Abstract
This study explores the zero-valent iron (ZVI) dechlorination of pentachlorophenol (PCP) and its dependence on the dissolved oxygen (O2), presence/formation of iron oxides, and presence of nickel metal on the ZVI surface. Compared to the anoxic system, PCP dechlorination was slower in the presence of O2, which is a potential competitive electron acceptor. Despite O2 presence, Ni0 deposited on the ZVI surfaces catalyzed the hydrogenation reactions and enhanced the PCP dechlorination by Ni-coated ZVI bimetal (Nic/Fe). The presence of O2 led to the formation of passivating oxides (maghemite, hematite, lepidocrocite, ferrihydrite) on the ZVI and Nic/Fe bimetallic surfaces. These passive oxides resulted in greater PCP incorporation (sorption, co-precipitation, and/or physical entrapment with the oxides) and decreased PCP dechlorination in the oxic systems compared to the anoxic systems. As received ZVI comprised of a wustite film, and in the presence of O2, only ≈ 17% PCP dechlorination observed after 25 days of exposure with tetrachlorophenol being detected as the end product. Wustite remained as the predominant oxide on as received ZVI during the 25 days of reaction with PCP under oxic and anoxic conditions. ZVI acid-pretreatment resulted in the replacement of wustite with magnetite and enhanced PCP degradation (e.g. ≈ 52% of the initial PCP dechlorinated after 25 days under oxic condition) with accumulation of mixtures of tetra-, tri-, and dichlorophenols. When the acid-washed ZVI was rinsed in NiSO4/H2SO4 solution, Ni0 deposited on the ZVI surface and all the wustite were replaced with magnetite. After 25 days of exposure to the Nic/Fe, ≈ 78% and 97% PCP dechlorination occurred under oxic and anoxic conditions, respectively, producing predominantly phenol. Wustite and magnetite are respectively electrically insulating and conducting oxides and influenced the dechlorination and H2 production. In conclusion, this study clearly demonstrates that the dissolved oxygen present in the aqueous solution decreases the PCP dechlorination and increases the PCP incorporation when using ZVI and Nic/Fe bimetallic systems. The findings provide novel insights towards deciphering and optimizing the performance of complex ZVI and bimetallic systems for PCP dechlorination in the presence of O2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pentachlorophenol (PCP) is an organochlorine compound, which has been widely used in the past as a wood preservative and biocide (UNEP 2014). Commercial PCP, which is currently used as a restricted biocide also contains other contaminants such as trace levels of tetrachlorophenols and trichlorophenols (ATSDR 1999). PCP is reported as a probable human carcinogen (IARC 1991; USEPA 2010), a priority pollutant (USEPA 2018a, b; EC 2016) and toxic to aquatic organisms (UNEP 2014). Considering the potential adverse effects of PCP for human health, the USEPA has stipulated the maximum contaminant level of PCP in drinking water as 1 ppb (USEPA 2018a, b). The use of PCP has been banned or restricted in many countries following the awareness of its toxicity and environmental impacts (UNEP 2014). Nevertheless, PCP’s recalcitrance has led to its continued ubiquitous presence as a contaminant in the environmental media (surface water, groundwater, soil, and sediment).
Various treatment approaches such as reductive dechlorination using zero-valent iron (ZVI) (Gunawardana et al. 2018; Kim and Carraway 2000), photocatalysis (Lan et al. 2011; Li et al. 2011; Ma et al. 2019), microbial-mediated reductive dechlorination (Xu et al. 2018; Yang and Chen 2016), and membrane biofilm reactors (Long et al. 2018) are being explored for PCP degradation. For passive treatment, permeable reactive barriers (PRBs) offer a cost-effective solution for the treatment of contaminants (Henderson and Demond 2007). Micrometer size ZVI has been widely used in PRBs as the reactive medium to reductively dechlorinate a range of chlorinated organics (Cheng et al. 2007; Choi et al. 2008; Chun et al. 2010; Feng and Lim 2005; Kim and Carraway 2000; Matheson and Tratnyek 1994). The process of reductive transformation of contaminants present in a ZVI/water system is driven by a combination of reducing agents that are concurrently present in the system, i.e., (1) direct reduction through electrons from elemental iron (Fe0) and (2) indirect reduction by secondary reductants (electrons from adsorbed/structural FeII, H/H2) and tertiary/quaternary reductants (such as Fe3O4 and green rust) (Hu et al. 2018).
On the other hand, when using ZVI systems, a decrease in PCP dechlorination kinetics has been observed over time as a result of various reasons such as presence/formation of surface passivating oxides on the iron surface and accumulation of lower chlorinated compounds (Gunawardana et al. 2018). Hence, the use of bimetallic particles formed by incorporating a second metal with a higher reduction potential than ZVI (such as Ni, Pd, or Pt) on the ZVI surface has been explored to overcome the limitations of ZVI systems. Bimetallics showed enhanced hydrodechlorination of chlorinated phenols (Choi et al. 2008; Ko et al. 2007; Liu et al. 2001; Xu et al. 2012; Zhou et al. 2010) and aliphatics (Feng and Lim 2005; Schrick et al. 2002). Further, some studies reported no PCP degradation with unmodified ZVI (Morales et al. 2002), while Pd/Fe, Pd/Mg (Morales et al. 2002), and Ni/Fe (Cheng et al. 2010; Zhang et al. 2006) dechlorinated PCP to lower chlorinated phenols and phenol. Also, nano-sized Pd/Fe converted 4-CP, 24-DCP, and 246-TCP to phenol (Zhou et al. 2010). In contrast, Kim and Carraway (2000) reported a marked decrease in PCP dechlorination with bimetallic systems (Pd/Fe, Pt/Fe, Ni/Fe, Cu/Fe) compared to unmodified ZVI. The increased activity of bimetals is attributed to several mechanisms, including catalysis of the hydrogenation reaction and the formation of galvanic cells, which enhance electron transfer on the metal surface (Tian et al. 2009). As the high cost of Pd or Pt can limit their use, an economical alternative such as Ni is preferable for real application of bimetals in PRB systems (Kim and Carraway 2000).
