Abstract
Diabetes-induced damages in brain are known as diabetic encephalopathy, which is well characterized by cellular, molecular and functional changes in the brain of diabetic subjects and rodents. However, little is known about the mechanism of damages and the therapeutic strategies in ameliorating those damages in the diabetic brain. In this study, we utilized a flavonoid, morin which is emerging as a potent drug against a wide range of free radical-mediated as well as neurodegenerative diseases. Morin (15 and 30 mg/kg body weight/day) was orally administered to two different groups of rats after 1 week of diabetes induction, and continued for five consecutive weeks. Two other untreated groups of diabetic and non-diabetic rats were used to compare with drug-treated groups. After drug treatments, cerebral cortex of the brain harvested and analyzed for different factors. Morin supplementation especially at high dose increased the levels of insulin, reduced glutathione, superoxide dismutase and catalase activities, and decreased fasting glucose and thiobarbituric acid reactive substances in the diabetic brain compared to untreated diabetic rats (P < 0.05). Morin also significantly decreased the level of inflammatory markers (TNFα, IL1β, IL-6) in the diabetic brain compared to untreated diabetic rats. Furthermore, the drug influenced an increase in the level of neurotrophic factors (BDNF, NGF and IGF-1) in the diabetic brain compared to untreated diabetic rats (P < 0.05). Thus, our results indicate a beneficial effect of morin by decreasing oxidative stress, inflammation and increasing the neurotrophic support in the diabetic brain, which may ameliorate diabetic encephalopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a metabolic disease which affects more than 371 million people worldwide and the number is increasing at an alarming rate (International Diabetes Federation, Diabetes Atlas 5th Edition, 2012). Diabetes induces various complications including neuropathy, nephropathy and retinopathy [1–3]. Among the neuropathy, autonomic and peripheral neuropathies are well studied, however, not much is known about the diabetes-induced damages in the central nervous system, known as diabetic encephalopathy. Diabetes-induced cellular, molecular and functional changes have been observed in the brain of diabetic subjects as well as in the experimental rodents [4, 5]. However, still the mechanism of the neuronal damage in the brain is unclear.
Increased oxidative stress is considered to play a central role in the diabetes-induced cellular damages including neurons in the brain [6]. A number of studies reported an increase in lipid peroxidation, nitrite levels, malondialdehyde and total oxidant status with a decrease in total antioxidant levels, and endogenous antioxidant marker enzymes in the brain of diabetic rats [7]. In addition, several studies suggest that diabetes-induced oxidative stress increases the level of proinflammatory cytokines including tumor necrosis factor (TNF-α), interleukin-6 (IL-6) [8] and also increases the expression of inflammatory molecules such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1(ICAM-1) and nuclear factor-kappa B (NF-kB) [8], which may cause degeneration of neurons, thus may initiate the development of lesions of diabetic encephalopathy.
Neurotrophic factors in brain are neuronal guidance molecules which play an important role in neuronal survival, growth, and functional maintenance. Studies in brain and neuronal cells suggest that neurotrophic factors including brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and insulin growth factor 1 (IGF-1) affect cell differentiation, synaptic connectivity, plasticity, growth, and cell survival [2, 9, 10]. However, a down-regulation of neurotrophins was observed in the diabetic brain [2, 4, 9–12], which may impair neuronal survival. Therefore, the purpose of this study was to find such a therapeutic drug which may be effective against oxidative stress, inflammation and to enhance the neurotrophic support to protect the damaging effect in the diabetic brain.
Among plant flavonoids, morin (3, 5, 7, 20, 40-pentahydroxyflavone) is a bioactive compound present in many herbs and fruits [13, 14]. Morin is considered a potential therapeutic drug to have properties including antioxidant [14], anti-inflammatory [15] and neuroprotective in neurodegenerative diseases with very minimal toxicity even at higher dose usage [14]. More recently, we have shown a number of beneficial effects of morin in hepatotoxicity, nephropathy [16] and osteoporosis [13] in the diabetic rats. Since, the treatment of diabetes involves chemical drugs and insulin which have various side effects with limited benefits, in contrast utilizing morin, a natural antioxidant would be effective, economical and safe for the better management of diabetes complications including encephalopathy. To our knowledge, no studies were reported on the protective effect of morin in the diabetic brain. Therefore, in this study we employed morin to treat streptozotocin-induced diabetic rats and analyzed its effect on oxidative stress, inflammation and neurotrophins in the diabetic brain. We anticipate that morin may exert beneficial effects within the brain by ameliorating those neurodegenerative factors in diabetes.
