Abstract
Hydroxyurea (HDU), a class of antineoplastic drugs, has a powerful efficacy in the treatment of several types of malignancies. However, it has multiple adverse effects including reduced fertility, especially in males. Thus, 60 male albino rats were used to investigate the chemoprotective potentials of royal jelly on HDU-induced testicular damage. Animals were gastro-gavaged with HDU (225 or 450 mg kg−1 bw day−1) before royal jelly (100 mg kg−1 bw day−1) for 60 days. Blood samples and testicles were collected, and spermatozoon was obtained. In a dose-dependent manner, the sperm count, motility and liveability, and testosterone, GSH, and catalase concentrations were decreased in HDU groups, whereas MDA, FSH, LH, IL-6, and IFN-γ expression levels were increased. Germinal epithelium degeneration, germ cell sloughing, reduction in the number of luminal spermatozoa, interstitial congestion, and severe leukocyte infiltration besides no glandular secretion in most of the acini were identified. However, royal jelly intake in HDU-treated rats successfully improved sperm quality, hormonal and antioxidant status, and reproductive organ histoarchitecture. Thus, it could be concluded that royal jelly is endowed with antioxidative and anti-inflammatory activities and could be, therefore, used as an adjuvant remedy to improve HDU-induced male subfertility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydroxyurea (HDU) is one of the antineoplastic drugs which are widely used in the treatment of neoplasm (as melanoma, prostatic carcinoma, ovarian cancer, transitional carcinoma of the urogenital tract, cervical cancer, and squamous neoplasm of head and neck), sickle cell disease, and thalassemia. Its action depends on inhibition of DNA production (Liebelt et al. 2007) through the inhibition of ribonucleotide reductase enzyme and decreasing deoxynucleotide triphosphates (especially dATP) pool (Charache et al. 1995). The side effects of HDU may include myelosuppression (anemia, leukopenia, and thrombocytopenia), genotoxicity, and cytotoxicity (Ahmad et al. 2018; Friedrisch et al. 2008). Also, spermatogenic arrest, decreased sperm production, and decreased male infertility have been reported in experimental animals (Gu et al. 2013; Shin et al. 1999; Wiger et al. 1995) and human (Berthaut et al. 2017; Berthaut et al. 2008; Grigg 2007). Cytotoxic effect of HDU may be attributed to the production of carbamoyl nitroso as a metabolite. Carbamoyl nitroso may be implicated in the reactive oxygen species (ROS) formation, thus oxidative stress induction (Cokic et al. 2003). Excessive production of ROS has a deleterious effect on spermatozoa, which associated with male sub-fertility. The plasma membrane of spermatozoa contains a high concentration of unsaturated fatty acids, which made it more vulnerable to oxidative injury (Gharagozloo and Aitken 2011). However, research studies regarding the impact of HDU on the male reproductive endpoints and fertility are still partial.
Royal jelly (RJ) is a creamy material secreted by the hypopharyngeal glands of worker honeybees, its ingredients consist of a mixture of glucose, lipids (fatty acids and sterols), protein, vitamins, and minerals. Royal jelly has several beneficial properties including antineoplastic, antioxidant, anti-inflammatory, anti-allergic, hypotensive, and immune-stimulatory potencies (Fujiwara et al. 1990; Guo et al. 2008; Hattori et al. 2007; Sver et al. 1996). Furthermore, several studies have proved that RJ has positive potentials on male reproductive organs and fertility besides its role against induced testicular oxidative damage (Amirshahi et al. 2014; Najafi et al. 2014; Shalizar Jalali et al. 2015; Zahmatkesh et al. 2014). Thus, this study was resumed to assess the adverse and/or toxic potentials of HDU on male reproductive organs and fertility and the probable protective role of RJ in diminishing its injurious effects in albino rats.
Materials and methods
Chemicals and reagents
Hydroxyurea was obtained as hard capsules (Hydrea®) containing 500 mg of hydroxycarbamide as the active ingredient (E.R. Squibb & Sons Ltd., England). Royal jelly was also obtained in the form of hard capsules (Royal Jelly®) containing 340 mg of lyophilized royal jelly (equivalent to 1000-mg-crude royal jelly) (Pharco Pharmaceuticals Company, Cairo, Egypt). HDU and RJ were dissolved in purified water immediately before administration.
Animals and experimental protocol
Sixty adults male Wistar albino rats (11-week old, weighing 190 ± 10 g) were utilized, which obtained from the closed random bred colony at the Alexandria Medical Research Institute, Egypt. They were raised in plastic cages, fed standard balanced diet, and allowed feed and water ad libitum. The Research Ethics Committee of Alexandria University as the instructions of “Care and Use of Laboratory Animals” prepared by the Faculty of Veterinary Medicine, Alexandria University, approved experimental procedures. The experimental rats were acclimatized for 2 weeks prior to experimentation and then randomly allocated into six groups (n = 10 per each) as follows:
-
Group 1:
(control; CTR): administered 2 ml saline solution/rat day−1.