ZVI corrosion due to Fe0 oxidation results in the formation of a range of iron oxides and/or oxyhydroxide precipitates (Matheson and Tratnyek 1994) that can affect the ZVI performance over time. ZVI reactivity was reported to be related to the characteristics of iron oxides initially present and formed (during the reaction with PCP) on the ZVI particles (Gunawardana et al. 2018). Magnetite has a high electrical conductivity and can enhance the interfacial electron transfer processes (Liu et al. 2006), while FeIII oxides and oxyhydroxides (e.g., lepidocrocite, goethite, maghemite), in comparison to magnetite, have a higher band gap between the valence and the conduction bands, causing iron surface passivation by hindering the transfer of both electrons and contaminant molecules across the solid/liquid interface (Farrell et al. 2000).
Dissolved oxygen (DO) is reported as the preferred electron acceptor in a ZVI/H2O system with chlorinated compounds under oxic conditions (Matheson and Tratnyek 1994). Hence, under oxic conditions, oxygen could compete with the chlorinated compounds for the electrons in the ZVI/H2O system (Junyapoon 2005). Such competition may result in significantly lower dechlorination rates under oxic conditions compared to that of in the anoxic systems (Ghauch et al. 2010; Ghauch and Tuqan 2009; Helland et al. 1995). Additionally, the presence of DO is expected to affect the composition of oxide phases form on the iron surface (Ritter et al. 2002), potentially enhancing or decreasing the reactivity of ZVI and bimetals. Therefore, PCP dechlorination by ZVI or bimetals is expected to be influenced by the presence of oxygen, and may be hindered, as DO could compete with PCP for the electrons generated during the iron corrosion. Only a few studies have assessed the reduction of chlorinated compounds using ZVI and bimetals under oxic and anoxic conditions (Ghauch et al. 2010; Ghauch and Tuqan 2009; Helland et al. 1995; Wan et al. 1999). However, studies have not been reported on the comparison of PCP dechlorination using ZVI and bimetals under oxic and anoxic conditions.
Although dechlorination is the preferred mechanism, incorporation of PCP with the iron oxide phases as they evolve during the reaction also plays a major role in the removal of PCP from aqueous solutions by ZVI-based systems (Gunawardana et al. 2011; Noubactep 2008). The incorporation process involves sorption, co-precipitation, and/or physical entrapment of PCP molecules with the iron oxides on the ZVI surface (Gunawardana et al. 2018). The incorporated PCP molecules with the oxide layers are not freely available in the aqueous phase for direct contact with the ZVI for dechlorination thus they are not further degraded. Since the availability of DO can vary in groundwater, the role of DO, in possible competition for electrons and oxides formation, during PCP dechlorination, would be relevant for the application of ZVI or bimetals in PRBs in groundwater treatment systems. However, at present, research is not available on the effect of DO presence on PCP dechlorination, iron oxides formation, and the effect of oxides formed in the presence of DO on PCP dechlorination and incorporation when using ZVI and bimetals.
Therefore, this study explores the influence of DO on the PCP dechlorination by ZVI and Ni-ZVI bimetals. Specifically, PCP dechlorination by unwashed (i.e., as received), acid washed, and Ni-coated electrolytic iron powder was investigated. Batch experiments were conducted with each material under oxic and anoxic conditions to explore the effect of DO on the performance of ZVI and bimetals. The formation of various oxides on ZVI and bimetals in the presence of DO and their potential impact on the PCP dechlorination and incorporation processes were also studied. The study design enabled the quantification of the different roles of dechlorination and incorporation in removing PCP from the solution as a function of the variables studied.
Materials and methods
Materials
Electrolytic iron powder with particle size 100 mesh and smaller (ACROS, Thermo Fisher Scientific) was used as ZVI. Analytical grade ethyl acetate, acetonitrile, acetone, sulfuric acid (H2SO4), and hydrochloric acid (HCl) were obtained from Ajax Finechem. PCP (98%), standard solutions of PCP, phenol, and chlorophenol isomers in methanol, and NiSO4.6H2O (> 98%) were purchased from Sigma-Aldrich. Deionized water with a resistivity of 18.20 MΩ cm (Millipore-Q system) was used in all experiments. For anoxic experiments, deoxygenated water (DW) was prepared by degassing deionized water at 80 °C for 1 h, followed by sparging with nitrogen for half an hour.
Acid-washed iron particles were prepared following the method of Liu et al. (2006). Iron powder (500 g) was added to 1.5 L of 1 N H2SO4 solution, and the mixture was agitated (100 rpm) at room temperature on a rotary shaker for 30 min, followed by rinsing with DW. After rinsing, the iron particles were dried under nitrogen for 4 h at 100 °C and then stored under nitrogen until use. Nickel-coated bimetallic iron particles (Nic/Fe) were prepared using the method explained by Kim and Carraway (2000). A Ni2+ solution was prepared by mixing 2 mL of 2.4% Ni stock solution (prepared using NiSO4.6H2O and 10% H2SO4) with 200 mL of DW (final solution pH 1.60), and 100.0 g of acid-washed iron powder was added to the mixture. The contents were placed on a rotary shaker for 1 h at 100 rpm, rinsed with DW and then acetone, air-dried at room temperature, and stored under nitrogen until use (Kim and Carraway 2000). Atomic absorption spectroscopy analysis of the Ni2+ solution before and after exposing to iron showed 92% removal of Ni from the solution, indicating that the bimetallic Ni content was 442 ppm (mg of Ni per kg of Fe).
Methods
A 5000 ppm PCP stock solution was prepared in ethyl acetate. Wheaton amber vials (30 mL) were used as batch reactors. To each reactor 1.00 (± 0.01) g of metal (unwashed-as received ZVI, or acid-washed ZVI, or Ni particles, or Nic/Fe bimetal) and 10 mL of water (oxygenated or deoxygenated) was added. Then the PCP stock solution (10 μL) was added to the reactors giving an initial concentration of 20 μM. Reactors were then immediately sealed with aluminum capped PTFE/silicone septa and agitated on a rotary shaker at 100 rpm at 23 (± 1) °C until analysis.
