Abstract
This paper studied the inhibitory effect of pomegranate peel (PP) extract on the growth of Microcystis aeruginosa, the model of harmful algal blooms in aquatic environment. The allelochemicals were identified by HPLC–MS/MS from PP and tested by batch experiment through measurement of algal density, chlorophyll a (Chl-a) concentration, maximum quantum yield of photosystem II (Fv/Fm), superoxide dismutase (SOD), and malondialdehyde (MDA) contents. Results showed that both PP powder and PP extract had obvious inhibitory effect on M. aeruginosa growth. Quercetin and luteolin were identified as the allelochemicals to M. aeruginosa growth. However, the inhibitory capacity of luteolin was stronger than that of quercetin. The growth inhibition ratio of luteolin can reach up to 98.7 and 99.1% of the control on day 7 at the dosages of 7 and 10 mg/L, respectively. Moreover, the changes of Chl-a, Fv/Fm, SOD, and MDA in M. aeruginosa confirmed jointly that the allelochemicals cause inhibition of photosystem and oxidative damage to M. aeruginosa cells with the antioxidant defense system being activated, which leads to the aggravation of membrane lipid peroxidation. Thus, luteolin could be used as a promising algaecide for emergency handling of M. aeruginosa blooms. This study might provide a new direction in the management of eutrophication in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pomegranate (Punica granatum L.) belongs to Punicacea family and grows well in semi-arid, mild-temperate to subtropical climates. At present, pomegranates are widely planted in China. Lintong, located in Shaanxi Province, is one of the largest pomegranate production bases in China. Pomegranate peel (PP) is the waste generated from the fruit-processing industry and accounts for approximately 40 to 50% of the whole fruit (Kazemi et al. 2016). One ton of fresh pomegranate would produce 669 kg of by-product containing 78% peel and 22% seeds (Qu et al. 2009). A large amount of PP is mainly discarded as useless residue (Shabtay et al. 2008). Some plants are able to release substances that inhibit the growth of algae (Haroon and Abdel-Aal 2016; Laue et al. 2014; Pichierri et al. 2016; Pakdel et al. 2013; Patino et al. 2018; Zak and Kosakowska 2015). Studies showed that PP was rich in phenols, ellagic tannins, proanthocyanidins, polysaccharides, flavonoids, and so on (Akhtar et al. 2015; Fischer et al. 2011; Li et al. 2015; Xi et al. 2017), which belong to allelochemicals as classified by Rice (1984) due to its inhibitory effect on the growth of algae. Therefore, it is reasonable to hypothesize that PP may contain the allelochemicals which have anti-algal effect on the rehabilitation of aquatic environment.
Among the issues of aquatic environment, harmful algal blooms (HABs) have happened frequently in recent years, which resulted from the input of excess nutrients, such as nitrogen and phosphorus (Glibert 2017; Yalcin et al. 2017), or climate changes. Cyanobacteria are one of the representative species of blooms that occur frequently in freshwater (Li et al. 2016). The outbreak of cyanobacteria results in the decrease of dissolved oxygen (DO) in the freshwater, the release of toxins, such as microcystin, the death of aquatic organisms, and the crash of aquatic ecosystem, which damage the water landscape effect (Mu et al. 2017; Sun et al. 2018). This phenomenon also endangers the human health by polluting the drinking water sources or the bioaccumulation of algal toxin. Therefore, how to control cyanobacterial blooms has been a significant topic in the field of aquatic environment control.
The traditional methods for algae control include physical, chemical, and biological treatments. However, physical methods, such as ultrasonic algae removal (Park et al. 2017) or mechanical algae removal, have high processing cost. Chemical methods, such as dosing coagulant (Magdaleno et al. 2014), produce secondary pollution and have poor durability. Biological methods, such as feeding of filter-feeding fish, would cause the imbalance of water ecosystem (Anderson 2009). Therefore, it is necessary to develop an efficient, rapid, economical, and green method for algae bloom control.