-
Group 2:
(RJ) received 100-mg royal jelly kg−1 bw day−1 (Amirshahi et al. 2014).
-
Group 3:
(HDU) received therapeutic dose 225 mg HDU kg−1 bw day−1 (Nair and Jacob 2016).
-
Group 4:
(2HDU) received double therapeutic dose 450 mg HDU kg−1 bw day−1.
-
Group 5:
(RJ + HDU) received 100-mg royal jelly plus 225 mg HDU kg−1 bw day−1.
-
Group 6:
(RJ + 2HDU) received 100-mg royal jelly plus 450 mg HDU kg−1 bw day−1.
The dosages were given orally using a stomach tube for 60 successive days taking in mind the period required to ensure a spermatogenic cycle. The following procedures were performed after 24 h of the last drug dose administration.
Determination of serum FSH, LH, and testosterone levels
Under the effect of ketamine (7.5:10 mg/kg) and xylazine (1 mg/kg i.p.) anesthesia, blood was collected from inner canthus of the eyes of all anesthetized rats. Blood samples were left to clot, then centrifuged to separate and collect serum aliquots, which were kept at − 4 °C. Levels of serum testosterone, FSH, and LH hormones were measured using rats’ highly sensitive ELISA Kits (Elabscience and Mybiosource, Texas, USA).
Determination of epididymal sperm count
Immediately after euthanization and abdominal opening, the right cauda epididymis was carefully dissected using anatomical scissors and pressed by fine surgical forceps in a small petri dish containing 1-ml phosphate buffer saline (PBS; pH 7.4 and 280 mOsm kg−1) to obtain the maximum amount of semen. Then, a part of the seminal suspension was diluted using formalin buffer saline and the sperm heads were calculated using a hemocytometer under a light microscope at × 200 magnification (Ciftci et al. 2012a).
Evaluation of sperm motility
A drop of semen suspension was diluted with warm (37 °C) sodium citrate solution 2.9% on a glass side and covered with a cover slide. The percentage of motile sperms in a forward progressive manner was recorded using a light microscope with a heated stage (37 °C) at × 400 magnification (Ciftci et al. 2012b). For accuracy, motility evaluation was assessed in three random fields in each sample and their mean was considered the result of the motility score.
Determination of epididymal alive sperm percent
To detect viability percentage of the sperms, 20 μl of epididymal semen suspension was mixed with 60 μl warm Eosin and Nigrosin stain (Nigrosin 5% and Eosin-Y 4% at ratio 3:1, 37 °C) for 30 s. Thereafter, smears were made and left to dry at room temperature and examined under a light microscope at × 400 magnification. Black-headed sperms were considered dead sperms (Bjorndahl et al. 2003).
Antioxidant/oxidant capacity
Later after euthanization, one testicle of each rat was rapidly excised, weighted, washed in warm normal saline, and preserved at − 80 °C for assessment of malondialdehyde (MDA) (Ohkawa et al. 1979), reduced glutathione (GSH) (Richardson and Murphy 1975), and catalase (CAT) (Aebi 1984) contents in the testicular homogenates. The homogenates were prepared by addition of 1 ml PBS for each 1 g of tissues and 9-ml ice-cold Tris–HCl buffer (10 mM, pH 7.4) with the aid of a tissue homogenizer (Glas-Col Tissues Homogenizer, Shanghai Konmix Mechanical & Electrical Equipment Co., Ltd., China) at 13,500 rpm for 3 min. The obtained homogenates were then centrifuged at 6000 rpm for 60 min at 4 °C. The supernatant fluids were carefully collected and stored at − 20 °C until the analysis time using commercially available kits (Biodiagnostic, Cairo, Egypt). The protein content of the obtained homogenate was detected using Bradford’s method.
Assessment of testicular pro-inflammatory biomarkers
As well, the testicular levels of interleukin-6 (IL-6) and interferon-γ (IFN-γ) were detected in the testicular homogenates by a quantitative sandwich enzyme immunoassay method using Rat High Sensitivity ELISA kits (Sigma-Aldrich, St Louis, MO, USA).
Histopathological examination
One testicle, epididymis, prostate, and seminal vesicle of each rat were excised and quickly fixed in 10% buffered formalin (24 h each). The samples were processed through the paraffin-embedding technique and cut into 4–5-μm-thick sections. The sections were deparaffinized with xylene and stained with hematoxylin-eosin for studying the pathological histoarchitecture.