Air and oxygen-free nitrogen gas were used as the headspace gases during setting up the oxic and anoxic experiments, respectively. The anoxic reactors were continuously purged with oxygen-free nitrogen gas during the addition of deoxygenated water and PCP stock solution, immediately sealed, and continuously agitated until sampled. The reactors for the oxic experiments were kept under oxic condition (open to air) only initially during the addition of DI water and PCP stock solution, then sealed, and continuously agitated until sampled. The oxic batch reactors were kept sealed during the experiment to avoid loss of PCP and degradation products through volatilization as well as to measure the hydrogen gas accumulated in the headspace. Under oxic conditions, reactions such as oxidation of ZVI, formation of lepidocrocite, and precipitation of Fe(OH)3 could consume the DO present in the system. Therefore, the amount of oxygen available in the oxic reactors was limited due to the sealed reactor system and available DO being consumed during the abovementioned reactions. Consequently, the oxidation process of PCP was not promoted and not considered under the oxic experiments in this study.
Control reactors were prepared similarly without adding metals (ZVI, Ni, or Nic/Fe). All experiments were performed in duplicate. During each sampling time over 25 days, the solution pH was recorded, and four reaction vials and two control vials were sacrificed for various analyses. At each sampling time, the remaining dissolved and total (i.e., dissolved plus incorporated and extracted) PCP concentrations were measured. To measure the total PCP concentration, 5 mL of ethyl acetate was added to each reactor containing reaction solution and metal particles. The mixture was then shaken at 100 rpm for 30 min, followed by the addition of 1 mL of concentrated HCl and mixing for an additional 10 min (Kim and Carraway 2000). For the determination of dissolved PCP in the solution, 9 mL aqueous solution, which was filtered using 0.2-μm RC (Regenerated Cellulose) membrane filters, was subjected to ethyl acetate/HCl extraction as previously explained for total PCP determination. Following this, 1.5 mL of the ethyl acetate was extracted, filtered (0.2 μm RC), and then transferred to autosampler vials for analysis of PCP and degradation products. The solid ZVI or Nic/Fe particles from the reactors used for quantifying the dissolved PCP concentration in the solution were retained for Raman analysis of the iron oxide phases formed/present.
Analysis
The analysis of PCP and its intermediates was performed using gas chromatography-mass spectrometer (Shimadzu model GCMS-QP2010S) equipped with a ZEBRON ZB5-msi capillary column (30 m L × 0.25 mm ID × 0.25 μm thickness). The Selected Ion Monitoring (SIM) analysis method with split mode injection of 2 μL of the sample at a ratio of 80:1 was used to quantify the PCP and the dechlorination products. Helium was used as the carrier gas at a flow rate of 1 mL/min. The injection temperature was set at 250 °C. The column temperature program was 70 °C for 2 min, ramped at 5 °C min−1 to 200 °C and held for 2 min, and then again ramped at 10 °C min−1 to 300 °C and held for 5 min. The hydrogen gas accumulating in the rectors was analyzed using an SRI 8610C gas chromatograph equipped with a HayeSep Q 80/100 column (Alltech, 6′ × 1/8″ × 0.085″ SS) and a thermal conductivity detector (TCD). The column temperature was maintained at 24 °C, and nitrogen was used as the carrier gas at a flow rate of 10 mL/min.
Statistical analysis was carried out using the SPSS statistical software package (IBM SPSS Statistics version 20.0.0 [SPSS Inc., USA]). The effects of different treatments on the amount of PCP degraded, PCP incorporated and extracted, as well as hydrogen gas accumulated at 25 days of the experiment were compared using a two-way analysis of variance model. Significance was determined at the 95% level.
Solid-phase characterization
The specific surface areas of the iron and Nic/Fe bimetallic particles were measured by Brunauer-Emmett-Teller (BET) N2 method using a Micromeritic Tristar 3000. Surface topography, characteristics and elemental information on selected regions of the iron surface as well as the distribution of Ni on the bimetal surface were obtained using FEI Quanta 200 F environmental scanning electron microscope (ESEM) coupled with a SiLi (lithium drifted) energy dispersive spectroscopy (EDS). Images were collected using the back-scattered detector at a beam potential of 20 kV. Renishaw Raman spectroscopy (Renishaw System 1000) was used to identify and quantify the iron oxides present on iron surfaces prior to and following contact with PCP. Eight reference iron oxides commonly found on iron metal surfaces [akaganeite, maghemite, hematite, magnetite, lepidocrocite, goethite, feroxyhyte, ferrihydrite] were prepared using established methods (Cornell and Schwertmann 2003). These reference oxide phases were characterized by Raman spectroscopy (Gunawardana et al. 2018). A Wustite mineral specimen was characterized by EDS and Raman analysis (Nadoll and Mauk 2011). The bands of different iron oxides present on all iron samples overlapped extensively indicating the presence of a mixture of oxide phases, making it difficult to clearly distinguish between them in the observed spectra. Hence, multivariate curve resolution with alternating least squares (MCR-ALS) analysis was performed in MATLAB (Mathworks®) to estimate the proportion of the different iron oxides present on the iron surface (Jaumot et al. 2005) using the reference spectra. The green rust phases were not stable under the sample storage conditions and therefore these spectra were excluded from the reference spectra.
