measured from 10 to 80° 2θ with a step size of 0.020° 2θ and collecting 10 s per step. Transmission electron microscopy (TEM) images were obtained with a Joel JEM-1230 electron microscope operated at 120 kV equipped with a Gatan UltraScan 4000SP 4K 9 4K CCD camera. Few drops from a diluted sample dispersion were deposited onto an amorphous carbon film on 400 mesh copper grids and left to evaporate at room temperature.
Animal use and dosages
All animal handling and experiments were conducted according to the procedures reviewed and approved by the Zagazig University Research Center Institutional Animal Care and Use Committee (IACUC) under number ZU-IACUC/3/F/89/2019. AgNPs was daily prepared and orally administered via a gastric tube at a dose of 50 mg/kg/day for 8 weeks. This dose and this route have been chosen based on pilot testing with different doses (1, 10, 50, and 100 mg/kg B.wt for 1 month) followed by evaluation of the different reproductive parameters. Furthermore, results from previous studies demonstrated a reduction in reproductive parameters (Baki et al. 2014; Miresmaeili et al. 2013) as well as elevation in the oxidative stress markers (Blanco et al. 2018). Morin hydrate was suspended in saline and was orally administered via gastric tube at a dose of 30 mg/kg/day as previously reported (Ola et al. 2014).
Experimental design
Forty-eight adult male Sprague-Dawley rats at 4–5 months of age and weighing 200 ± 10 g were obtained from the Animal Research Unit, Faculty of Veterinary Medicine, Zagazig University, Egypt. The animals were housed in a 12-h light/dark cycle, at appropriate humidity (50–60%) and temperature (21–24 °C) in polycarbonate cages. Access to standard diet and water throughout the experimental period was allowed ad libitum. The animals were allowed 2 weeks for acclimation to the laboratory conditions before starting the experiment. The animals were divided into four groups (12 rats per group). Group I (control group) orally received physiological saline (1 ml/kg b.wt) daily for eight weeks. Group II (morin-treated group) was gavaged with morin dissolved in normal saline at a dose of 30 mg/kg b.wt daily for 8 weeks (Ola et al. 2014). Group III (AgNP-treated group) was orally administered AgNPs at a dose of 50 mg kg b.wt daily for 8 weeks. Group IV (AgNP + morin-treated group) was simultaneously treated with AgNPs (50 mg kg b.wt) and morin (30 mg kg b.wt) via oral route and for the same duration.
Sample collection
At the end of the experiment, rats from the different experimental groups were sacrificed by decapitation followed by exsanguination after routine rat weighting recordings. Blood samples were collected from the control and treated groups in a BD Vacutainer PST II Tube (Chance et al. 2009), left for coagulation, followed by centrifugation at 3,000 rpm for 15 min. The serum samples were preserved at − 20 °C until used for the different biochemical analysis. The pair of testes and epididymis from all rats of the different experimental groups were rapidly excised, dissected free of adhering tissue, rinsed in ice-cold saline, and weighed. The hypothalamus was dissected from each brain tissue following the Glowinski and Iversen technique (Glowinski and Iversen 1966).The hypothalamus and 30 mg of testicular tissue were snap frozen in liquid nitrogen and stored in − 80 °C for subsequent RT-PCR procedures. Part of one testis from each experimental animal was homogenized by a WiseTis HG-15D homogenizer (Daihan Scientific Co., Seoul, Korea) for the measurement of antioxidant. The other part was digested in nitric acid and used for quantification of silver concentration. The other testis specimens from all groups were preserved in 10% neutral buffered formalin for histopathological examination.
Food intake and body weight measurement and absolute and relative organ weight quantification
Food intake and body weight were monitored weekly throughout the experimental period. The testes and epididymis were immediately harvested and weighted following decapitation (absolute weight). The relative weight of the testes or epididymis (gonadosomatic or epididymal somatic indices) were calculated as the percentage of testicular or epididymal weight in relation to the total body weight.
Biochemical assays
The serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), uric acid, and creatinine levels were performed using commercially available kits obtained from Spectrum kits (Egyptian Company for Biotechnology, Cairo, Egypt). The oxidative status and antioxidant enzymes have been measured as reported in our previous studies (Hussein et al. 2015; Hussein et al. 2016). Briefly, a suitable aliquot of homogenate was ultra-centrifuged at 10,000×g at 4 °C for 30 min. The resulting supernatant was used for estimation of the malondialdehyde (MDA) the lipid peroxidation marker, catalase (CAT) (EC 1.11.1.6), superoxide dismutase (SOD) (EC1.15.1.1), and glutathione peroxidase (GPx) (EC 1.11.1.9). Spectrophotometrical determination of the levels of MDA (Nair and Turner 1984), CAT activity at a wavelength of 570 nm (Sinha 1972), SOD activity at a wavelength of 480 nm (Misra and Fridovich 1972), and GPx activity (Paglia and Valentine 1967) were measured using a Shimadzu-type spectrophotometer (UV 120-02). All respective kits for antioxidant status were performed using Biodiagnostic kits (Biodiagnostic Company, Dokki, Giza, Egypt) and all the guidelines of the manufacturer’s instructions were followed.
Hormonal analysis
The serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and total and free testosterone were measured by method using commercially available rat enzyme-linked immunosorbent assay (ELISA) kits purchased from MyBioSource (San Diego, CA, USA) using a plate reader (DNM–9602; Beijing Perlong Medical Instrument Ltd., China) as we previously reported (Arisha and Moustafa 2019).