In this paper, Microcystis aeruginosa, one of the models of Cyanobacteria, was selected as the representative algae for the study of algae bloom control. The effects of PP on the growth of M. aeruginosa were investigated firstly, and the allelochemicals of PP were extracted, isolated, and identified. Furthermore, the inhibitory effects of the allelochemicals were tested under different concentrations and periods. In addition, the biochemical effects were explored by measuring the algal density, chlorophyll a (Chl-a) concentration, maximum quantum yield of photosystem II (Fv/Fm), superoxide dismutase (SOD) content, and malondialdehyde (MDA) content in the treated sample with the allelochemicals. Based on the above-mentioned jobs, the following two questions need to be cleared: (1) Is the PP inhibited harmful algal blooms valid? (2) How do the allelochemicals inhibit harmful algal growth? So, this study would provide a promising algaecide for HAB control in aquatic environment.
Materials and methods
Experimental materials
PP was obtained from fresh pomegranate purchased from Lintong, Shaanxi Province, China. The reagents (methanol, chloroform, and ethyl acetate) used for the extraction and isolation of allelochemicals were of analytical grade purchased from Tianjin Kermel Chemical Reagent Co., Ltd. Quercetin (purity 97%) and luteolin (purity ≥ 98%) for exposure experiment of M. aeruginosa were both purchased from Shanghai Macklin Biochemical Technology Co., Ltd. Column chromatography (60 cm × 4 cm), silica gel (200–300 mesh), and thin layer of silica gel plate (GF254) used in this experiment were purchased from Qingdao Ocean Chemical Co., Ltd.
Algal incubation
The M. aeruginosa specimen used in this experiment was purchased from the Institute of Hydrobiology, Chinese Academy of Sciences. The algae were cultured in the autoclaved BG-11 medium. The culture flasks were placed in a light incubator (MGC-250P, China) under illumination intensity of 3000–4000 Lux and a light/dark ratio of 12(h):12(h) at 25 ± 1 °C. The flasks were shaken three times during the light culture period every day and randomly swapped positions to prevent the algal cells from caking and uneven illumination.
Exposure of M. aeruginosa to PP powder
Preparation of the PP powder
The fresh PP was rinsed with distilled water three times, and the excess water was drained at room temperature. The washed PP was crushed with a crusher (FW-80, China), and the chips were placed in a vacuum freeze-dryer (FD-1A-50, China) for 24 h to remove the moisture. The cold-dried crumbs were pulverized into powder (60 mesh sieve) and kept inside a dry brown bottle.
Effect of PP powder on the growth of M. aeruginosa
Different dosages of PP powder, which was sterilized by the ultraviolet lamp, were packaged with the sterile gauzes. The M. aeruginosa in exponential phase and the packaged powder were added into a 500 mL Erlenmeyer flask at the same time. The initial algal density was 5 × 105 cells/mL for all the flasks, and the dosages of the powder were set at 0, 2, 4, 6, and 8 g/L, and the flask without any PP powder was taken as the control. The conical flasks were placed in a light incubator under the same condition as in the “Algal incubation” section. The algal density was determined on days 0, 2, 4, and 6.
Preparation and isolation of the PP extract
Preparation of the PP extract
The PP powder was mixed with methanol (1:20 w/v) and subjected to ultrasonic (60 W, 40 kHz) treatment for 30 min at room temperature. Then, the solution was aliquoted in 50 mL centrifuge tubes and centrifuged at 4000 ×g for 10 min at 4 °C. The supernatant was filtered with a Millipore glass filter (0.22 μm pore size). The filtrate was concentrated by a rotary evaporator (RE-2000, China) at 200 rpm and 60 °C, and the crude extract was gained.