Results and discussion
PCP dichlorination by ZVI and Nic/Fe bimetal under oxic and anoxic conditions
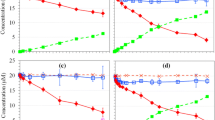
The change in the total concentration of PCP and its dechlorination products as PCP reacts with ZVI (unwashed, acid washed) and Nic/Fe under oxic and anoxic conditions are presented in Fig. 1. The ZVI in the as-received state is termed as unwashed or UW. The ZVI after treatment with H2SO4 is termed as acid washed or AW. For each solid phase, the presence of DO significantly decreased (p < 0.05) the PCP dechlorination. The unwashed ZVI showed the lowest reactivity of all treatments (except Ni only treatment); only 17% (Fig. 1a) and 34% (Fig. 1b) of the PCP dechlorinated over 25 days under oxic and anoxic conditions, respectively. The acid washing of ZVI led to an enhancement in performance with 52% (Fig. 1c) and 62% (Fig. 1d) PCP dechlorination over 25 days under oxic and anoxic conditions, respectively. The Nic/Fe bimetallic particles showed the fastest degradation; over 25 days of exposure, 78% (Fig. 1e) and 97% (Fig. 1f) PCP dechlorination were observed under oxic and anoxic conditions, respectively. In contrast, compared to ZVI (UW and AW) and Nic/Fe bimetallic systems, the Ni particles alone (1.0 g), when used without ZVI, exhibited the least reactivity with significantly lower PCP dechlorination (p < 0.05). When PCP reacted with Ni particles alone under anoxic conditions, only 10% of the PCP dechlorinated over 25 days of reaction. The standard reduction potential of Ni (− 0.26 V) is higher than that of iron (− 0.44 V) (Arning and Minteer 2007). Hence, Ni is a poorer electron donor compared to ZVI for dechlorination as also evidenced by the lower PCP degradation with only Ni in the reactors.
Total concentrations of PCP and its dechlorination products remaining either in solution or incorporated in the systems with a unwashed ZVI (oxic), b unwashed ZVI (anoxic), c acid-washed ZVI (oxic), d acid-washed ZVI (anoxic), e Nic/Fe bimetal (oxic), and f Nic/Fe bimetal (anoxic). Error bars indicate ± one standard deviation. Some error bars are smaller than data symbols. Total TeCPs, TCPs, DCPs, and MCPs were the sum of all the TeCPs, TCPs, DCPs, and MCPs measured, respectively
Mass balance recoveries in all the systems were within 96–98% demonstrating that (1) the PCP degradation by ZVI and Nic/Fe under oxic and anoxic conditions was due to reductive dechlorination, and (2) incorporated PCP and degradation products were efficiently extracted from the solution and also from the oxide phases by the HCl and ethyl acetate extraction. As confirmed by the detection and quantification of degradation products and mass balance, the observations provide evidence that the reduction of total PCP concentration (as shown in Fig. 1) is basically entirely due to the PCP dechlorination process.
The different ZVI treatments and the presence or absence of DO affected the formation of PCP degradation products. Unwashed ZVI transformed PCP only to tetrachlorophenols (TeCPs) (Fig. 1a, b). Acid-washed ZVI showed PCP conversion to tetra-, tri- (TCPs), di- (DCPs), and mono-chlorophenols (MCPs). With acid-washed ZVI under oxic conditions, TeCPs were the dominant product over the whole reaction (Fig. 1c) while a large amount of MCPs formed under anoxic conditions after 25 days of reaction (Fig. 1d). Interestingly, after 25 days under oxic and anoxic conditions, the Nic/Fe bimetal transformed PCP predominantly to phenol with relatively low concentrations of the lower chlorinated phenols finally remaining in the reactors (Fig. 1e, f). In the following sections, the present study explores the significance of the presence of DO initially present in the oxic reactors on the iron oxides formation, the effects of oxides formed in the presence of DO on PCP dechlorination, as well as incorporation with the iron oxides formed in the ZVI and Nic/Fe bimetal systems observed in the experiments.
Characterization of ZVI and Nic/Fe material
Surface characteristics and elemental information of selected regions of the ZVI surfaces and the distribution of Ni on the Nic/Fe bimetal surface were obtained using the ESEM-EDS analysis (Fig. 2). Both acid-washed ZVI and Nic/Fe bimetal particles demonstrated larger oxygen content (24–27%) compared to unwashed ZVI (oxygen content 9.5%). Despite this higher oxygen content, the acid-washed ZVI and the Nic/Fe were effective than the unwashed ZVI in PCP dechlorination under both oxic and anoxic conditions (Fig. 1).
Environmental scanning electron microscopy images and elemental analysis on the iron surfaces using ESEM/EDS before exposed to PCP a unwashed ZVI, b acid washed ZVI, c Nic/Fe bimetal. The areas marked in red squares were randomly selected on the ZVI and Nic/Fe surfaces and scanned using the EDS for elemental analysis
The ESEM/EDS analysis showed that Ni was deposited as small clusters on the acid-washed ZVI surface (Fig. 2c). The reduction potential and reduction kinetics of the metals are known to play a major role in determining the structure of the bimetallic catalysts (De et al. 2016). In this study, the two metallic precursors (Ni and ZVI) were mixed at the same time, and the Nic/Fe bimetallic particles were prepared by reductive deposition of Ni0 on the ZVI surface (Fe0 + Ni2+ → Ni0 + Fe2+). Under such circumstance, the reduction potentials of the metals are reported as important in determining the final structure of the Ni-Fe bimetallic particles (De et al. 2016). Other than the preparation method, the relative position of the two metals in the periodic table is also known to govern the structure of a bimetallic system. In general, the metals that are located further away from Ni (e.g., Fe) in the periodic table are known to form intermetallic compounds with Ni (De et al. 2016). Hence, under the preparation methods and other conditions followed in this study, Ni seems to deposit as clusters of Ni0 on the acid-washed ZVI surfaces with electrostatic forces leading to the formation of Ni-Fe intermetallic compounds. However, a straightforward relationship is yet to be established between the reduction kinetics and reduction potential for determining the structure of the bimetallic particles. Advanced characterization techniques [e.g., Auger electron spectroscopy (AES), X-ray photoelectron spectroscopy (XPS), and extended X-ray absorption fine structure (EXAFS)] would be required to understand the definite structure of the Nic/Fe bimetallic particles.