Measurement of the testicular level of Ag
The collected testicular tissue from AgNP-treated and untreated groups respectively were weighed and treated with 5 mL of analytical grade nitric acid (69%) for 4 h at 50 °C, and at 100 °C for the same period. The digested samples were then diluted with 2% nitric acid and analyzed using ICP-OES (5100, Agilent Technologies, Santa Clara, CA, USA) with synchronous vertical dual view (SVDV) to detect the testicular content of Ag.
Semen evaluation and sperm parameters
The caudal part of the epididymis from one testis was accurately excised and transferred to a sterilized Petri dish where it was macerated in 2 mL normal saline; the suspension of epididymal contents was handled exactly as the semen (Hafez 1970). All the solutions and equipment used in the various steps of handling this suspension were pre-warmed at 37 °C. For sperm motility assessment, a drop of this suspension was placed on a clean glass slide, covered by a glass cover slide, and examined under high power (× 40) of a light microscope to evaluate the individual motility of the spermatozoa. Several microscopical fields were examined to evaluate the total percentage of motile spermatozoa. One drop of the suspension was mixed with eosin–nigrosine stain on a glass slide to evaluate the ratio of live/dead sperms (viability ratio). For the sperm cell concentration per milliliter of the semen, semen was diluted with normal saline at a ratio of 1:4 and few drops of formalin (40%) were added. An improved Neubauer hemocytometer counting chamber was used to count the spermatozoa in the preformed mixture (Robb et al. 1978). The sperm cell count equals the total number of spermatozoa in four squares × 2500 × dilution factor. Eosin–nigrosine-stained smears were examined under oil immersion lens for evaluating the percentage of sperm abnormalities after randomly examining at least 100 spermatozoa (Filler 1993).
Relative quantitative RT-PCR analysis
Total RNA was extracted using Trizol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s standard instructions. The resulting RNA pellet was then resuspended in 50 μl of RNase/DNase free water. Both quality and concentration of the extracted RNA were determined using the NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, Delaware USA) by estimating the ratios of absorbance at 230 nm, 260 nm, and 280 nm. The assessed RNA purity ranged between 1.8 and 2. The following reverse transcription of complementary DNA (cDNA) synthesis was performed with 1 μg total RNA using the HiSenScript™ RH (-) cDNA Synthesis Kit (iNtRON Biotechnology Co., South Korea) in a Veriti 96-well thermal cycler (Applied Biosystems, Foster City, CA) for 60 min at 45 °C followed by 10 min at 85 °C. For analysis of gene expression (Table 1), the real-time RT-PCR was performed in a Mx3005P Real-Time PCR System (Agilent Stratagene, USA) using 5x HOT FIRE Pol EvaGreen qPCR Mix Plus (Solis BioDyne, Tartu, Estonia) following the manufacturer’s instructions. The PCR cycling conditions included an initial denaturation at 95 °C for 12 min followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. The oligonucleotide-specific primers were synthesized by Sangon Biotech (Beijing, China) as shown (Table 1). A melting curve analysis was performed following PCR amplification. All RT-PCR processes and reporting comply with MIQE guidelines (Bustin et al. 2009). The expression level of the target genes was normalized to that of Gapdh, and the relative fold changes in gene expression were calculated based on the 2−ΔΔCT comparative method (Livak and Schmittgen 2001; Schmittgen and Livak 2008).
Histopathological examination
The fixed testes specimens were properly processed and embedded in paraffin via standard histological procedure. Briefly, tissues were washed in 70% alcohol, dehydrated in an ascending ethanol percentage washes until reaching 100% ethanol. This was cleared in xylene, infiltrated with soft paraffin, and subsequently embedded into molten paraffin wax. The resulting blocks were sectioned at 5-μm thickness. These sections were dewaxed in xylene and then rehydrated through a series of descending ethanol percentage washes. The sections were then stained with hematoxylin and eosin (H&E) dyes (Bancroft and Gamble 2007) and examined using a light microscope.
Statistical analysis
All the data were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed by using t test when comparing two experimental groups or one-way analysis of variance (ANOVA) followed by the Bonferroni test for post hoc comparisons between different experimental groups by GraphPad prism 7 (GraphPad Software Inc., San Diego, 189 CA, USA). A probability level of less than 0.05 was considered to be statistically significant.
Results
Characterization of the biologically synthesized AgNPs
Allowing the AgNO3 solution added to an aqueous solution of baker’s yeast extract to react under visible light–induced extracellular reduction of the Ag ions to Ag nanoparticles (Fig. 1). The biosynthesized AgNPs were characterized by optical absorption analysis. The absorption spectrum of the synthesized AgNPs showed maximum absorption at 407 nm (Fig. 1a) indicating the formation of AgNPs. The XRD pattern, characterizing the crystalline natures of AgNPs, showed four intense peaks in the spectrum ranging from 20 to 80 (Fig. 1b). XRD patterns corresponding to the (111), (200), (220), and (311) planes of the formed AgNPs were observed at 2θ angles of 38.27°, 44.39°, 64.59°, and 77.67°, respectively (El-shazly et al. 2017; Mohamed et al. 2018). The morphology of the produced AgNPs was analyzed using TEM (Fig. 1c). The as-prepared AgNPs showed spherical shape with an average size of 20.0 ± 1.3 nm (the total count of NPs = 100 particles).