Materials and methods
Animals and experimental model
Three months aged male Wistar albino rats, weighing 250–270 g were received from Experimental Animal Care Center (King Saud University, Riyadh, Saudi Arabia). Single dose of streptozotocin 65 mg/kg body weight made in citrate buffer injected intraperitoneally to make rats diabetic. Diabetes was confirmed after 3 days by measuring fasting blood glucose level more than 300 mg/dl. For drug treatments, animals were divided into four groups (n = 6) as follows; (1) control (C), (2) diabetic (D), (3) diabetic treated with morin at a dose 15 mg/kg/day (D + M15), and (4) diabetic treated with morin 30 mg/kg/day (D + M30). Morin was suspended in 0.5 % (w/v) carboxymethyl cellulose (CMC) (vehicle) and administered orally by gavage to those rats. Vehicle and morin treatments were started once a day, after 1 week of diabetes induction and continued for five consecutive weeks. All procedures including euthanasia were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the Ethical Guidelines of the Experimental Animal Care Center, King Saud University, Saudi Arabia.
Tissue harvesting and sample preparation
At the end of the treatments, animals were fasted overnight and blood samples were collected though cardiac puncture under deep anesthesia. Cerebral cortex was quickly removed, rinsed in ice-cold saline, flash frozen in liquid nitrogen and stored at −80 °C until use. Blood samples were centrifuged at 3,000 rpm for 10 min, serum removed and stored at −20 °C until analysis. Brain tissues were homogenized in a cold 50 mM phosphate-buffered saline (pH 7.4) by a glass homogenizer. The homogenates were then centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatants were separated and used for enzyme activities assays and for other biochemical analyses.
Assay of glucose and insulin levels
Serum glucose levels were assayed using commercially available kit (RANDOX Laboratories Ltd., UK), while insulin serum level was assayed using ELISA kit (Bio-Source, Europe S.A., Belgium).
Estimation of thiobarbituric acid reactive substances (TBARS) levels
The lipid peroxidation products TBARS levels were estimated using commercially available assay kit (ZeptoMetrix, USA). Briefly, 2.5 ml of the kit provided reaction buffer was mixed with 100 μl of the homogenate. The mixture was then heated at 95 °C for 60 min. After cooling and centrifugation, the absorbance of the supernatant was measured at 532 nm using a spectrophotometer.
Estimations of glutathione (GSH) levels
The total GSH levels were measured in the brain of morin-treated and untreated diabetic and non-diabetic rats using the method described by Sedlak and Lindsay [17] with slight modification. Brain homogenate samples were deproteinized by adding an equal volume of metaphosphoric acid (2.5 % w/v). After 5 min, the mixture was centrifuged at 10,000 rpm and supernatant was collected. In the supernatant, 5 μl of 4 M triethanolamine per 100 μl was added and assay performed using 50 μl supernatant from brain. To this mixture, 100 μl of 0.01 M Ellman’s reagent, [5,5′-dithiobis-(2-nitro-benzoic acid)] (DTNB) was added. The absorbance of the clear supernatants was recorded to measure the concentration of GSH using spectrophotometer at 412 nm within 5 min.
Estimations of superoxide dismutase (SOD) and catalase (CAT) activities
SOD activity in brain was estimated according to the method described by Kono [18] using nitroblue tetrazolium as an indicator. The CAT activity was estimated by the method of Aebi [19] using H2O2 as substrate.
Assay for inflammatory cytokines levels
Concentrations of interleukin-1β (IL-1β), IL-6, and TNF-α were determined in the brain homogenate of control, diabetic and diabetic rats supplemented with morin using commercially available ELISA kits (USCN LIFE, Wuhan EIAab Science Co., Ltd) according to the manufacturer’s instructions.