Raman spectroscopy was used to identify and quantify the individual oxide phases present before reaction and formed during the reaction. The effect of DO on iron oxides formation and the role of formed oxides (in the presence of DO) on PCP dechlorination and incorporation were elaborated using the findings of iron oxides. Typical Raman spectra of the ZVI (unwashed and acid washed) and Nic/Fe bimetal at the start of experiments and after 25 days of reaction are shown in Fig. 3 with spectra of the relevant reference oxide phases. The amounts of individual oxides detected on the iron surfaces, prior to reaction and following the reaction period of 1, 12, and 25 days with PCP, are shown in Table 1.
Raman spectra of pure reference iron oxides. (a) Maghemite. (b) Hematite. (c) Lepidocrocite. (d) Wustite. (e) Magnetite. (f) Ferrihydrite, typical sample spectra that was unreacted. (g) Unwashed ZVI. (h) Acid-washed ZVI. (i) Nic/Fe bimetal, and typical sample spectra after 25 days of reaction. (j) Unwashed ZVI (oxic). (k) Unwashed ZVI (anoxic). (l) Acid-washed (oxic). (m) Acid-washed (anoxic). (n) Nic/Fe bimetal (oxic). (o) Nic/Fe bimetal (anoxic)
The major oxide on the unwashed ZVI was wustite, at the start of the experiment as well as during and after 25 days of reaction under both oxic and anoxic conditions (Table 1). After 25 days of reaction, there was an increase in the amount of magnetite detected on the unwashed ZVI surface; however, this increase was smaller in the presence of DO (Table 1). The pervasiveness of wustite iron oxide on unwashed ZVI surfaces could be because wustite form on the iron surfaces at high temperatures, (e.g., 600 °C), is metastable for extended periods at ambient conditions (Redl et al. 2004). The surface of the acid washed ZVI initially contained ≈ 80% magnetite with ≈ 20% wustite (Table 1). During the reaction of acid-washed ZVI with PCP, in the presence of DO, a small decrease in the amount of magnetite was observed. Concurrently, small amounts of maghemite, hematite, and lepidocrocite were detected on the acid-washed ZVI surface over the 25 days of reaction (Table 1). The surface of the Nic/Fe bimetal contained ≈ 90% magnetite and ≈ 10% of wustite and ferrihydrite at the start of the experiment. In comparison to ZVI systems, Nic/Fe particles contained a higher amount of magnetite initially as well as after 1 and 12 days of reaction with PCP (Table 1). Interestingly, with the Nic/Fe particle, magnetite was maintained as the main oxide phase over the 25 days of reaction both in the presence and absence of the DO. However, when DO was present with the Nic/Fe system, a small decrease in the magnetite amount and formation of passivating oxides (maghemite and lepidocrocite) was observed after 1 and 12 days of reaction (Table 1). These passivating oxides seemed to remain on the Nic/Fe surface during the 25 days of reaction (Table 1).
The findings of this study demonstrated a clear relationship between the surface iron oxide coatings and the efficacy of the iron materials towards the reductive dechlorination of PCP. Iron surfaces that are dominated by wustite have reductive dechlorination performance that is substantially worse than iron surfaces that are dominated by magnetite. The as-received iron powder used in this study had wustite present on the surface. Wustite (FeIIO) acts as a passivating iron oxide due to a large band gap (2.3 eV) (Cornell and Schwertmann 2003) between its valence and conduction bands. Thus, the low electrical conductivity of wustite can be linked to the poor PCP dechlorination reactivity which requires electron transfer from the Fe0, Fe2+ ions, or other tertiary/quaternary reducing agents (Hu et al. 2018). Conversely, the large amounts of magnetite present on the acid washed samples, and even larger amounts in the Nic/Fe bimetal can be linked to their superior dechlorination characteristics. Magnetite (FeIII2FeIIO4) is formally a semiconductor but it does not lead to passivating the metal surface as the band gap (0.1 eV) between its valence and conduction bands is small (Cornell and Schwertmann 2003; Farrell et al. 2000). This distinctive characteristic gives magnetite an electrical conductivity close to that of metals, thus facilitating the electron transfer process through the oxide film and at the ZVI surface to reduce the organic compounds (Liu et al. 2006). The observations discussed above are consistent with previous studies on the effect of the oxidation state of iron oxides [i.e., Fe(II):Fe(III) ratio] on the kinetics of electron transfer process (Gorski et al. 2010; Gorski and Scherer 2009; Stratmann and Müller 1994).
When DO was present, the amount of magnetite detected on the unwashed ZVI surface was less compared to the amount of magnetite detected with the anoxic unwashed ZVI system (Table 1). This finding further supports the reduced PCP dechlorination efficiency of unwashed ZVI in the presence of DO. Furthermore, the presence of DO resulted in the formation of passivating oxides such as maghemite, hematite, lepidocrocite, and ferrihydrite on the acid-washed ZVI and Nic/Fe surfaces (Table 1). These oxides have a lower conductivity at room temperature compared to the conductivity of magnetite (Cornell and Schwertmann 2003). Therefore, the formation of these passivating oxides could also result in lower PCP dechlorination by acid-washed ZVI and Nic/Fe systems in the presence of DO compared to the corresponding anoxic systems.