a–c Characterization of AgNPs synthesized in baker’s yeast. a Absorption spectrum of the supernatant solution after completion of the reaction that indicates the formation of AgNPs. b X-ray diffraction (XRD) pattern of the formed AgNPs. c Morphology of the formed AgNPs is pictured by transmission electron microscopy (TEM)
Effect of oral administration of AgNPs and/or morin on food consumption, body and organ weights, and testicular concentration of silver
There were no statistically significant changes in weight gain between the control and any of the treatment groups (P < 0.05) (Fig. 2a). Also, no significant difference in daily food consumption between the different experimental groups during the entire experimental period was noted as shown in (Fig. 2b). Absolute and relative weights of the reproductive organ (testes and epididymis) of the male rats in the four groups are summarized in (Table 2). A statistically significant reduction in both absolute and relative right, left, or net testicular weights of the AgNP-treated groups was noted (P < 0.05) that was reversed by oral morin coadministration in group 4. No significant alterations in the weights of the right, left, or total epididymis were observed. Furthermore, the testicular level of Ag showed a significantly higher amount of silver deposited in the AgNP-treated groups in comparison with the untreated control (P < 0.05) (Fig. 2c). Interestingly, morin cotreatment significantly decreased the amount of detected silver in the testicular tissue. These data suggest that ingested AgNPs are capable of reaching and accumulating in testicular tissues following oral exposure. Furthermore, the implications of the blood–testis barrier (BTB) permeability and the expression of tight junction proteins (occludin, claudin-11, and tight junction protein 1 (Tjp1)) in reducing the testicular levels of silver with the coadministration of morin was suggested. A reduced mRNA expression of occludin, claudin 11, and Tjp1 was detected in the testis of rats orally exposed to the AgNPs (Fig. 2e–g). Significant upregulation of both occludin and claudin-11 was noticed. Also, the expression of the transforming growth factor (TGF)-β, secreted by testicular cells and can alter the tightness of the tight junctions, was reduced in AgNP-treated animals and elevated in the administered rats (Fig. 2d). Animals from group 3 displayed a significant elevation in serum levels of ALT, AST, ALP, BUN, uric acid, and creatinine levels compared to those in control animals. These parameters were significantly reduced in animals from the cotreated group (Fig. 3)
a–g Effect of oral administration of AgNPs and/or morin on body weight, daily feed intake, testicular concentration, and blood–testis barrier in adult male rats. a Body weight change. b Average daily food intake (g). c Testicular concentration of silver. d Testicular mRNA expression of TGFβ1. e Testicular mRNA expression of Tjp-1. f Testicular mRNA expression of claudin-11. g Testicular mRNA expression of occludin. Values are mean ± SEM of 8–10 animals per experimental group. Means bearing different superscripts were significantly different at P < 0.05
a–f Effect of oral administration of AgNPs and/or morin on serum urea, creatinine, uric acid and liver function tests in adult male rats. a BUN (mg/dl). b Creatinine (mg/dl). c Uric acid (mg/dl). d ALT (U/L). e AST (U/L). f ALP (U/L). Values are mean ± SEM of 8–10 animals per experimental group. Means bearing different superscripts were significantly different at P < 0.05
Effect of oral administration of AgNPs and/or morin on semen parameters
Oral exposure of rats to AgNPs showed a significant reduction in sperm motility, epididymal sperm count, live/dead ratio, and elevation in sperm abnormalities when compared with that of the control group (P < 0.05) (Table 3). The cotreatment of morin and AgNPs resulted in a significant elevation in the sperm motility, epididymal sperm count, live/dead ratio, and reduction in the percentage of morphologically abnormal spermatozoa compared to the AgNP-treated group. This improvement in testicular function, although significant, did not reverse the changes in either sperm count or live sperm ratio to the control values (P < 0.05). The different observed shapes of sperm morphology are shown in (Fig. 4) which included detached head, broken head, short tail, bent tail, curved tail, detached tail, looped tail, and protoplasmic droplet.
a–j Effect of oral administration of AgNPs and/or morin on sperm morphology. a normal spermatozoon. b Short tail. c Detached head with protoplasmic droplet. d Looped tail. e detached tail. f Protoplasmic droplet. g Bent tail. h Broken head. i Curved tail. j Detached head. Images of the eosin–nigrosine-stained smears were captured at × 100 magnification
Effect of orally administered AgNPs and/or morin on the hypothalamic–pituitary–gonadal axis
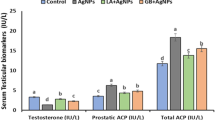
AgNP treatment significantly reduced the mRNA expression of hypothalamic GnRH1, testicular AR, and serum levels of FSH, LH and testosterone (total and free) concentrations (Fig. 5) and increased the mRNA expression of hypothalamic AR suggesting downregulation of the hypothalamic–pituitary–gonadal axis. Morin cotreatment significantly alleviated the decrease in mRNA expression of hypothalamic GnRH1, testicular AR, serum LH, and free testosterone but not serum FSH or total testosterone although an upward tendency was noted (Fig. 5). There were no significant alterations in the hypothalamic expression of GnIH between the different groups.
a–g Effect of orally administered AgNPs and/or morin on the hypothalamic–pituitary–gonadal axis in adult male rats. a Hypothalamic mRNA expression of GnRH1. b Hypothalamic mRNA expression of GnIH. c Hypothalamic and testicular mRNA expression of Androgen receptor. d Serum FSH (ng/mL). e Serum LH (mIU/mL). f Serum Total Testosterone (ng/mL). g Serum-free testosterone (ng/mL). Values are mean ± SEM of 8–10 animals per experimental group. Means bearing different superscripts were significantly different at P < 0.05
Expression of apoptosis-related genes in testicular tissue
The possibility of AgNP-induced spermatogenic cell death was explored by measuring the expression levels of PCNA—a marker for proliferation, caspase-3, Bcl-2, Bax, Fas, and FasL. There was a significant reduction in the mRNA expression of both proliferating cell nuclear antigen (PCNA) and Bcl-2 levels in the AgNP-treated rats. Also, a significant downregulation in the mRNA expression of Fas, FasL, caspase-3, and Bax was evident. these actions were significantly reversed in the AgNPs morin cotreated rats (Fig. 6).