Estimation of the levels of BDNF, NGF and insulin growth factor (IGF-1)
Brain levels of BDNF, NGF and IGF-1, were assessed and quantified by using enzyme-linked immunosorbent assay (ELISA) (R & D systems, USA) according to the manufacturer’s instructions.
Results
Effect of morin on body weight, blood glucose and insulin level
Table 1 shows the levels of mean body weights and insulin levels are significantly decreased while fasting blood glucose level increased in diabetic rats as compared to control animals (P < 0.01). Oral treatments with morin could not correct the body weights. However, morin treatment at high dose (30 mg/kg) showed a significant decrease in the levels of fasting blood glucose and an increase in the insulin level (P < 0.001) compared with untreated diabetic rats. At lower dose (15 mg/kg), morin could neither affect fasting glucose nor insulin levels in the diabetic rats compared to those untreated diabetic rats.
Effect of morin on oxidative stress
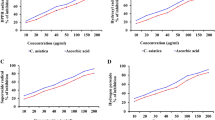
The levels of TBARS were increased while GSH levels significantly decreased (P < 0.01) as compared to control rats (Fig. 1). Morin treatments at both doses reduced the elevated TBARS levels in diabetic rats (Fig. 1a); however, significant reduction was only at high dose compared to untreated diabetic rats. Furthermore, high dose of morin increased the endogenous level of brain GSH in diabetic rats as compared to untreated diabetic rats (P < 0.05) (Fig. 1b). Activities of free radical scavenging enzymes, SOD and CAT were reduced in the brain of diabetic animals as compared to control group (P < 0.01) (Fig. 1c, d). The decreased SOD and CAT activities in the diabetic brain were restored by morin treatment at high doses.
Effects of morin on TBARS and GSH levels as well as SOD and CAT activities in brain of diabetic and non-diabetic animals. Values are expressed as mean ± SEM, n = 6/group. For statistical analysis, one-way ANOVA followed by Student–Newman–Keuls multiple comparisons test were applied. D (diabetic) group was compared with C (non-diabetic control) group (**P < 0.01) and morin-treated groups (#P < 0.05 and ## P < 0.01). Morin (15 mg or 30 mg/kg body weight) treated diabetic groups (D + M15, D + M30)
Effect of morin on inflammatory cytokines
The levels of TNF-α, IL-1β and IL-6 were significantly increased compared in diabetic brain compared to non-diabetic control (P < 0.01). Both doses of morin treatments to diabetic rats reduced the elevated level of cytokines in diabetic brain compared to without treatments (Fig. 2). However, the high dose of the drug was more effective in bringing back the elevated level of those cytokines in the brain of diabetic rats to control levels. In case of IL-6, both doses of the drug significantly decreased the level of IL-6 to control level. This suggests that the low dose of morin was sufficient to completely block the receptor or the pathway(s) which triggered an increase in the IL-6 level in diabetes compared to normal level. Therefore, high dose might have saturation level to further inhibit the receptor or pathway(s), thus cannot reduce the IL-6 level below the control.
Effects of morin on TNF-α, IL-1β and IL-6 levels in brain of diabetic and non-diabetic animals. Values are expressed as mean ± SEM, n = 6/group. For statistical analysis, one-way ANOVA followed by Student–Newman–Keuls multiple comparisons test were applied. D (diabetic) group was compared with C (non-diabetic control) group (*P < 0.05 and **P < 0.01) and morin-treated groups (# P < 0.05 and ## P < 0.01). Morin (15 mg or 30 mg/kg body weight) treated diabetic groups (D + M15, D + M30)
Effect of morin on the level of neurotrophic factor
The levels of BDNF, NGF and IGF-1 were significantly low in diabetic rats as compared to control rats (P < 0.05) (Fig. 3). High dose of the drug treatment significantly increased the levels of those neurotrophic factors in the diabetic brain compared to the untreated diabetic rats (Fig. 3a). However, low dose of morin treatments could not affect the levels of these neurotrophic factors in the brain of diabetic rats.