Isolation of the PP extract
The crude extract was separated by a column chromatography. The column was filled with the silica gel, in which the effective height was 40 ± 1 cm. Then, the column was eluted with a step-wise gradient of chloroform–methanol–ethyl acetate (4.5:4.5:1, 3:6:1, 2:7:1, and 1:8:1) before methanol elution. The total volume of each eluent was 600 mL, and the eluate was collected using 50 mL cuvette in succession. First, thin layer chromatography was used to search for the similar substances roughly from the different fractions, and, then, the similar fractions were gathered together for the next step. Fourteen final mixed fractions (numbered Me-01 to Me-14) were obtained and concentrated by the rotary evaporator to remove the organic solvent separately. Finally, the concentrated fractions were re-dissolved in dimethyl sulfoxide (DMSO, analytical grade) for reserve or as the PP extract.
Exposure of M. aeruginosa to PP extract
M. aeruginosa in exponential phase and the fractions were added into a 500 mL Erlenmeyer flask together. The flask without any PP extract was taken as the control. The initial algal density was 5 × 105 cells/mL, and the concentrations of the 14 evaporated fractions were set at 50 mg/L. The concentration of DMSO in the culture medium was less than 1% (v/v) which confirmed that it had no effect on the growth of M. aeruginosa. The culture condition was same as in the “Algal incubation” section. The algal density was determined on days 0, 2, 4, and 6.
Identification of the PP compositions
Me-13 fraction was selected to identify with HPLC–MS/MS (Thermo Fisher Scientific, USA) due to its strong inhibition effect after the anti-algal experiment of 14 different fractions in the PP extract. HPLC–MS/MS analysis was performed in the positive and negative ion mode for ion extraction, respectively. Chromatographic separation was performed on C18 column (100 mm × 2.1 mm, 2.6 μm), analytical column with gradient elute. A mobile phase consisting of A (0.1% formic acid in water) and B (100% methanol) was programmed as follows: 0–5 min, 5% B; 5–15 min, 5–70% B; 15–20 min, 70% B; 20–30 min, 70–90% B; 30–34 min, 90% B; 34–35 min, 90–100% B; 35–38 min, 100% B; 38–38.1 min, 100–5% B; 38.1–40 min, 5% B. The flow rate was 0.3 mL/min, while the column temperature was set at 35 °C. The instrument was equipped with an ESI ion source. Full-scan data was obtained by scanning from 80 to 1200 m/z. Operating parameters included the ion spray voltage of + 3.2 KV and − 3.0 KV, atomization gas temperature of 400 °C, capillary temperature of 300 °C, sheath gas pressure of 35 bar, auxiliary gas pressure of 5 bar, and resolution of FullMS–7000 and ddMS2–17,500.
Exposure of M. aeruginosa to quercetin and luteolin
The allelochemicals of the Me-13 fraction in the PP extract were identified as quercetin and luteolin. The two compounds were subjected to the anti-algal test and were dissolved in DMSO. The experimental concentrations of the two compounds were set at 0, 3, 5, 7, and 10 mg/L according to the preliminary test. The flasks with only same volume DMSO and without any quercetin and luteolin were taken as the controls. The concentration of DMSO in the culture medium was less than 1% (v/v). The initial algal density of M. aeruginosa in exponential phase was 5 × 105 cells/mL. The culture condition was same as in the “Algal incubation” section. Sampling times were on days 1, 3, 5, and 7. The algal density, Chl-a concentration, Fv/Fm, SOD, and MDA contents were measured for each sample.
Analysis and test
Algal density
The number of M. aeruginosa was counted using a hemocytometer with a Nikon microscope (Nikon 50i, Japan). The relative inhibition ratio (IR) of M. aeruginosa growth was calculated according to the following Eq. (1):
where C0 and Ct are the cell numbers in the control and exposed groups, respectively.