a–f Effect of oral administration of AgNPs and/or morin on testicular apoptosis in adult male rats. a Testicular mRNA expression of Fas. b Testicular mRNA expression of FasL. c Testicular mRNA expression of Caspase 3. d Testicular mRNA expression of PCNA. e Testicular mRNA expression of Bax. f Testicular mRNA expression of Bcl2. Values are mean ± SEM of 8–10 animals per experimental group. Means bearing different superscripts are significantly different at P < 0.05
Effect of oral administration of AgNPs on lipid peroxidation, oxidative stress marker and antioxidant status in testes
The level of MDA was significantly elevated following AgNPs oral administration (Table 4). Also, a significant decline was observed in rats administered with morin and AgNPs simultaneously when compared to group 3 (Table 4). On the other hand, the activity of GPx, SOD, and CAT was significantly lower in AgNP-treated group when compared with the control group (Table 4). These impairments were significantly ameliorated by morin co-administration in group 4.
Effect of oral administration of AgNPs on testicular histopathology
Histopathological examination was performed in all of the experimental groups and the results are shown in (Fig. 7). Sections from control rats and rats from the morin-treated groups revealed a normal histological structure of the seminiferous tubules in testis tissue by light photomicrography. A prominent atrophy of seminiferous tubules, thinning of the tubule wall, and disorganization and vacuolization of germinal epithelium, and loss of spermatogenic cells in testis tissue of the rats received AgNPs. Relative normalization of the testicular tissue with preservation of the normal histological structure of most of the seminiferous tubules, mild edema in intertubular space and moderately number of spermatozoa were seen within the tubular lumen in rats that received both morin and AgNPs.
a–d Photomicrograph of testicular cross section stained with hematoxylin and eosin of adult male rat. a normal histological appearance of seminiferous tubules of rat testis tissue of the control group with spermatozoa (indicated by an asterisk) seen within the tubular lumen. b normal histological appearance of seminiferous tubules of rat testes tissue of the morin-treated group with spermatozoa (indicated by an asterisk) seen within the tubular lumen. c Testes of the orally administered AgNP group showing atrophy in the seminiferous tubule, disorganization and vacuolization (indicated by arrows) of germinal epithelium, and loss of spermatogenic cells. d Testes of AgNP + morin-treated group showing mild edema in intertubular space and moderately number of spermatozoa (indicated by an asterisk) seen within the tubular lumen and mild vacuolization (indicated by arrows) of germinal epithelium
Discussion
Exposure to nanoparticle causes various health effects that have been the main focus of several nanotoxicology studies (Aillon et al. 2009; Fischer and Chan 2007). Recently, green chemistry is developed to produce less toxic environmentally friendly nanomaterials. Nanomaterials including silver and gold were shown to impair key reproductive and sperm functions (Taylor et al. 2014; Tiedemann et al. 2014). Morin is one of the naturally occurring bioflavonoids that has been recognized for its dietary health benefits and pharmacological properties such as antioxidant (Merwid-Lad et al. 2012; Prahalathan et al. 2012), anti-inflammatory (Fang et al. 2003), chemoprotective (Kawabata et al. 1999), and anticancer (Kuo et al. 2007) activities. This study focuses on the potential impact of orally administered AgNPs on several pathways and mechanisms regulating the reproductive performance including the HPG axis, spermatogenesis, and blood–testis barrier as well as testicular apoptosis and antioxidant defense mechanisms. Furthermore, the results of the current study demonstrate the potential ameliorating effect of morin against AgNP-induced reproductive toxicity in male rats.
Results of the current study showed that repeated oral administrations of AgNPs for 8 weeks did not affect body weight and food consumption (Espinosa-Cristobal et al. 2013), although absolute and relative reductions in testicular weight in male rats (Yang et al. 2017) could be attributed to testosterone withdrawal. Indeed, the effects of AgNP administration on body weight and food consumption was expected. Previous studies demonstrated that body weight and weight gain of rats in 28-day studies were not affected by oral doses of AgNPs up to 1 g/kg (Kim et al. 2008). Other studies with various doses also observed no significant alterations in the sense of food intake or weight gain in rats (Adeyemi and Adewumi 2014) or mice (Yang et al. 2017). Meanwhile, a reduction in weight gain has been reported by (Zhang et al. 2013) with different doses of AgNPs. The oral route of exposure should cause less toxicity as compared to other routes of administration due to reduced bioavailability. Nevertheless, some oral exposure studies reported high distribution to the testes following 28 days of exposure (Kim et al. 2008; Park et al. 2010; Tiwari et al. 2011). Interestingly, morin cotreatment reduced the level of testicular nanosilver deposition that might be a result of the upregulation in some tight junction molecules of the BTB or increased hepatic or renal excretion. Semen analysis studies are valuable for the assessment of the male reproductive performance following administration of any therapeutic agents (Coetzee et al. 1999; Graves et al. 2005) or to assess the effect of treatment of any underlying condition or even the protective effects of certain medication. AgNP treatment decreases the live sperm ratio (Gromadzka-Ostrowska et al. 2012) and the number of primary spermatocyte and spermatid cells (Miresmaeili et al. 2013)as well as spermatogonial stem cell proliferation (Braydich-Stolle et al. 2010).The hypothalamic–pituitary–gonadal (HPG) axis represents an important hormone system that contributes to the reproductive efficiency both in male and female. The hypothalamus secrets GnRH to the pituitary gland that induces the synthesis and secretion of gonadotropins (LH and FSH). FSH and LH are transported in the systemic circulation to the gonads (testes in male). In males, LH stimulates Leydig cells to secrete testosterone. Both FSH and testosterone regulate the function of Sertoli cells that is responsible for supporting and nourishing the sperm cells during their maturation (Egwurugwu et al. 2013, Faccio et al. 2013). Any interference with the normal functioning of the HPG axis can lead to reduced fertility, and if this interference persists, infertility could be developed. Here, we described the effects of AgNPs on the different levels of the HPG axis including aspects of serum sex hormone levels in male rats. Our results suggest that the decreased level of testosterone, mainly due to testicular damage, has affected testicular spermatogenesis. Testicular damage was exacerbated by reduced FSH and TGF-β leading to downregulation in the BTB function. Other in vivo studies have demonstrated how NPs can penetrate the BTB (Lan and Yang 2012) and the accumulation of nanosized metals including AgNPs in the testis (van der Zande et al. 2012; Yoshida et al. 2009) eventually could impair the endocrine and reproductive functions. The beneficial effect of morin on spermatogenesis is also evident by the significantly improving some of the HPG axis parameters as well as its novel protective action on BTB molecules when co-administered with AgNPs.