Effects of morin on BDNF, NGF and IGF-1 levels in brain of diabetic and non-diabetic rats. Values are expressed as mean ± SEM, n = 6/group. For statistical analysis, one-way ANOVA followed by Student–Newman–Keuls multiple comparisons test were applied. D (diabetic) group was compared with C (control) group (*P < 0.05) and morin (15 mg or 30 mg/kg body weight) treated diabetic groups (D + M15, D + M30) (# P < 0.05)
Discussion
Numerous studies suggest that diabetes causes brain injury which is evidenced from cellular, molecular and functional changes in the brain of diabetic animals [4, 20]. However, little is known about the mechanism of the brain damage and therapeutic strategies in ameliorating those damages. In continuation with our previous studies about the beneficial effects of the potential drug, morin in attenuating diabetes-induced oxidative and inflammatory damages in several rat tissues, in this study, we investigated the protective effect of the drug in ameliorating the damage in the cerebral cortex of diabetic rats. Therefore, first, we analyzed the effect of the drug on antioxidant status by measuring the level of reduced GSH and TBARS, and also the level of free radical scavenging enzymes (CAT and SOD). Second, we investigated the anti-inflammatory role of morin in diabetic brain by measuring important proinflammatory cytokines and factors after supplementation with morin. Third, we measured the level of potential neurotrophic factors (BDNF, NGF and IGF-1) in the diabetic brain after morin treatments. Our findings clearly suggest that morin administrations to the diabetic rats could be beneficial as the drug increases levels of antioxidant status, decreases the inflammation and increases the level of neurotrophic support in the diabetic brain.
Increasing evidence suggests that increased oxidative stress in diabetes is the central to pathogenesis of tissue damage including brain [6]. We and others have shown that diabetes induces oxidative stress in several tissues including retina, kidney, liver and bone of diabetic rodents [13, 16, 21]. In the present study, TBARS levels were significantly increased whereas the level of reduced GSH and activities of superoxide dismutase and catalase were markedly decreased in the cerebral cortex of diabetic rats. Treatment with morin to diabetic rats returned the levels of TBARS, GSH and activities of SOD and CAT in the brain towards their control values. These protective effects of morin against oxidative stress and lipid peroxidation are in agreement with our published reports and also as reported by others [13, 14, 16]. Thus, we suggest that morin may have cerebroprotective action against diabetes-induced injury through antioxidative mechanism.
Proinflammatory cytokines are known to be elevated in several neuropathological states such as in the case of diabetes. Among proinflammatory cytokines, TNF-α, IL-1β, and IL-6 are the major cytokines which initiate inflammatory reactions, and induce expression of other cytokines [22]. The increased levels of these cytokines measured in the brain of diabetic rats may contribute to glial-induced neuronal death. The levels of those cytokines were significantly reduced in the diabetic brain after morin treatments. These results are in accordance with the earlier reports that morin reduced the production of proinflammatory cytokines [13, 23, 24]. Similarly, a number of other flavonoids have been shown to inhibit IL-1 β, TNF-α and IL-6 production in activated microglial cells [25]. Therefore, morin may have cerebroprotective action through anti-inflammatory effects.
Neurotrophic factors play an important role in neuronal survival and maintenance. In the present study, we found a decreased level of BDNF, NGF and IGF-1 in the brain of diabetic rats consistent with previous studies which may cause neurodegeneration in the brain [4, 9, 12]. Treatments with morin could markedly increase their levels. Our results are in agreement that flavonoids induce synthesis and secretion of neurotrophic factors as reported in glial cells and brain [26, 27]. Thus, increased levels of neurotrophic factors induced by morin may protect neuronal damage by their antiapoptotic and antioxidants actions. Therefore, morin might exert cerebroprotective action by inducing neurotrophic support to the neuronal cells under diabetic conditions. Taken together, the findings of this study suggest that the flavonoid, morin possesses strong potential as antioxidant, anti-inflammatory and neurotrophic support actions in the diabetic brain. Therefore, the drug may represent an important anti-diabetic molecule with its potential to limit neurodegeneration and prevent or reverse diabetic encephalopathy.