Fv/Fm and Chl-a concentration
The Fv/Fm was measured using a Water-PAM fluorometer (Walz, Germany) after 1.5 mL algal suspension was incubated in the dark for 15 min. Chl-a concentration was determined according to Hung et al. (2014). The solution containing algae was filtered through a cellulose acetate microporous membrane, and, then, the membrane was placed in a refrigerator to be dried for 6 to 8 h. The dried membrane was then removed into mortar and grinded with a small amount of basic magnesium carbonate powder and 90% acetone. The mixture was poured into a centrifuge tube of 10 mL and centrifuged at 4000 ×g for 10 min at 4 °C. Afterwards, the supernatant was removed into a volumetric flask of 10 mL. The grinding process was repeated 2–3 times. All the supernatants were gathered into the same volumetric flask, where acetone was added to make the total volume of 10 mL. Finally, the absorbance was measured at 750, 663, 645, and 630 nm with a spectrophotometer (722, China) and acetone which were used as a blank solution. Chl-a concentration was calculated according to the following Eq. (2):
where V and V1 are the volumes of algae solution (L) and the extract (mL), respectively. The δ represents the cuvette optical path (cm).
Enzyme extract and measurement
The cell-free enzyme extract was used for SOD and MDA measurements. Algal cells were harvested by centrifugation at 4000 ×g for 10 min at 4 °C. After the supernatant was removed, the cell pellets were re-suspended in 2 mL of pre-cooled PBS solution (containing 1% PVP, 50 mM, pH 7.8). The cells were homogenized by an ultrasonic cell disruption system (JY96-II, China) at 200 W with a total time of 5 min (ultrasonic time 3 s; rest time 5 s) under ice-bath cooling. Then, the homogenate was centrifuged at 10,000 ×g for 15 min at 4 °C. The supernatant was a cell-free enzyme extract. MDA was determined according to the method of Mihara and Uchiyama (1978). SOD was assayed by a nitroblue tetrazolium method (Beauchamp and Fridovich 1971).
Statistical analysis
Each treatment set was composed of three parallel tests. The test data was expressed as the mean ± standard deviation. The analysis, calculation, and plot of experimental data were processed using Origin 9.0. The variance was analyzed by two-way ANOVA, and the multiple comparisons are performed on the means by the Tukey by SPSS 22.0. p < 0.05 was considered statistically significant.
Results and discussion
Effect of PP powder on the growth of M. aeruginosa
The effect of PP powder on the growth of M. aeruginosa was shown in Table 1, which showed that the algal growth was inhibited obviously by the PP powder and the inhibitory effect was dosage-dependent. On day 2, the algal densities of the treatments with PP powder were half of the control and the IR was about 50.0% although the algal density of different treatments was indistinctive. On day 4, the dosage of PP had striking effect on the IRs, which was 71.5, 86.7, 93.1, and 93.9% for the dosage of 2, 4, 6, and 8 g/L, respectively. Micrographic examination showed that most of the algal cells exposed to PP powder died due to lysis and M. aeruginosa with intact cell structure could not be found on day 6. Thus, PP has the inhibitory effect on M. aeruginosa growth. So, it is necessary to explore the main allelochemicals of PP, which made it to have an inhibitory effect.
Isolation of PP extract and their effects on the growth of M. aeruginosa
The PP extracts were separated by column chromatography preliminary, and 14 eluates, namely Me-01 to Me-14, were obtained. The 14 fractions were carried out to test algal inhabitation, and the bioassay results were shown in Table 2. It can be seen that all of 14 fractions had inhibitory effect on the growth of M. aeruginosa compared with the control during the experimental period. The relative IR for most of the fractions exhibited an increasing trend with time. The relative IR of Me-11, Me-12, and Me-13 were higher than other fractions and reached more than 80.0% on day 6 of the experiment. The relative IR of Me-12 was the highest, while the relative volume of Me-11 was the highest among Me-11, Me-12, and Me-13. However, Me-13 was selected as the final fraction for further identification because Me-13 was placed in the middle for both of the relative content and IR.