Statistical analyses
The obtained data were performed by one-way ANOVA followed by post hoc multiple comparisons Duncan’s test using the SPSS statistical package v22.0 for Windows (IBM, Armonk, NY, USA). p values less than 0.05 were regarded as statistically significant differences. The analyzed data are presented as the mean ± standard error of mean (SEM).
Results
Hormonal changes
As shown in Table 1, comparatively with control rats, treatment with RJ alone did not show a significant effect (p > 0.05) on the testosterone and FSH and LH hormonal levels. However, serum concentration of testosterone showed a significant reduction (p < 0.05) in HDU-treated rats, which came parallel with significant enhancement (p < 0.05) in FSH and LH levels. These hormonal disturbances were much greater in the 2HDU-treated group. Fortunately, they were significantly attenuated (p < 0.05) with RJ co-treatment compared with HDU-treated groups.
Sperm parameters
Compared with control rats, the animals that were given RJ showed normal sperm motility, sperm count, and a significantly improved (p < 0.05) sperm liveability. However, these parameters were significantly reduced (p < 0.05) in HDU-treated rats, with a higher reduction in the 2HDU-treated group. The RJ co-treatment was significantly modified (p < 0.05) HDU-induced impairment in sperm parameters, compared with HDU-treated rats (Table 2).
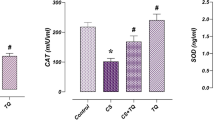
Testicular oxidative stress parameters
Data recorded in Table 3 illustrated that the levels of MDA were significantly elevated (p < 0.05) in testicular tissues in HDU-treated groups. Meanwhile, the activity of testicular CAT enzyme and content of GSH were significantly decreased (p < 0.05) compared with controls that were more severe in 2HDU-treated rats. These alterations were remarkably reversed when RJ was administrated with HDU. RJ alone significantly enhanced (p < 0.05) antioxidant status of testicular tissues—GSH concentration and CAT activity were significantly increased (p < 0.05) and the MDA level recorded a significant decline (p < 0.05) in testicular tissues compared with control.
Testicular inflammatory cytokines level
The level of pro-inflammatory cytokine IL-6 in testicular homogenates of HDU-treated rats recorded a significant increment (p < 0.05), but the magnitude of this increase was higher in 2HDU-treated animals as compared with controls. Combined treatment with RJ significantly decreased (p < 0.05) the testicular level of IL-6 when compared with HDU-treated rats. Also, the treatment with HDU significantly increased (p < 0.05) the level of IFN-γ nearly by the same extent; administration of RJ with HDU significantly augmented (p < 0.05) the increase in the testicular IFN-γ level compared with HDU-treated groups. The sole treatment with RJ did not seem to have any effect on the levels of these pro-inflammatory cytokines comparatively with control values (Table 3).
Histological findings
Testis
Histopathological examinations of the testicles of control and RJ-received rats had normal histoarchitecture, displaying complete spermatogenesis in well-organized seminiferous tubules and interstitium connective tissue (Fig. 1a). The seminiferous tubules had stratified germinal epithelium of spermatogenic and Sertoli cells, and their lumina were loaded with matured spermatozoa. The primary spermatocytes were markedly observed with their prominent nuclei having dark colored–stained chromatin material; meanwhile, subsidiary spermatocytes were rarely observed on the grounds. Spermatids in both round and elongated stages were observed. The testicles of HDU-treated rats showed that the lumina of some seminiferous tubules contained sloughed germinal epithelial (Fig. 1b), with depletion of germinal cells and coagulative necrosis with hyalinized luminal contents (Fig. 1c) and giant cell formations (Fig. 1d). On the other hand, interstitium exhibited edema that appeared as faint eosinophilic material and congestion of the interstitial blood vessels (Fig. 1e). In HDU + RJ rats, the presence of spermatids and spermatozoa in most of the seminiferous tubules declared amelioration of spermatogenesis (Fig. 1f). Histopathological alterations of 2HDU-treated rats composed of disorganized, shrunken seminiferous tubules with buckled and irregular basement membrane (Fig. 2a) besides clear arresting of spermatogenesis. Majorities of seminiferous tubules were lined with single or double cell layers, which are devoid of spermatids and spermatozoa (Fig. 2b) with giant cells (Fig. 2c). Some seminiferous tubules exhibit coagulative necrosis with hyalinization of the luminal contents. Additionally, there was an obvious thickening of interstitium by congestion of the blood vessels, fibroplasia, hyperplasia of Leydig cells (Fig. 2d), and faint eosinophilic albuminous material (Fig. 2e). On the contrary, interstitium of 2HDU + RJ-treated rats showed congestion of the blood vessels and mildly edematous. Some seminiferous tubules contained sloughed necrotic germinal epithelium, while the majority had complete spermatogenesis (Fig. 2f).