References
Ola MS, Nawaz MI, Siddiquei MM et al (2012) Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complicat 26:56–64
Ola MS, Nawaz MI, Khan HA et al (2013) Neurodegeneration and neuroprotection in diabetic retinopathy. Int J Mol Sci 14:2559–2572
Sun YM, Su Y, Li J et al (2013) Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem Biophys Res Commun 433:359–361
Navaratna D, Guo SZ, Hayakawa K et al (2011) Decreased cerebrovascular brain-derived neurotrophic factor-mediated neuroprotection in the diabetic brain. Diabetes 60:1789–1796
Francis GJ, Martinez JA, Liu WQ et al (2008) Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain 131:3311–3334
Reagan LP, Magariños AM, McEwen BS (2000) Neurological changes induced by stress in streptozotocin diabetic rats. Nature 404:787–790
Acar A, Akil E, Alp H et al (2012) Oxidative damage is ameliorated by curcumin treatment in brain and sciatic nerve of diabetic rats. Int J Neurosci 122:367–372
Prabhakar O (2013) Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 386(8):705–710
Li ZG, Zhang W, Sima AA (2005) The role of impaired insulin/IGF action in primary diabetic encephalopathy. Brain Res 1037:12–24
Tirassa P, Maccarone M, Florenzano F et al (2013) Vascular and neuronal protection induced by the ocular administration of nerve growth factor in diabetic-induced rat encephalopathy. CNS Neurosci Ther 19:307–318
Nitta A, Murai R, Suzuki N et al (2002) Diabetic neuropathies in brain are induced by deficiency of BDNF. Neurotoxicol Teratol 24:695–701
Sposato V, Manni L, Chaldakov GN et al (2007) Streptozotocin-induced diabetes is associated with changes in NGF levels in pancreas and brain. Arch Ital Biol 145:87–97
Abuohashish HM, Al-Rejaie SS, Al-Hosaini KA et al (2013) Alleviating effects of morin against experimentally-induced diabetic osteopenia. Diabetol Metab Syndr 5:5
Prahalathan P, Kumar S, Raja B (2012) Morin attenuates blood pressure and oxidative stress in deoxycorticosterone acetate-salt hypertensive rats: a biochemical and histopathological evaluation. Metabolism 61:1087–1099
Fang SH, Hou YC, Chang WC et al (2003) Morin sulfates/glucuronides exert anti-inflammatory activity on activated macrophages and decreased the incidence of septic shock. Life Sci 74:743–756
Aleisa AM, Al-Rejaie SS, Abuohashish HM et al (2013) Nephro-protective role of morin against experimentally induced diabetic retinopathy. Digest J Nanomaterials Biostructureol 8:395–401
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684
Huang M, Gao L, Yang L et al (2012) Abnormalities in the brain of streptozotocin-induced type 1 diabetic rats revealed by diffusion tensor imaging. Neuroimage Clin 1:57–65
Ola MS, Ahmed MM, Abuohashish HM et al (2013) Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem Res 38:1572–1579
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 17:97
Chen WP, Wang YL, Tang JL et al (2012) Morin inhibits interleukin-1β-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Int Immunopharmacol 12:447–452
Manna SK, Aggarwal RS, Sethi G et al (2007) Morin (3,5,7,2′,4′-pentahydroxyflavone) abolishes nuclear factor-kappaB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-kappaB-regulated gene expression and up-regulation of apoptosis. Clin Cancer Res 13:2290–2297
Li R, Huang YG, Fang D et al (2004) (−)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res 78:723–731
Xu SL, Bi CW, Choi RC et al (2013) Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: a signaling response mediated by estrogen receptor. Evid Based Complement Alternat Med 2013:127075
De Nicoló S, Tarani L, Ceccanti M et al (2013) Effects of olive polyphenols administration on nerve growth factor and brain-derived neurotrophic factor in the mouse brain. Nutrition 29:681–687
Acknowledgments
The authors would like to extend their sincere appreciation to Deanship of Scientific Research at King Saud University for its funding of this research through the Research Project No. RGP-VPP-263.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ola, M.S., Aleisa, A.M., Al-Rejaie, S.S. et al. Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol Sci 35, 1003–1008 (2014). https://doi.org/10.1007/s10072-014-1628-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1628-5