Identification of Me-13 with HPLC–MS/MS
The total ion chromatogram of Me-13 was obtained in positive mode. According to related reports about the compositions of PP (Akhtar et al. 2015; Fischer et al. 2011; Li et al. 2015; Xi et al. 2017), the mass-to-charge ratios of the selected target components were used for MS with full scan. After the parent ions of the object ingredients appeared and the intensity reached the set threshold, MS/MS was triggered automatically. The relative mass spectrometry data was shown in Table 3.
MS data of full scan was 287.05357–287.05645 m/z and 303.04841–303.05145 m/z for Me-13. In the MS full-scan spectrum of 287.05357–287.05645 m/z, the high intensity of ion peak was found at the retention time of 16.56 min. The ion peak at 287.05563 m/z could be assigned to the molecular ion of [M + H]+. The signal intensity of [M + H]+ was significantly higher than other impurity peaks with an accuracy of 2.1233 ppm in the MS/MS spectrum. Therefore, the molecular formula of the substance was C15H10O6. Thus, the substance was hypothesized as kaempferol or luteolin. After comparing with the standard substance retention time, the compound was determined as luteolin. In the MS full-scan spectrum of 303.04841–303.05145 m/z, there was a specific peak at the retention time of 16.74 min, and the ion peak at 303.05066 m/z could be assigned to molecular ion of [M + H]+. The ion peak of [M + H]+ was found to be of high intensity at 303.05066 m/z, and the accuracy was 2.40012 ppm in the MS/MS spectrum. Consequently, the molecular formula was C15H10O7 and the substance was speculated to be quercetin.
Both quercetin and luteolin belong to flavonoids, which are given attention to medicine and food industry due to the properties of antioxidant, anti-inflammatory, and antibacterial. However, their interactions with aquatic organisms in the natural environment are not adequately studied. A few studies have suggested that the extracts of plants containing flavonoids have negative effects on the growth of cyanobacteria. Huang et al. (2015, 2016) reported that the natural flavonoids (5,4′-dihydroxy flavone (DHF), apigenin, and luteolin) could induce growth inhibition by disrupting the membrane integrity and the photosynthetic system of M. aeruginosa. Yan et al. (2011) also found that the flavonoids from the decoction of Radix Astragali appeared to be responsible for the inhibition of M. aeruginosa growth, even the dosage of the decoction was less than 20 mL/L. Thus, it could infer that the allelochemicals with strong anti-algal effect might be luteolin or quercetin. So, both quercetin and luteolin derived from the PP extractive might be regarded as the allelochemicals that are capable of inhibiting the algal growth.
Inhibitory effects of quercetin and luteolin on the growth of M. aeruginosa
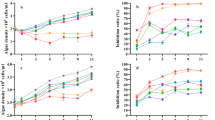
The inhibitory effects of quercetin and luteolin on M. aeruginosa growth were illustrated in Fig. 1. It showed that the difference of the algal density between the control and the sample treated with DMSO was tiny (p > 0.05), which confirmed that the effect of the solvent DMSO on the growth of M. aeruginosa could be ruled out just as Wang et al. (2016a) reported. It also exhibited that the samples treated with both quercetin and luteolin could inhibit the growth of M. aeruginosa compared with the control group (p < 0.01). Moreover, the inhibitory effects of both quercetin and luteolin presented concentration-dependent and time-dependent trends during the whole experiment.
Figure 1a illustrates that the maximum IR reached 34.5, 47.5, 53.0, and 69.8% when the concentrations of quercetin were 3, 5, 7, and 10 mg/L on day 5, respectively. Figure 1b displays that the inhibitory effect of luteolin was similar to that of quercetin. The algal densities were decreased from the beginning of the experiment, and an obvious inhibition was observed in the samples treated with luteolin of 7 and 10 mg/L (p < 0.01), where the IR of 73.7 and 98.7% was attained for the former while the IR of 80.6 and 99.1% was attained for the latter on day 3 and day 7. Thus, the minimum algal densities in the samples treated with luteolin of 7 and 10 mg/L were 0.1 × 105 and 0.09 × 105 cells/mL, respectively. Meanwhile, most of the M. aeruginosa exposed to luteolin of 7 and 10 mg/L were settled at the bottom of the flask, which led the culture medium to transparent.