PCNA expression, a useful cellular proliferation marker (Angelopoulou et al. 2008; Zhao et al. 2018), is decreased in the AgNP-treated rats and was increased in the AgNP and morin co-administered ones. The proapoptotic Bax and antiapoptotic Bcl-2 are two critical molecules involved in cell death, and the ratio of Bax/Bcl-2 is the denominator that decides whether cells will undergo apoptosis. Rats from the NP-treated group had increased Bax and decreased Bcl-2 expression in their testicular tissues (therefore, increased ratio of Bax/Bcl-2). The other apoptotic pathway elements including caspase 3 as well as the mitochondria-dependent intrinsic apoptotic pathway were also decreased. Cotreatment with morin significantly changed the equilibrium of the proapoptotic (represented by Bax) and antiapoptotic (represented by Bcl-2) molecules and effectively inhibited apoptosis of the testicular cells. Under normal conditions reactive oxygen species (ROS) and free radicals are effectively eliminated by several systems including antioxidant enzymes (Hu et al. 2005). These enzymes including GPx, SOD, and CAT represent a biochemical defense system (Yao et al. 2007) against ROS. When the balance between the generation and elimination of ROS is broken, several cellular components can be damaged (Adi et al. 2016). Spermatozoa are extremely sensitive to the various reactive oxygen metabolites (Begum et al. 2018). The level of ROS has the ability to attack sperms and induce MDA production which in turn initiates the lipid peroxidation cascade and results in loss of unsaturated fatty acids from the plasma membrane and subsequent reduction in the fertilizing capacity of these sperm cells (Ryu et al. 2017). The results of this study demonstrated that AgNP oral administration has reduced the activity of antioxidant enzymes while promoting MDA level resulting in oxidative stress that could explain the decline in fertility of spermatozoa. On the other hand, morin is known to possess a potent antioxidant power via inhibiting the generation of ROS (Kapoor and Kakkar 2012). Morin significantly attenuated oxidative stress and improved the SOD, CAT, and GPx activities while lowering that of MDA concentration when administered alongside the AgNPs. Our aforementioned results were corroborated with the histopathological studies of the testes. In a histopathological examination of the testes, AgNP-treated rats showed testicular damage and absence of spermatogenesis. Our results were concordant with other reports (Thakur et al. 2014). These testicular lesions were restored towards the normal histological architecture by co-administration of morin.
Conclusions and future prospective
In conclusion, morin has exhibited a certain protective effect on the different reproductive parameters of male rats orally exposed to AgNPs. A potential involvement of mechanisms including the induction of testicular apoptosis, oxidative stress, and higher levels of lipid peroxidation in AgNP-induced toxicity and ameliorative effect of morin was observed in the present study. Overall, based on our results, it is postulated that AgNPs, although biogenically synthesized, negatively modulate the testicular function of male rats. Further investigations are needed to discern the toxicologic potential of the biologically produced AgNPs from other forms of physical and chemical synthesis. Also, a detailed investigation to describe the underlying molecular mechanism by which morin improves the reproductive function that was deteriorated by exposure to biologically synthesized AgNPs in male rats is needed.