The findings of this study established that, despite the presence of DO, the use of a Nic/Fe bimetallic system resulted in a significantly higher PCP dechlorination (p < 0.05) compared to the ZVI systems (Fig. 1). In the Nic/Fe systems, Ni can act as a catalyst by promoting the formation of atomic hydrogen or a metal hydride phase on the ZVI surface. In this way, Ni can enhance PCP degradation via hydrodechlorination (Ko et al. 2007; Tian et al. 2009). In addition, Ni can catalytically enhance the hydrogen gas (H2) production by accelerated ZVI corrosion as well as improve the efficiency of hydrogen as a reductant (Feng and Lim 2005; Matheson and Tratnyek 1994). Furthermore, magnetite, which is present in larger quantities in the Nic/Fe media, can also promote the formation of H2 gas (Odziemkowski et al. 1998), and thereby contribute to the enhancement in PCP dechlorination. Figure 4 presents the H2 gas accumulation in the batch reactors (Fig. 4a). The data confirm significantly higher (p < 0.05) H2 gas accumulation with Nic/Fe bimetallics compared to acid-washed ZVI under oxic and anoxic conditions. In addition, Nic/Fe showed a larger BET specific surface area (4.46 ± 0.02 m2/g) compared to the unwashed (0.250 ± 0.004 m2/g) and acid-washed (2.31 ± 0.01 m2/g) iron. The difference in surface areas of iron and bimetallic particles also suggests the possibility of compositional or morphological variations between the materials, which could have influenced the PCP dechlorination (Cwiertny et al. 2007). It has also been suggested that Ni can prolong the ZVI activity by preventing the formation and precipitation of passivating oxide films on the iron surface (Feng and Lim 2005). This is supported by the Raman analysis, which showed that, in comparison to ZVI particles, Nic/Fe bimetallic particles contained a larger amount of magnetite (Table 1) and was maintained over 25 days of reaction despite the presence or absence of DO.
Incorporation of PCP into and onto the different oxide phases of ZVI and Nic/Fe materials
The difference between the total and dissolved chlorinated phenol concentrations was calculated to quantify the amount of extractable PCP incorporated with the ZVI and oxides to assess the significance of the incorporation processes. The amounts of extractable PCP incorporated on unmodified and modified iron particles are shown in Fig. 5. Incorporation (sorption, co-precipitation, and/or physical entrapment) of PCP with the oxides was a significant process with incorporated and extracted PCP ranging up to 0.06 μmole/g ZVI, i.e., 6 μM PCP removed from the reaction solution (Fig. 5). When DO was present, the PCP incorporation onto ZVI and Nic/Fe surfaces were greater compared to that of the corresponding anoxic systems. Higher PCP incorporation occurred in the presence of DO could be a result of the formation of non-conducting surface passivating oxides and greater incorporation of PCP with these oxides (Noubactep 2013). Iron oxides that are forming on the ZVI or bimetallic surfaces during the exposure to ZVI/H2O/PCP system act as sorption sites for PCP molecules. The PCP molecules can also be co-precipitated within the oxide films as the oxide phases evolve. Further, some non-conducting oxide films forming on the ZVI surface also result in hindrance to the electron transfer process (Gunawardana et al. 2018; Noubactep 2013). After 25 days of reaction, all but one system had the amount of PCP incorporated and extracted between 0.03 and 0.04 μmole/g ZVI, despite the differences in the total amount of PCP in the systems (Fig. 5). After 25 days, the incorporated PCP in the oxic Nic/Fe system was significantly higher (p < 0.05) (0.038 μmole/g ZVI) compared to the anoxic Nic/Fe system (0.005 μmole/g ZVI) (Fig. 5). On the other hand, after 25 days of reaction in the presence of DO, the amount of extractable PCP incorporated with the ZVI systems was not statistically different from that of the corresponding anoxic systems.
Amount of extractable PCP incorporated on unmodified and modified iron particles under oxic and anoxic conditions. Error bars indicate ± one standard deviation. The initial amount of PCP available in each system = 0.19 μmole/g ZVI. The level of 0.06 μmole/g ZVI of extractable PCP incorporated corresponds to 6 μM PCP removed from the solution
In addition to its role in various oxides formation on the ZVI surface, DO is an electron acceptor (Yang and Lee 2005) and expected to inhibit PCP dechlorination by competing for electrons. To assess the magnitude of this effect, the PCP degradation rates were estimated by fitting the experimental data to a first-order reaction. The rate of PCP degradation was evaluated using the first-order rate model:C = C0e−kt, where t is the reaction time [h], C is the PCP concentration [μM] at time t, C0 is the initial PCP concentration [μM], and k is the first-order rate constant [k, h−1]. The resulting rate constants (kobs) are presented in Table 2. These results show that PCP dechlorination is slower in the presence of DO. This conclusion is supported by significantly lower (p < 0.05) hydrogen gas accumulation observed in the presence of DO with the acid-washed ZVI and Nic/Fe systems (Fig. 4a)—no hydrogen gas was detected with unwashed ZVI. In the presence of a catalyst, hydrogen can efficiently transform chlorinated chemicals, producing reduced daughter products along with chloride and hydrogen ions. The pH of the reaction (Fig. 4b) was not controlled and there was a general increase in pH over the reaction time from pH 6 to 7 initially to pH 6.7 to 7.8 after 25 days of reaction. There was a slight decrease in pH between 15 and 25 days for Nic/Fe systems, and this may reflect the greater role of dechlorination of PCP by H/H2 in the Nic/Fe bimetallic systems; i.e., H/H2 + RCl → H+ + RH + Cl− (Hu et al. 2018).
Conclusions
PCP dechlorination using ZVI (unwashed and acid washed) and nickel-coated ZVI (Nic/Fe) bimetal was examined in the presence and absence of DO. The presence of DO resulted in slower dechlorination rates and led to significantly decreased PCP dechlorination by ZVI and Nic/Fe. This effect could be attributed to (1) the competition of DO with PCP for the electrons generated during ZVI corrosion and (2) decreased ZVI reactivity due to the formation of non-conducting surface passivating oxides such as maghemite, hematite, and lepidocrocite. The as-received unwashed ZVI demonstrated significantly lower PCP dechlorination while acid washing of ZVI significantly enhanced the PCP dechlorination under oxic condition. Despite the presence of DO, the Nic/Fe resulted in significantly higher PCP dechlorination compared to the ZVI systems. When DO was present, tetrachlorophenols were the main degradation product with ZVI systems and Nic/Fe resulted in phenol being the major end product along with small concentrations of lower chlorophenols after 25 days of reaction. The unwashed ZVI surface was mainly covered with wustite during the reaction under oxic condition, while the increase in the amount of magnetite detected was slower in the presence of DO compared to the anoxic system. The presence of DO resulted in the formation of passivating oxides (maghemite, hematite, lepidocrocite, ferrihydrite) on the acid-washed ZVI and Nic/Fe surfaces. These passive oxides resulted in high PCP incorporation and decreased PCP dechlorination efficiency in acid-washed ZVI and Nic/Fe systems under oxic conditions. The availability of DO can vary in groundwater. Therefore, from a practical perspective, the findings provide greater insight regarding the influence of DO on PCP dechlorination and on the formation of iron oxides and their impact on PCP dechlorination/incorporation when using ZVI and bimetals. In particular, the results are of interest for the application of ZVI and Nic/Fe for in-situ remediation of PCP-contaminated groundwater and determining the dechlorination efficiency of these systems. Further, this study offers evidence on the use of Nic/Fe bimetal that allows for enhanced PCP dechlorination despite the presence of DO.