Photomicrograph of rat testes stained with HE × 400, scale bar = 50 μm). a Normal testes histoarchitecture of control rats. b, c, d, e Therapeutic HDU rats showing sloughing of the germinal epithelium in the lumen of seminiferous tubules (arrowheads), depletion of germinal cells and hyalinization of the luminal contents (stars) with single or double cell layers (arrows) beside giant cell formations in the lumen of seminiferous tubules (red arrowhead) and interstitial edema which represented by faint eosinophilic albuminous material (asterisks). f Therapeutic HDU + RJ rats showing normal histoarchitecture of seminiferous tubules
Photomicrograph of rat testes stained with HE (× 200, scale bar = 100 μm). a, b, c, d, e Double therapeutic HDU rats showing shrunken, buckled, and disorganized seminiferous tubules (black arrowheads), the majority of seminiferous tubules had single or double cell layers (black arrows), giant cell formations in the lumen of seminiferous tubules (red arrowheads), coagulative necrosis of seminiferous tubule, depletion of germinal cells, and hyalinization of the luminal contents (stars) with hyperplasia of interstitial endocrine cells (short black arrows), congestion of the interstitial blood vessels (white arrow), and interstitial edema (asterisks). f Double therapeutic HDU + RJ rats showing normal histoarchitecture of seminiferous tubules with presence of spermatids and spermatozoa in their lumen
Epididymis
Epididymal sections of the control and RJ-treated rats showed a normal histoarchitecture with normal sperm density (Fig. 3a). Epididymis of HDU rats exhibited sloughed germ cells in some cauda epididymal ductules (Fig. 3b). In HDU + RJ-treated rats, cleared improvement in sperm density and epididymal picture (Fig. 3c) was noticed. Epididymis of 2HDU-treated rats showed sloughing of lining epithelial cells, vacuolation of few germinal epithelial cells (Fig. 3d), and some of the epididymal ductules had no or low numbers of spermatozoa in their lumina beside mild mononuclear cell infiltration in interstitial connective tissue (Fig. 3e). Also, 2HDU + RJ rat’s epididymis showed a normal histoarchitecture with normal sperm density in the majority of epididymal ductules (Fig. 3f).
Photomicrograph of rat epididymis stained with HE (× 200, scale bar = 100 μm). a Normal histological structure with normal sperm density of cauda epididymis of control rats. b Therapeutic HDU rats showing some cauda epididymal ductules contained sloughed germ cells (black arrows). c Therapeutic HDU + RJ rats showing normal histological structure with normal sperm density of cauda epididymis. d, e Double therapeutic HDU rats showing sloughed germ cells (black arrows), vacuolation of few lining germinal epithelial cells (black arrowhead), and mild epididymitis with inflammatory cell infiltration (asterisks). f Double therapeutic HDU + RJ rats showing normal histological structure with normal sperm density
Prostate gland
The prostate gland sections of the control and RJ-treated rats had no obvious histopathological alterations (Fig. 4a). Interstitial congestion of blood vessel and moderate leukocyte infiltration including neutrophils, lymphocytes, and plasma cells besides no luminal secretions in most of the glandular acini (Fig. 4b) were the commonly observed lesions in HDU rats while co-treatment with RJ showed only interstitial congestion of blood vessel and moderate glandular secretions (Fig. 4c). The histoarchitecture of prostatitis was obvious in 2HDU-treated rats, which composed of interstitial congestion and severe leukocyte infiltration besides no glandular secretion in most of the acini (Fig. 4d and e) while co-treatment with RJ showed mild leukocyte infiltration with an adequate quantity of glandular secretions (Fig. 4f).
Photomicrograph of rat prostate stained with HE (× 200, scale bar = 100 μm). a Normal histological structure of control rats’ prostate. b Therapeutic HDU rats showing interstitial congestion of blood vessel (black arrow) and moderate leukocytes infiltration (asterisks). c Therapeutic HDU plus royal jelly rats showing roughly equal normal histological structure with mild interstitial congestion of blood vessel (black arrow). d, e Double therapeutic HDU rats showing interstitial congestion of blood vessel (black arrow) and severe leukocytes infiltration (asterisks) f double therapeutic HDU plus royal jelly rats showing mild interstitial leukocytes infiltration (asterisks)
Seminal vesicle
The seminal vesicle sections of the control and RJ-treated rats were histologically normal (Fig. 5a). The histoarchitecture of seminal vesiculitis was marked in all HDU-treated rats, with higher severity in 2HDU-treated rats, which represented by interstitial congestion of blood vessel and WBC infiltrations mainly neutrophils, lymphocytes, and plasma cells (Fig. 5b and d). A smaller number of epithelium folds with pyknosis of the epithelium nuclei were noticed. The RJ + HDU rats exhibited normal histological structure (Fig. 5c). Meanwhile, in 2HDU-treated rats, there were mild WBC infiltrations (Fig. 5e) and most of seminal vesicle sections had normal histology that was roughly like the controls (Fig. 5f).