The above-mentioned results revealed that both quercetin and luteolin did inhibit the growth of M. aeruginosa effectively and luteolin had a stronger inhibitory activity than that of quercetin.
Effects of quercetin and luteolin on Chl-a concentration
Figure 2 shows the Chl-a concentration changes in the M. aeruginosa culture treated with quercetin and luteolin over time. Figure 2a exhibits that the concentration of Chl-a was decreased with the increased concentration of quercetin (p < 0.01). However, the concentration of Chl-a was increased with the extended exposure time for a certain concentration of quercetin (p < 0.01). Meanwhile, the Chl-a concentrations in all the samples treated with quercetin were lower than the control one at the same exposure time (p < 0.05) although their original values were similar. On day 7, Chl-a concentration of the control was increased to 502 μg/L, whereas the corresponding values were 87.4, 80.3, 66.6, and 44.1% of the control for the samples treated with quercetin of 3, 5, 7, and 10 mg/L, respectively.
Figure 2b shows that luteolin had a similar effect on Chl-a concentration in the M. aeruginosa culture. However, Chl-a concentrations were decreased with the extended exposure time at the high concentration of luteolin (7 and 10 mg/L) (p < 0.01). It had no difference between the control and the treatment groups at the early stage but distinguished evidently in the subsequent exposure time (p < 0.01). On day 7, Chl-a concentration was 55.0, 35.2, 10.5, and 8.30% of the control for the samples treated with luteolin of 3, 5, 7, and 10 mg/L, respectively.
Chl-a is the major photosynthetic pigment in algae, and the concentration of Chl-a is closely related to the growth and photosynthesis of algae (Chen et al. 2016; Ni et al. 2015). The above-mentioned results indicated that the photosynthetic system of M. aeruginosa was restrained or destroyed, which may then affect its growth and proliferation, leading to the change of algal density as described in Fig. 1. Therefore, the changes of Chl-a concentration were consistent with the variation of algal density.
Effects of quercetin and luteolin on Fv/Fm
Fv/Fm, the maximum quantum yield of photosystem II (PS II), indicates the photosystem capacity of phytoplankton to convert light energy into chemical energy and represents the potential maximum photosynthetic activity of phytoplankton. In general, a low Fv/Fm value means lower photosynthetic efficiency, which meant PS II damage was caused by environmental stresses (Yang et al. 2015). Wang et al. (2016b) suggested that Fv/Fm was inhibited significantly when M. aeruginosa was exposed to ferulic acid of more than 2.32 mM. Figure 3 shows the varying trend of Fv/Fm with the concentration of quercetin and luteolin. It exhibits that the trend of Fv/Fm was decreased with the increased concentration of quercetin and luteolin (p < 0.01). As shown in Fig. 3a, Fv/Fm reached up to maximum inhibition of 15.4% on day 5 at the concentration of 10 mg/L quercetin. For luteolin (Fig. 3b), the maximum quantum yield of M. aeruginosa was totally inhibited (Fv/Fm = 0) after 1 day exposure at the concentration of 7 and 10 mg/L.
In a word, the fall of Fv/Fm indicated that the inhibition of photosynthetic capacity of M. aeruginosa occurred when exposed to quercetin and luteolin, which was coincident with the corresponding result of Chl-a concentration. Moreover, the maximum quantum yield of M. aeruginosa also proved that the optimal inhibitory effect occurred when M. aeruginosa was exposed to luteolin at the concentration of 7 and 10 mg/L as the “Inhibitory effects of quercetin and luteolin on the growth of M. aeruginosa” section presented.