References
Arning MD, Minteer SD (2007) Handbook of Electrochemistry, pp 813–827
ATSDR (1999) Toxicological profile for chlorophenols. Agency for Toxic Substances and Disease Registry, U.S Department of Health and Human Services
Cheng R, Wang J, Zhang W-x (2007) Comparison of reductive dechlorination of p-chlorophenol using Fe0 and nanosized Fe0. J Hazard Mater 144:334–339
Cheng R, Zhou W, Wang J-L, Qi D, Guo L, Zhang W-X, Qian Y (2010) Dechlorination of pentachlorophenol using nanoscale Fe/Ni particles: role of nano-Ni and its size effect. J Hazard Mater 180:79–85
Choi JH, Choi SJ, Kim YH (2008) Hydrodechlorination of 2,4,6-trichlorophenol for a permeable reactive barrier using zero-valent iron and catalyzed iron. Korean J Chem Eng 25:493–500
Chun CL, Baer DR, Matson DW, Amonette JE, Penn RL (2010) Characterization and reactivity of iron nanoparticles prepared with added Cu, Pd, and Ni. Environ Sci Technol 44:5079–5085
Cornell RM, Schwertmann U (2003) The iron oxides structure, properties, reactions, occurrences, and uses. Wiley-VCH, Weinheim
Cwiertny DM, Bransfield SJ, Roberts AL (2007) Influence of the oxidizing species on the reactivity of iron-based bimetallic reductants. Environ Sci Technol 41:3734–3740
De S, Zhang J, Luque R, Yan N (2016) Ni-based bimetallic heterogeneous catalysts for energy and environmental application. Energy Environ Sci 9:3314–3347
EC (2016) List of priority substances in the field of water policy. European Commission http://ec.europa.eu/environment/water/waterframework/priority_substances.htm Accessed 12 June 2017
Farrell J, Kason M, Melitas N, Li T (2000) Investigation of the long-term performance of zero-valent iron for reductive dechlorination of trichloroethylene. Environ Sci Technol 34:514–521
Feng J, Lim T-T (2005) Pathways and kinetics of carbon tetrachloride and chloroform reductions by nano-scale Fe and Fe/Ni particles: comparison with commercial micro-scale Fe and Zn. Chemosphere 59:1267–1277
Ghauch A, Tuqan A (2009) Reductive destruction and decontamination of aqueous solutions of chlorinated antimicrobial agent using bimetallic systems. J Hazard Mater 164:665–674
Ghauch A, Assi AH, Bdeir S (2010) Aqueous removal of diclofenac by plated elemental iron: bimetallic systems. J Hazard Mater 182:64–74
Gorski CA, Scherer MM (2009) Influence of magnetite stoichiometry on FeII uptake and nitrobenzene reduction. Environ Sci Technol 43:3675–3680
Gorski CA, Nurmi JT, Tratnyek PG, Hofstetter TB, Scherer MM (2010) Redox behavior of magnetite: implications for contaminant reduction. Environ Sci Technol 44:55–60
Gunawardana B, Singhal N, Swedlund P (2011) Degradation of chlorinated phenols by zero valent iron and bimetals of iron: a review. Environ Eng Res 16:187–203
Gunawardana B, Swedlund PJ, Singhal N, Nieuwoudt MK (2018) Pentachlorophenol dechlorination with zero valent iron: a Raman and GCMS study of the complex role of surficial iron oxides. Environ Sci Pollut Res 25:17797–17806
Helland BR, Alvarez PJJ, Schnoor JL (1995) Reductive dechlorination of carbon tetrachloride with elemental iron. J Hazard Mater 41:205–216
Henderson AD, Demond AH (2007) Long-term performance of zero-valent iron permeable reactive barriers: a critical review. Environ Eng Sci 24:401–423
Hu R, Cui X, Gwenzi W, Wu S, Noubactep C (2018) Fe0/H2O systems for environmental remediation: the scientific history and future research directions. Water 10:1739
IARC (1991) IARC monographs on the evaluation of carcinogenic risks to humans: occupational exposures in insecticide application, and some pesticides 53. IARC, Lyon
Jaumot J, Gargallo R, De Juan A, Tauler R (2005) A graphical user-friendly interface for MCR-ALS: a new tool for multivariate curve resolution in MATLAB. Chemom Intell Lab Syst 76:101–110
Junyapoon S (2005) Use of zero-valent iron for wastewater treatment. KMITL Sci Tech J 5:587–595
Kim YH, Carraway ER (2000) Dechlorination of pentachlorophenol by zero valent iron and modified zero valent irons. Environ Sci Technol 34:2014–2017
Ko SO, Lee DH, Kim YH (2007) Kinetic studies of reductive dechlorination of chlorophenols with Ni/Fe bimetallic particles. Environ Technol 28:583–593
Lan Q, Liu H, Li FB, Zeng F, Liu CS (2011) Effect of pH on pentachlorophenol degradation in irradiated iron/oxalate systems. Chem Eng J 168:1209–1216
Li Y, Niu J, Yin L, Wang W, Bao Y, Chen J, Duan Y (2011) Photocatalytic degradation kinetics and mechanism of pentachlorophenol based on superoxide radicals. J Environ Sci 23:1911–1918
Liu Y, Yang F, Yue PL, Chen G (2001) Catalytic dechlorination of chlorophenols in water by palladium/iron. Water Res 35:1887–1890
Liu CC, Tseng DH, Wang CY (2006) Effects of ferrous ions on the reductive dechlorination of trichloroethylene by zero-valent iron. J Hazard Mater 136:706–713