Photomicrograph of rat seminal vesicles stained with HE (× 200, scale bar = 100 μm). a Normal histological structure of control rats seminal vesicles. b Therapeutic HDU rats showing interstitial congestion of blood vessel (black arrows) and moderate leukocytes infiltration (asterisks). c Therapeutic HDU + RJ rats showing normal histological structure. d Double therapeutic HDU rats showing severe interstitial congestion of blood vessel (black arrows) and severe leukocytes infiltration (asterisks). e, f Double therapeutic HDU + RJ rats showing mild interstitial congestion of blood vessel (black arrows), mild interstitial leukocytes infiltration (asterisk), and normal histoarchitecture
Discussion
Chemotherapy-induced testicular damage is an emerging reason for azoospermia and disrupted hormonal status in males. HDU is widely used as an effective chemotherapeutic agent to manage cancerous and non-cancerous diseases. It is considered the first-choice antineoplastic medication for sickle cell anemia (Agrawal et al. 2014) and another neoplasm (Liebelt et al. 2007). Nevertheless, its clinical applications were currently restricted due to its declared cytotoxic adverse effects including testicular derangement (Perreault et al. 2008). Herein, HDU treatment induced a significant decline in the blood testosterone level which may be explained based on testicular Leydig cell dysfunction or oxidative damage. Primarily, it has been shown that HDU suppresses testosterone synthesis and release (Jones et al. 2009) through the suppressive effect of nitric oxide, HDU metabolite (Huang et al. 2006) on the testosterone production by Leydig cells (Lamanna et al. 2007). Moreover, HDU is proven to have the capability to generate ROS and free radicals (Cokic et al. 2003), which expressed through an increased MDA (the end product of lipid peroxidation) (Ahmad et al. 2018) with lowered GSH and CAT contents in testicular tissues (El-Maddawy and El-Sayed 2018; El-Neweshy et al. 2013). Besides HDU, its substrate is proven as metal chelators and inhibitors of CAT activity (Italia et al. 2013; Juul et al. 2010). The significant increase in the serum FSH and LH levels following HDU treatment may be due to a lack of negative feedback mechanism of testosterone on GnRH production that stimulates the pituitary gland to secrete FSH and LH to enhance (Corradi et al. 2016) and thus impairing testosterone production and spermatogenesis process. Co-treatment with RJ significantly ameliorated the hormonal imbalance and this may be attributed to its antioxidant properties that was derived from the short-chain peptides (Guo et al. 2008), phenolic compounds (flavonoids and cinnamic acid derivatives) (Kolayli et al. 2016), some vitamins (A, E, and C), minerals (Fe, Zn, and Cu), and fatty acids (trans-10-hydroxy-2-decenoic acid) (Xue et al. 2017) in the rebalancing of oxidant-antioxidant status (Ghanbari et al. 2015; Zahmatkesh et al. 2014), as reflected on the MDA and GSH concentrations and CAT activity in testicular tissues.
Sperms have an abundance of polyunsaturated fatty acids in their membrane and lack enzymatic-linked antioxidant defensive systems; this collectively makes them more sensitive to oxidative damage (Hsu et al. 1998). This could provide an acceptable explanation for decreased sperm motility, count, and liveability in HDU-treated (Grigg 2007), which is primarily mediated through oxidative stress induced by HDU-reactive metabolites, carbamoyl nitroso that involved in ROS generation (Cokic et al. 2003). The reduced number of spermatozoa in HDU-treated rats may be also attributed to the direct effect of HDU on spermatogenesis, which can arrest mitotic division of spermatogonia through inhibition of DNA synthesis (Florensa et al. 2003) or its apoptotic effect upon germ cell (Zhou et al. 2015). It has been described that treatment with HDU may cause a significant reduction in total sperm count (Berthaut et al. 2017; Berthaut et al. 2008), which can reach to azoospermia (Garozzo et al. 2000) and deterioration of sperm motility and morphology (Berthaut et al. 2008; Grigg 2007) and sperm livability (Grigg 2007) in human patients. Treatment of HDU-treated rats with RJ significantly improved sperm motility, count, and liveability, which might be mediated through its reputable antioxidant activity that remarkably protects sperms against oxidative damage (Ghanbari et al. 2015; Shalizar Jalali et al. 2015). Testicular inflammatory state related to oxidative stress in HDU-treated rats was clearly highlighted by the increment in pro-inflammatory cytokine IL-6, the major inflammatory mediator (Hodge et al. 2005) and IFN-γ, and has several anti-inflammatory properties that modify and control the degree of inflammatory conditions (Muhl and Pfeilschifter 2003). This inflammatory state was approximately alleviated upon administration of RJ, indicating that it has anti-inflammatory properties and an inhibitory effect on the release of pro-inflammatory cytokines (Pasupuleti et al. 2017), which might be related to its component of trans-10-hydroxy-2-decenoic acid, 10-hydroxydecanoic acid, and sebacic acid (Chen et al. 2016).