Effects of quercetin and luteolin on the content of SOD and MDA in M. aeruginosa
Effects of quercetin and luteolin on SOD content of M. aeruginosa
The SOD content per unit algal cell exposed to the different concentrations of quercetin was shown in Fig. 4a. It displays that the SOD content per unit algal cell was almost positively correlated with the concentration of quercetin at the beginning of the experiment but arrived at an approaching value at the end of the experiment. The slightly high level of SOD occurred initially, which was possibly due to the adjustment of the algal cells to the inoculated medium. This is similar with the phenomenon studied by Li et al. (2016). However, compared with the control, all the SOD content of the algal cells exposed to quercetin were high and the maximum values could reach 1.10-, 2.20-, 1.80-, and 2.90-fold of the control for the quercetin concentrations of 3, 5, 7, and 10 mg/L, respectively, on day 1. Generally, all the SOD content per unit algal cell appeared a downward trend during the experiment. For the algal cells exposed to quercetin, the SOD content dropped sharply with time, especially at 10 mg/L quercetin. The above-mentioned results demonstrated that M. aeruginosa was subject to oxidative stress at the early stage, and the antioxidant system was activated to produce SOD to scavenge ROS. Thereafter, the SOD content was reduced on the account of the removal of a portion of excess ROS (Zheng et al. 2018).
The SOD content per unit algal cell exposed to the different concentrations of luteolin was shown in Fig. 4b, which showed different scenes compared with Fig. 4a. The SOD content per unit algal cell kept increasing at the high concentration of luteolin (7 and 10 mg/L), while it kept decreasing at the low concentration of luteolin (3 mg/L) during the whole experiment. The latter was similar to the exposure of quercetin at low concentration (3 and 5 mg/L). The content of maximum SOD reached 4.40 × 10−5 and 5.30 × 10−5 U/cell at the high concentration of luteolin (7 and 10 mg/L) on day 7, respectively, which were 27.7- and 33.3-fold of the control at the same period. This indicated that the content of excess ROS was increased and M. aeruginosa cells were subject to oxidative damage which was caused by the production and reaction of reactive oxygen species (ROS). Meanwhile, more SOD was produced to scavenge O2·− as a cellular detoxification response when M. aeruginosa cells were exposed to high concentrations of luteolin.
The process of cell metabolism leads to the production of ROS (Mallick et al. 2000). Normally, the cells maintain their ROS levels to a homeostasis via an antioxidant system (Yang et al. 2011). When the homeostasis is disrupted, excessive ROS is produced, which results in cellular peroxidation, cell lysis, and death (Ali and Alqurainy 2006). Superoxide radical (O2·−) is the precursor of several ROS, which mainly limits molecular oxygen (O2) reactivity (Hong et al. 2009). SOD is the only enzyme with free radical as substrate and plays a key role in maintaining the dynamic metabolic balance in living organisms. It is mainly responsible for the removal of O2·− by disproportioning two molecules of O2·− to hydrogen peroxide (H2O2) and O2. H2O2 is further decomposed by catalase (Ken et al. 2005). Thus, the cells are protected from oxidative damage (Zamocky et al. 2008).
This study stated clearly that high concentrations of luteolin (7 and 10 mg/L) led to more SOD production and the maximum SOD could reach 27.7- and 33.3-fold of the control at the same period. Thus, it can be inferred that the luteolin concentration of more than 7 mg/L might cause the homeostasis of M. aeruginosa disrupted and result in the increase of SOD production, which was reported as the first line of defense against ROS (Hassan and Scandalios 1990).
Effects of quercetin and luteolin on MDA content of M. aeruginosa
MDA is one of the important products of the membrane lipid peroxidation (Busch and Binder 2017) and can be used as an important index of membrane lipid peroxidation that indirectly characterizes the damage degree of the membrane system and the stress resistance of the plant (Adams et al. 1999; Davey et al. 2005).