Long M, Ilhan ZE, Xia S, Zhou C, Rittmann BE (2018) Complete dechlorination and mineralization of pentachlorophenol (PCP) in a hydrogen-based membrane biofilm reactor (MBfR). Water Res 144:134–144
Ma H-Y, Zhao L, Guo L-H, Zhang H, Chen F-J, Yu W-C (2019) Roles of reactive oxygen species (ROS) in the photocatalytic degradation of pentachlorophenol and its main toxic intermediates by TiO2/UV. J Hazard Mater 369:719–726
Matheson LJ, Tratnyek PG (1994) Reductive dehalogenation of chlorinated methanes by iron metal. Environ Sci Technol 28:2045–2053
Morales J, Hutcheson R, Cheng IF (2002) Dechlorination of chlorinated phenols by catalyzed and uncatalyzed Fe(0) and Mg(0) particles. J Hazard Mater 90:97–108
Nadoll P, Mauk JL (2011) Wüstite in a hydrothermal silver-lead-zinc vein, Lucky Friday mine, Coeur d’Alene mining district, USA. Am Mineral 96:261–267
Noubactep C (2008) A critical review on the process of contaminant removal in Fe0-H2O systems. Environ Technol 29:909–920
Noubactep C (2013) Metallic iron for water treatment: a critical review. CLEAN Soil Air Water 41:1–9
Odziemkowski MS, Schuhmacher TT, Gillham RW, Reardon EJ (1998) Mechanism of oxide film formation on iron in simulating groundwater solutions: Raman spectroscopic studies. Corros Sci 40:371–389
Redl FX, Black CT, Papaefthymiou GC, Sandstrom RL, Yin M, Zeng H, Murray CB, O'Brien SP (2004) Magnetic, electronic, and structural characterization of nonstoichiometric iron oxides at the nanoscale. J Am Chem Soc 126:14583–14599
Ritter K, Odziemkowski MS, Gillham RW (2002) An in-situ study of the role of surface films on granular iron in the permeable iron wall technology. J Contam Hydrol 55:87–111
Schrick B, Blough JL, Jones AD, Mallouk TE (2002) Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic nickel-Iron nanoparticles. Chem Mater 14:5140–5147
Stratmann M, Müller J (1994) The mechanism of the oxygen reduction on rust-covered metal substrates. Corros Sci 36:327–359
Tian H, Li J, Mu Z, Li L, Hao Z (2009) Effect of pH on DDT degradation in aqueous solution using bimetallic Ni/Fe nanoparticles. Sep Purif Technol 66:84–89
UNEP (2014) Pentachlorophenol and its salts and esters: draft risk management evaluation, UNEP/POPS/POPRC.10/2, Persistent Organic Pollutants Review Committee, United Nations Stockholm Convention on Persistent Organic Pollutants
USEPA (2010) IRIS toxicological review of pentachlorophenol (final report): in support of summary information on the integrated risk information system (IRIS). The United States Environmental Protection Agency, Washington, DC
USEPA (2018a) Drinking water contaminants—standards and regulations. National Primary Drinking Water Regulations. https://www.epa.gov/dwstandardsregulations. Accessed 18 March 2019
USEPA (2018b) Groundwater & drinking water. National primary drinking water regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-waterregulations#Organic. Accessed 18 March 2019
Wan C, Chen YH, Wei R (1999) Dechlorination of chloromethanes on iron and palladium-iron bimetallic surface in aqueous systems. Environ Toxicol Chem 18:1091–1096
Xu F, Deng S, Xu J, Zhang W, Wu M, Wang B, Huang J, Yu G (2012) Highly active and stable Ni–Fe bimetal prepared by ball milling for catalytic hydrodechlorination of 4-chlorophenol. Environ Sci Technol 46:4576–4582
Xu Y, Xue L, Ye Q, Franks AE, Zhu M, Feng X, Xu J, He Y (2018) Inhibitory effects of sulfate and nitrate reduction on reductive dechlorination of PCP in a flooded paddy soil. Front Microbiol 9:567. Published online 2018 Mar 28. https://doi.org/10.3389/fmicb.2018.00567
Yang BR, Chen AH (2016) Effects of pentachlorophenol on the bacterial denitrification process. Chem Speciat Bioavailab 28:163–169
Yang GCC, Lee H-L (2005) Chemical reduction of nitrate by nanosized iron: kinetics and pathways. Water Res 39:884–894
Zhang W, Quan X, Wang J, Zhang Z, Chen S (2006) Rapid and complete dechlorination of PCP in aqueous solution using Ni-Fe nanoparticles under assistance of ultrasound. Chemosphere 65:58–64
Zhou T, Li Y, Lim T-T (2010) Catalytic hydrodechlorination of chlorophenols by Pd/Fe nanoparticles: comparisons with other bimetallic systems, kinetics and mechanism. Sep Purif Technol 76:206–214
Acknowledgments
The authors acknowledge the assistance of Dr. Michel Nieuwoudt with Raman spectroscopic analysis.
Funding
The study was funded by grants from the New Zealand Foundation for Research, Science and Technology, University of Auckland International Doctoral Scholarship and New Zealand International Doctoral Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gunawardana, B., Swedlund, P.J. & Singhal, N. Effect of O2, Ni0 coatings, and iron oxide phases on pentachlorophenol dechlorination by zero-valent iron. Environ Sci Pollut Res 26, 27687–27698 (2019). https://doi.org/10.1007/s11356-019-06009-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06009-w