References
Adeyemi OS, Adewumi I (2014) Biochemical evaluation of silver nanoparticles in Wistar rats. Int Sch Res Not 2014:196091
Adi PJ, Burra SP, Vataparti AR, Matcha B (2016) Calcium, zinc and vitamin E ameliorate cadmium-induced renal oxidative damage in albino Wistar rats. Toxicol Rep 3:591–597
Ahamed M, Alsalhi MS, Siddiqui MK (2010) Silver nanoparticle applications and human health. Clin Chim Acta 411:1841–1848
Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML (2009) Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Deliv Rev 61:457–466
Al Gurabi MA, Ali D, Alkahtani S, Alarifi S (2015) In vivo DNA damaging and apoptotic potential of silver nanoparticles in Swiss albino mice. OncoTargets Ther 8:295–302
Angelopoulou R, Balla M, Lavranos G, Chalikias M, Kitsos C, Baka S, Kittas C (2008) Evaluation of immunohistochemical markers of germ cells' proliferation in the developing rat testis: a comparative study. Tissue Cell 40:43–50
Arisha AH, Moustafa A (2019) Potential inhibitory effect of swimming exercise on the Kisspeptin–GnRH signaling pathway in male rats. Theriogenology 133:87–96
Arora S, Jain J, Rajwade JM, Paknikar KM (2008) Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol Lett 179:93–100
Attia YA, Farag YE, Mohamed YMA, Hussien AT, Youssef T (2016a) Photo-extracellular synthesis of gold nanoparticles using Baker's yeast and their anticancer evaluation against Ehrlich ascites carcinoma cells. New J Chem 40:9395–9402
Attia YA, Mohamed YMA, Altalhi TA (2016b) Photobiosynthesis of metal/graphene nanocomposites: new materials for water desalination and purification. Desalin Water Treat 57:26014–26021
Baki ME, Miresmaili SM, Pourentezari M, Amraii E, Yousefi V, Spenani HR, Talebi AR, Anvari M, Fazilati M, Fallah AA, Mangoli E (2014) Effects of silver nano-particles on sperm parameters, number of Leydig cells and sex hormones in rats. Iran J Reprod Med 12:139–144
Bancroft J, Gamble M (2007) Theory and practice of histological technique. Churchill, Livingston, New York
Begum R, Bajgai J, Fadriquela A, Kim C-S, Kim S-K, Lee K-J (2018) Molecular hydrogen may enhance the production of testosterone hormone in male infertility through hormone signal modulation and redox balance. Med Hypotheses 121:6–9
Birla SS, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK (2009) Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol 48:173–179
Blanco J, Tomas-Hernandez S, Garcia T, Mulero M, Gomez M, Domingo JL, Sanchez DJ (2018) Oral exposure to silver nanoparticles increases oxidative stress markers in the liver of male rats and deregulates the insulin signalling pathway and p53 and cleaved caspase 3 protein expression. Food Chem Toxicol 115:398–404
Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ, Hussain SM, Hofmann MC (2010) Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci 116:577–589
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Chance J, Berube J, Vandersmissen M, Blanckaert N (2009) Evaluation of the BD Vacutainer PST II blood collection tube for special chemistry analytes. Clin Chem Lab Med 47:358–361
Chen X, Schluesener HJ (2008) Nanosilver: a nanoproduct in medical application. Toxicol Lett 176:1–12
Cheng CY, Mruk DD (2012) The blood-testis barrier and its implications for male contraception. Pharmacol Rev 64:16–64
Cheng CY, Wong EWP, Yan HHN, Mruk DD (2010) Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol Cell Endocrinol 315:49–56
Coetzee K, Kruger TF, Lombard CJ, Shaughnessy D, Oehninger S, Ozgur K, Pomeroy KO, Muller CH (1999) Assessment of interlaboratory and intralaboratory sperm morphology readings with the use of a Hamilton Thorne Research integrated visual optical system semen analyzer. Fertil Steril 71:80–84
de Lima R, Seabra AB, Duran N (2012) Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol : JAT 32:867–879
Dziendzikowska K, Gromadzka-Ostrowska J, Lankoff A, Oczkowski M, Krawczynska A, Chwastowska J, Sadowska-Bratek M, Chajduk E, Wojewodzka M, Dusinska M, Kruszewski M (2012) Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J Appl Toxicol : JAT 32:920–928
Egwurugwu JN, Ifedi CU, Uchefuna RC, Ezeokafor EN, Alagwu EA (2013) Effects of zinc on male sex hormones and semen quality in rats. Niger J Physiol Sci 28:17–22
El-shazly M, Attia Y, Kabil F, Anis E, Hazman M (2017) Inhibitory Effects of Salicylic Acid and Silver Nanoparticles on Potato Virus Y-Infected Potato Plants in Egypt. 6:835–848
Espinosa-Cristobal LF, Martinez-Castañon GA, Loyola-Rodriguez JP, Patiño-Marin N, Reyes-Macías JF, Vargas-Morales JM, Ruiz F (2013) Toxicity, distribution, and accumulation of silver nanoparticles in Wistar rats. J Nanopart Res 15:1702
Faccio L, Da Silva AS, Tonin AA, Franca RT, Gressler LT, Copetti MM, Oliveira CB, Sangoi MB, Moresco RN, Bottari NB, Duarte MM, Monteiro SG (2013) Serum levels of LH, FSH, estradiol and progesterone in female rats experimentally infected by Trypanosoma evansi. Exp Parasitol 135:110–115
Fang SH, Hou YC, Chang WC, Hsiu SL, Chao PD, Chiang BL (2003) Morin sulfates/glucuronides exert anti-inflammatory activity on activated macrophages and decreased the incidence of septic shock. Life Sci 74:743–756
Filler R (1993) Methods for evaluation of rat epididymal sperm morphology. Methods Toxicol 3(part A):334–343
Fischer HC, Chan WC (2007) Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol 18:565–571
Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT (2012) Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol 763:237–259
Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20:8856–8874
Galehdari H, Negahdari S, Kesmati M, Rezaie A, Shariati G (2016) Effect of the herbal mixture composed of Aloe Vera, Henna, Adiantum capillus-veneris, and Myrrha on wound healing in streptozotocin-induced diabetic rats. BMC Complement Altern Med 16:386
Gautam M, Bhattacharya I, Rai U, Majumdar SS (2018) Hormone induced differential transcriptome analysis of Sertoli cells during postnatal maturation of rat testes. PloS one 13:e0191201
Glowinski J, Iversen LL (1966) Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem 13:655–669
González-Mariscal L, Betanzos A, Ávila-Flores A (2000) MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 11:315–324
Graves JE, Higdon HL 3rd, Boone WR, Blackhurst DW (2005) Developing techniques for determining sperm morphology in today's andrology laboratory. J Assist Reprod Genet 22:219–225
Greco F, Courbiere B, Rose J, Orsiere T, Sari-Minodier I, Bottero JY, Auffan M, Perrin J (2015) Toxicity of nanoparticles on reproduction. Gynecol Obstet Fertil 43:49–55
Gromadzka-Ostrowska J, Dziendzikowska K, Lankoff A, Dobrzynska M, Instanes C, Brunborg G, Gajowik A, Radzikowska J, Wojewodzka M, Kruszewski M (2012) Silver nanoparticles effects on epididymal sperm in rats. Toxicol Lett 214:251–258
Hadrup N, Lam HR (2014) Oral toxicity of silver ions, silver nanoparticles and colloidal silver--a review. Regul Toxicol Pharmacol : RTP 68:1–7
Hafez E (1970) Reproduction and breeding techniques for laboratory animals. Lea and Fabiger, Philadelphia, PA, pp 301–310
Hou Q, Huang Y, Zhu S, Li P, Chen X, Hou Z, Liu F (2017) MiR-144 Increases Intestinal Permeability in IBS-D Rats by Targeting OCLN and ZO1. Cell Physiol Biochem 44:2256–2268
Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, Huang P (2005) Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem 280:39485–39492
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol in Vitro 19:975–983
Hussein MM, Ali HA, Ahmed MM (2015) Ameliorative effects of phycocyanin against gibberellic acid induced hepatotoxicity. Pestic Biochem Physiol 119:28–32
Hussein MM, Ali HA, Saadeldin IM, Ahmed MM (2016) Querectin alleviates zinc oxide nanoreprotoxicity in male albino rats. J Biochem Mol Toxicol 30:489–496
Hutz RJ, Carvan MJ 3rd, Larson JK, Liu Q, Stelzer RV, King-Heiden TC, Baldridge MG, Shahnoor N, Julien K (2014) Familiar and novel reproductive endocrine disruptors: xenoestrogens, dioxins and nanoparticles. Curr Trends Endocrinol 7:111–122
Jiang Z, Zhou B, Li X,Kirby GM, Zhang X (2018) Echinacoside increases sperm quantity in rats by targeting the hypothalamic androgen receptor, 8
Kapoor R, Kakkar P (2012) Protective role of morin, a flavonoid, against high glucose induced oxidative stress mediated apoptosis in primary rat hepatocytes. PloS one 7:e41663
Kawabata K, Tanaka T, Honjo S, Kakumoto M, Hara A, Makita H, Tatematsu N, Ushida J, Tsuda H, Mori H (1999) Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int J Cancer 83:381–386
Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon IH, Jeong J, Han BS, Yu IJ (2008) Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol 20:575–583
Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE (2008) Structure and function of claudins, 1778, 631-645 pp
Kuo HM, Chang LS, Lin YL, Lu HF, Yang JS, Lee JH, Chung JG (2007) Morin inhibits the growth of human leukemia HL-60 cells via cell cycle arrest and induction of apoptosis through mitochondria dependent pathway. Anticancer Res 27:395–405
Lan Z, Yang W-X (2012) Nanoparticles and spermatogenesis: How do nanoparticles affect spermatogenesis and penetrate the blood-testis barrier, 7, 579-96 pp
Lee KS, Asgar J, Zhang Y, Chung MK, Ro JY (2013) The role of androgen receptor in transcriptional modulation of cannabinoid receptor type 1 gene in rat trigeminal ganglia. Neuroscience 254:395–403
Livak KJ, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method, 25, 402–408 pp
Ma Y, Ge A, Zhu W, Liu YN, Ji NF, Zha WJ, Zhang JX, Zeng XN, Huang M (2016) Morin attenuates ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling. Oxidative Med Cell Longev 2016:5843672
Merwid-Lad A, Trocha M, Chlebda E, Sozanski T, Magdalan J, Ksiadzyna D, Kopacz M, Kuzniar A, Nowak D, Piesniewska M, Fereniec-Golebiewska L, Kwiatkowska J, Szelag A (2012) Effects of morin-5'-sulfonic acid sodium salt (NaMSA) on cyclophosphamide-induced changes in oxido-redox state in rat liver and kidney. Hum Exp Toxicol 31:812–819
Miresmaeili SM, Halvaei I, Fesahat F, Fallah A, Nikonahad N, Taherinejad M (2013) Evaluating the role of silver nanoparticles on acrosomal reaction and spermatogenic cells in rat. Iran J Reprod Med 11:423–430
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mital P, Hinton BT, Dufour JM (2011) The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 84:851–858
Mohamed Y, Attia Y, Johansson Solum E (2018) Photoinduced one-pot synthesis of hydroxamic acids from aldehydes through in-situ generated silver nanoclusters, 71
Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, Tsukita S (1998) Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cell testes, 274, C1708-17 pp
Mruk Dolores D, Cheng CY (2010) Tight junctions in the testis: new perspectives. Philos Trans R Soc B: Biol Sci 365:1621–1635
Nair V, Turner G (1984) The thiobarbituric acid test for lipidperoxidation: structure of the adduct with malondialde-hyde. Lipids 19:804–805