The HDU induced degenerated, irregular, buckled, thick, and hyalinized basement membrane of seminiferous tubules. Vacuolization of the germinal and Sertoli cells may be attributed to smooth endoplasmic reticulum dilatation that possibly signifies cellular permeability changes (Creasy and Chapin 2013). Sertoli cells which provide an abundance of factors required for spermatogenesis are the supportive cells within the seminiferous tubule (O'Donnell et al. 2011). Consequently, the detachment of germinal cells was induced as a result of severe Sertoli cell impairment due to microtubule morbidness (Kumar et al. 2006) in seminiferous tubules’ lumina. The incomplete spermatogenesis with many seminiferous tubules having single or double cell layers may be referred to reduce testosterone levels upon HDU treatment, which is critical for the attachment of various generations of germ cells. Thus, a low level of intra-testicular testosterone may initiate sloughing of germ cells from seminiferous epithelium and may induce cell apoptosis and thereby male infertility (Blanco-Rodriguez and Martinez-Garcia 1998). The marked thickening of the interstitium was seen particularly in 2HDU because HDU may cause androgen receptor (AR) antagonist. Accordingly, there is generally congestion of the interstitial blood vessels, edema, fibroplasia, and Leydig cell hyperplasia. Increased LH secretion from the anterior pituitary stimulates the Leydig cells, increasing testosterone production to maintain androgenic homeostasis (Jones et al. 2009; O'Connor et al. 2002), which are essential for differentiation, development, and maintenance of epithelial cell of prostate glands and seminal vesicles (Ono et al. 2004). The epididymal histoarchitecture reflected the arrest of spermatogenesis, where there is no or low number of spermatozoa in most of the epididymal ducts. Degenerating detached germ cells in the lumina of the epididymal tubule signifies testicular dysfunction (El-Neweshy et al. 2013; Roy et al. 2001). Our result reveals HDU treatment decreased the serum testosterone level leading to hyposecretion of both glands beside prostatitis and seminal vesiculitis. Co-treatment with RJ ameliorated the pathological changes of testicles, epididymis, and accessory sex glands, which might be mediated through scavenging generated ROS and protecting against inflammatory reactions.
Conclusion
Taken together, these findings provide additional information that royal jelly (RJ) prevented testicular injury, oxidative stress, and inflammation associated with HDU antineoplastic medication. Hence, RJ can be used as an adjuvant remedy to improve HDU-induced toxicity and male subfertility.
Abbreviations
- AR:
-
androgen receptor
- CAT:
-
catalase
- CTR:
-
control
- GSH:
-
reduced glutathione
- HDU:
-
hydroxyurea
- HE:
-
hematoxylin and eosin
- IL-6:
-
interleukin-6
- IFN-γ:
-
interferon-γ
- FSH:
-
follicular stimulating hormone
- LH:
-
luteinizing hormone
- MDA:
-
malondialdehyde
- PBS:
-
phosphate buffer saline
- RJ:
-
Royal jelly
- ROS:
-
reactive oxygen species
- SEM:
-
standard error of mean
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agrawal RK, Patel RK, Shah V, Nainiwal L, Trivedi B (2014) Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus 30:91–96
Ahmad MF, Ansari MO, Jameel S, Wani AL, Parveen N, Siddique HR, Shadab G (2018) Protective role of nimbolide against chemotherapeutic drug hydroxyurea induced genetic and oxidative damage in an animal model. Environ Toxicol Pharmacol 60:91–99
Amirshahi T, Najafi G, Nejati V (2014) Protective effect of royal jelly on fertility and biochemical parameters in bleomycin-induced male rats. Iran J Reprod Med 12:209–216
Berthaut I, Guignedoux G, Kirsch-Noir F, de Larouziere V, Ravel C, Bachir D, Galacteros F, Ancel PY, Kunstmann JM, Levy L, Jouannet P, Girot R, Mandelbaum J (2008) Influence of sickle cell disease and treatment with hydroxyurea on sperm parameters and fertility of human males. Haematologica 93:988–993
Berthaut I, Bachir D, Kotti S, Chalas C, Stankovic K, Eustache F, Ravel C, Habibi A, Brailly-Tabard S, Levy-Dutel L, Bleibtreu A, Simon T, Galacteros F, Lionnet F, Mandelbaum J (2017) Adverse effect of hydroxyurea on spermatogenesis in patients with sickle cell anemia after 6 months of treatment. Blood 130:2354–2356
Bjorndahl L, Soderlund I, Kvist U (2003) Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum Reprod 18:813–816
Blanco-Rodriguez J, Martinez-Garcia C (1998) Apoptosis precedes detachment of germ cells from the seminiferous epithelium after hormone suppression by short-term oestradiol treatment of rats. Int J Androl 21:109–115
Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR (1995): Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 332, 1317–1322
Chen YF, Wang K, Zhang YZ, Zheng YF, Hu FL (2016) In vitro anti-inflammatory effects of three fatty acids from royal jelly. Mediat Inflamm 2016:3583684
Ciftci O, Aydin M, Ozdemir I, Vardi N (2012a) Quercetin prevents 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced testicular damage in rats. Andrologia 44:164–173
Ciftci O, Ozdemir I, Aydin M, Beytur A (2012b) Beneficial effects of chrysin on the reproductive system of adult male rats. Andrologia 44:181–186
Cokic VP, Smith RD, Beleslin-Cokic BB, Njoroge JM, Miller JL, Gladwin MT, Schechter AN (2003) Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest 111:231–239
Corradi PF, Corradi RB, Greene LW (2016) Physiology of the hypothalamic pituitary gonadal axis in the male. Urol Clin North Am 43:151–162
Creasy DM, Chapin RE (2013) Chapter 59 - male reproductive system. In: Haschek WM, Rousseaux CG, Wallig MA (eds) Haschek and Rousseaux’s handbook of toxicologic pathology, Third edn. Academic Press, Boston, pp 2493–2598
El-Maddawy ZK, El-Sayed YS (2018) Comparative analysis of the protective effects of curcumin and N-acetyl cysteine against paracetamol-induced hepatic, renal, and testicular toxicity in Wistar rats. Environ Sci Pollut Res Int 25:3468–3479
El-Neweshy MS, El-Maddawy ZK, El-Sayed YS (2013) Therapeutic effects of date palm (Phoenix dactylifera L.) pollen extract on cadmium-induced testicular toxicity. Andrologia 45:369–378
Florensa R, Bachs O, Agell N (2003) ATM/ATR-independent inhibition of cyclin B accumulation in response to hydroxyurea in nontransformed cell lines is altered in tumour cell lines. Oncogene 22:8283–8292
Friedrisch JR, Pra D, Maluf SW, Bittar CM, Mergener M, Pollo T, Kayser M, da Silva MA, Henriques JA, da Rocha Silla LM (2008) DNA damage in blood leukocytes of individuals with sickle cell disease treated with hydroxyurea. Mutat Res 649:213–220
Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi K (1990) A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem 265:11333–11337
Garozzo G, Disca S, Fidone C, Bonomo P (2000) Azoospermia in a patient with sickle cell disease treated with hydroxyurea. Haematologica 85:1216–1218
Ghanbari E, Nejati V, Najafi G, Khazaei M, Babaei M (2015) Study on the effect of royal jelly on reproductive parameters in streptozotocin-induced diabetic rats. Int J Fertil Steril 9:113–120
Gharagozloo P, Aitken RJ (2011) The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 26:1628–1640
Grigg A (2007) Effect of hydroxyurea on sperm count, motility and morphology in adult men with sickle cell or myeloproliferative disease. Intern Med J 37:190–192
Gu L, Xiong WT, Wang C, Sun HX, Li GF, Liu X (2013) Cistanche deserticola decoction alleviates the testicular toxicity induced by hydroxyurea in male mice. Asian J Androl 15:838–840
Guo H, Ekusa A, Iwai K, Yonekura M, Takahata Y, Morimatsu F (2008) Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J Nutr Sci Vitaminol (Tokyo) 54:191–195
Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S (2007) Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed Res 28:261–266
Hodge DR, Hurt EM, Farrar WL (2005) The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 41:2502–2512
Hsu PC, Liu MY, Hsu CC, Chen LY, Guo YL (1998) Effects of vitamin E and/or C on reactive oxygen species-related lead toxicity in the rat sperm. Toxicology 128:169–179
Huang J, Yakubu M, Kim-Shapiro DB, King SB (2006) Rat liver-mediated metabolism of hydroxyurea to nitric oxide. Free Radic Biol Med 40:1675–1681
Italia K, Colah R, Ghosh K (2013) Hydroxyurea could be a good clinically relevant iron chelator. PLoS One 8:e82928
Jones KM, Niaz MS, Brooks CM, Roberson SI, Aguinaga MP, Hills ER, Rice VM, Bourne P, Bruce D, Archibong AE (2009) Adverse effects of a clinically relevant dose of hydroxyurea used for the treatment of sickle cell disease on male fertility endpoints. Int J Environ Res Public Health 6:1124–1144