Ola MS, Aleisa AM, Al-Rejaie SS, Abuohashish HM, Parmar MY, Alhomida AS, Ahmed MM (2014) Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol Sci 35:1003–1008
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee SH, Yoon J, Lee BC, Park K (2010) Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol 30:162–168
Prahalathan P, Kumar S, Raja B (2012) Morin attenuates blood pressure and oxidative stress in deoxycorticosterone acetate-salt hypertensive rats: a biochemical and histopathological evaluation. Metab Clin Exp 61:1087–1099
Rai M, Yadav A, Gade A (2008) Current [corrected] trends in phytosynthesis of metal nanoparticles. Crit Rev Biotechnol 28:277–284
Rai M, Ingle AP, Birla S, Yadav A, Santos CA (2016) Strategic role of selected noble metal nanoparticles in medicine. Crit Rev Microbiol 42:696–719
Robb GW, Amann RP, Killian GJ (1978) Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil 54:103–107
Ryu S, Lee S-H, Kim S, Yoon B-W (2016) Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res 11:298–304
Ryu D-Y, Kim K-U, Kwon W-S, Rahman MS, Khatun A, Pang M-G (2017) Peroxiredoxin activity is a major landmark of male fertility. Sci Rep 7:17174–17174
Schmittgen T, Livak KJ (2008) Schmittgen TD, Livak KJAnalyzing real-time PCR data by the comparative C(T) method. Nat Protocols 3(6):1101–1108 3, 1101-8 pp
Schrand AM, Braydich-Stolle LK, Schlager JJ, Dai L, Hussain SM (2008) Can silver nanoparticles be useful as potential biological labels? Nanotechnology 19:235104
Sharma VK, Yngard RA, Linm YA (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interf Sci 145:83–96
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Soga T, Teo CH, Cham KL, Idris MM, Parhar IS (2015) Early-Life Social Isolation Impairs the Gonadotropin-Inhibitory Hormone Neuronal Activity and Serotonergic System in Male Rats. Front Endocrinol 6
Taylor U, Barchanski A, Petersen S, Kues WA, Baulain U, Gamrad L, Sajti L, Barcikowski S, Rath D (2014) Gold nanoparticles interfere with sperm functionality by membrane adsorption without penetration. Nanotoxicology 8(Suppl 1):118–127
Thakur M, Gupta H, Singh D, Mohanty IR, Maheswari U, Vanage G, Joshi DS (2014) Histopathological and ultra structural effects of nanoparticles on rat testis following 90 days (Chronic study) of repeated oral administration. J Nanobiotechnol 12:42
Tiedemann D, Taylor U, Rehbock C, Jakobi J, Klein S, Kues WA, Barcikowski S, Rath D (2014) Reprotoxicity of gold, silver, and gold-silver alloy nanoparticles on mammalian gametes. Analyst 139:931–942
Tiwari DK, Jin T, Behari J (2011) Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol Mech Methods 21:13–24
van der Zande M, Vandebriel RJ, Van Doren E, Kramer E, Herrera Rivera Z, Serrano-Rojero CS, Gremmer ER, Mast J, Peters RJ, Hollman PC, Hendriksen PJ, Marvin HJ, Peijnenburg AA, Bouwmeester H (2012) Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 6:7427–7442
Wijnhoven S, Peijnenburg W, Herberts C, Hagens W, Oomen A, Heugens E, Roszek B, Bisschops J, Gosens I, van de Meent D, Dekkers S, deJong W, van Zijverden M, Sips A, Geertsma R (2009) Nano-silver: A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicol 2009:109–138
Yang G, Zhou Z, Cen Y, Gui X, Zeng Q, Ao Y, Li Q, Wang S, Li J, Zhang A (2015) Death receptor and mitochondria-mediated hepatocyte apoptosis underlies liver dysfunction in rats exposed to organic pollutants from drinking water. Drug Des Dev Ther 9:4719–4733
Yang L, Kuang H, Zhang W, Aguilar ZP, Wei H, Xu H (2017) Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci Rep 7:3303
Yao P, Li K, Song F, Zhou S, Sun X, Zhang X, Nussler AK, Liu L (2007) Heme oxygenase-1 upregulated by Ginkgo biloba extract: potential protection against ethanol-induced oxidative liver damage. Food Chem Toxicol 45:1333–1342
Yoshida S, Hiyoshi K, Ichinose T, Takano H, Oshio S, Sugawara I, Takeda K, Shibamoto T (2009) Effect of nanoparticles on the male reproductive system of mice. Int J Androl 32:337–342
Zhang R, Kang KA, Kang SS, Park JW, Hyun JW (2011) Morin (2',3,4',5,7-pentahydroxyflavone) protected cells against gamma-radiation-induced oxidative stress. Basic Clin Pharmacol Toxicol 108:63–72
Zhang Y, Ferguson SA, Watanabe F, Jones Y, Xu Y, Biris AS, Hussain S, Ali SF (2013) Silver nanoparticles decrease body weight and locomotor activity in adult male rats. Small 9:1715–1720
Zhang H, Yin Y, Wang G, Liu Z, Liu L, Sun F (2014a) Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells, 4, 4260 pp
Zhang T, Wang L, Chen Q, Chen C (2014b) Cytotoxic potential of silver nanoparticles. Yonsei Med J 55:283–291
Zhang XF, Choi YJ, Han JW, Kim E, Park JH, Gurunathan S, Kim JH (2015) Differential nanoreprotoxicity of silver nanoparticles in male somatic cells and spermatogonial stem cells. Int J Nanomedicine 10:1335–1357
Zhao W-p, Wang H-w, Liu J, Tan P-p, Luo X-l, Zhu S-q, Chen X-l, Zhou B-h (2018) Positive PCNA and Ki-67 expression in the testis correlates with spermatogenesis dysfunction in fluoride-treated rats. Biol Trace Elem Res 186:489–497
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arisha, A.H., Ahmed, M.M., Kamel, M.A. et al. Morin ameliorates the testicular apoptosis, oxidative stress, and impact on blood–testis barrier induced by photo-extracellularly synthesized silver nanoparticles. Environ Sci Pollut Res 26, 28749–28762 (2019). https://doi.org/10.1007/s11356-019-06066-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06066-1