Juul T, Malolepszy A, Dybkaer K, Kidmose R, Rasmussen JT, Andersen GR, Johnsen HE, Jorgensen JE, Andersen SU (2010) The in vivo toxicity of hydroxyurea depends on its direct target catalase. J Biol Chem 285:21411–21415
Kolayli S, Sahin H, Can Z, Yildiz O, Malkoc M, Asadov A (2016) A member of complementary medicinal food: anatolian royal jellies, their chemical compositions, and antioxidant properties. J Evid Based Complementary Altern Med 21:NP43–NP48
Kumar SG, Narayana K, Bairy KL, D'Souza UJ, Samuel VP, Gopalakrishna K (2006) Dacarbazine induces genotoxic and cytotoxic germ cell damage with concomitant decrease in testosterone and increase in lactate dehydrogenase concentration in the testis. Mutat Res 607:240–252
Lamanna C, Assisi L, Vittoria A, Botte V, Di Fiore MM (2007) D-Aspartic acid and nitric oxide as regulators of androgen production in boar testis. Theriogenology 67:249–254
Liebelt EL, Balk SJ, Faber W, Fisher JW, Hughes CL, Lanzkron SM, Lewis KM, Marchetti F, Mehendale HM, Rogers JM, Shad AT, Skalko RG, Stanek EJ (2007) NTP-CERHR expert panel report on the reproductive and developmental toxicity of hydroxyurea. Birth Defects Res B, Dev Reprod Toxicol 80:259–366
Muhl H, Pfeilschifter J (2003) Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol 3:1247–1255
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31
Najafi G, Nejati V, Shalizar Jalali A, Zahmatkesh E (2014) Protective role of royal jelly in oxymetholone-induced oxidative injury in mouse testis. Iranian J Toxicol 8:1073–1080
O'Connor JC, Frame SR, Ladics GS (2002) Evaluation of a 15-day screening assay using intact male rats for identifying antiandrogens. Toxicol Sci 69:92–108
O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG (2011) Spermiation: the process of sperm release. Spermatogenesis 1:14–35
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ono Y, Suzuki K, Kashiwagi B, Shibata Y, Ito K, Fukabori Y, Yamanaka H (2004) Role of androgen on blood flow and capillary structure in rat seminal vesicles. Tohoku J Exp Med 202:193–201
Pasupuleti VR, Sammugam L, Ramesh N, Gan SH (2017) Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxidative Med Cell Longev 2017:1259510
Perreault S, Klinefelter G, Clegg E (2008) Assessment of male reproductive toxicity. In: Hayes AW (ed) Principles and methods of toxicology. Raven Press, New York, NY, pp 1605–1640
Richardson RJ, Murphy SD (1975) Effect of glutathione depletion on tissue deposition of methylmercury in rats. Toxicol Appl Pharmacol 31:505–519
Roy S, Banerjee A, Pandey HC, Singh G, Kumari GL (2001) Application of seminal germ cell morphology and semen biochemistry in the diagnosis and management of azoospermic subjects. Asian J Androl 3:55–62
Shalizar Jalali A, Najafi G, Hosseinchi M, Sedighnia A (2015) Royal jelly alleviates sperm toxicity and improves in vitro fertilization outcome in stanozolol-treated mice. Iran J Reprod Med 13:15–22
Shin JH, Mori C, Shiota K (1999) Involvement of germ cell apoptosis in the induction of testicular toxicity following hydroxyurea treatment. Toxicol Appl Pharmacol 155:139–149
Sver L, Orsolic N, Tadic Z, Njari B, Valpotic I, Basic I (1996) A royal jelly as a new potential immunomodulator in rats and mice. Comp Immunol Microbiol Infect Dis 19:31–38
Wiger R, Hongslo JK, Evenson DP, De Angelis P, Schwarze PE, Holme JA (1995) Effects of acetaminophen and hydroxyurea on spermatogenesis and sperm chromatin structure in laboratory mice. Reprod Toxicol 9:21–33
Xue X, Wu L, Wang K (2017) Chemical composition of royal jelly. In: Alvarez-Suarez JM (ed) Bee products - chemical and biological properties. Springer International Publishing, Cham, pp 181–190
Zahmatkesh E, Najafi G, Nejati V, Heidari R (2014) Protective effect of royal jelly on the sperm parameters and testosterone level and lipid peroxidation in adult mice treated with oxymetholone. Avicenna J Phytomed 4:43–52
Zhou L, Wu CQ, Luo YW, Liao MY, Sun ZY (2015) Studies on the characteristics and mechanisms of testicular toxicity induced by hydroxyurea. Toxicol Mech Methods 25:396–401
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tohamy, H.G., Gad El-Karim, D.R. & El-Sayed, Y.S. Attenuation potentials of royal jelly against hydroxyurea-induced infertility through inhibiting oxidation and release of pro-inflammatory cytokines in male rats. Environ Sci Pollut Res 26, 21524–21534 (2019). https://doi.org/10.1007/s11356-019-05521-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05521-3