The MDA content per unit algal cell exposed to the different concentrations of quercetin was shown in Fig. 5a. It presents that the variation of MDA was similar to that of SOD content when M. aeruginosa cells were exposed to quercetin. The highest content of MDA occurred initially for all the M. aeruginosa samples. All the MDA content of the algal cells exposed to quercetin was higher than the control one, and the maximum values reached 1.70-, 1.20-, and 1.60-fold relative to that of the control one for the concentration of quercetin being 5, 7, and 10 mg/L, respectively, on day 1. However, there was no obvious difference between the control and the 3 mg/L of quercetin exposed. Afterwards, all the MDA content per unit algal cell either exposed to quercetin were decreased gradually and eventually remained stable.
The MDA content per unit algal cell exposed to the different concentrations of luteolin was shown in Fig. 5b. It presents that the variation of MDA content per unit algal cell was similar to that of SOD content when M. aeruginosa cells were exposed to luteolin. The MDA content per unit algal cell kept increasing at the high concentrations of luteolin (7 and 10 mg/L) while kept decreasing at the low concentration of luteolin (3 mg/L) during the whole experiment. The latter was similar to the exposure of quercetin at low concentrations (3 and 5 mg/L). The content of maximum MDA was 38.6- and 43.2-fold relative to that of the control one on day 7 at the luteolin concentrations of 7 and 10 mg/L, respectively.
Zheng et al. (2018) pointed out that there was a positive correlation between ROS and MDA. With the prolongation of exposure time, a small amount of ROS was eliminated by SOD, which led to the decrease of ROS content. Therefore, the MDA content was decreased gradually in the samples treated with the quercetin and with low concentrations (3 and 5 mg/L) of luteolin. M. aeruginosa cells, however, were subject to oxidative damage severely at the high concentrations (7 and 10 mg/L) of luteolin and produced excessive ROS, which led to the aggravation of membrane lipid peroxidation, thereby increasing the MDA content.
The above-mentioned results showed that both quercetin and luteolin could cause oxidative damage to M. aeruginosa cells with the antioxidant defense system being activated. Moreover, the oxidative damage of M. aeruginosa cells exposed to luteolin was stronger than that exposed to quercetin even at the same concentration. The changes of SOD and MDA content per unit algal cell were also consistent with the variation of the algal density. Thus, the growth inhibition and biochemical reactions of M. aeruginosa cells vary under different stresses because different types of stress can cause different damage degrees to the algal cells, just as other researchers reported (Li et al. 2016).
Conclusions
PP has inhibitory effect on M. aeruginosa growth. Quercetin and luteolin were identified as the allelochemicals to M. aeruginosa. The inhibitory capacity of luteolin was stronger than that of quercetin. The IR of M. aeruginosa exposed to luteolin of 7 and 10 mg/L can reach up to 98.7 and 99.1%, respectively, on day 7. Moreover, Chl-a, Fv/Fm, and the maximum contents of SOD and MDA in M. aeruginosa confirmed jointly that both quercetin and luteolin caused inhibition of photosystem and oxidative damage to M. aeruginosa cells with the antioxidant defense system being activated, which led to the aggravation of membrane lipid peroxidation. The maximum quantum yield of M. aeruginosa was totally inhibited (Fv/Fm = 0) after 1 day exposure at the concentration of 7 and 10 mg/L. On the other side, the maximum content of SOD and MDA in M. aeruginosa could reach 33.3- and 43.2-fold of that in control while when exposed to luteolin of 10 mg/L on day 7. Therefore, this study would provide new insight into the inhibitory effects to algal by allelochemicals, especially quercetin and luteolin belong to flavonoids.
References
Adams LK, Benson EE, Staines HJ, Bremner DH, Millam S, Deighton N (1999) Effects of the lipid peroxidation products 4-hydroxy-2-nonenal and malondialdehyde on the proliferation and morphogenetic development of in vitro plant cells. J Plant Physiol 155(3):376–386
Akhtar S, Ismail T, Fraternale D, Sestili P (2015) Pomegranate peel and peel extracts: chemistry and food features. Food Chem 174:417–425
Ali AA, Alqurainy F (2006) Activities of antioxidants in plants under environmental stress. In: Motohashi N (ed) The lutein—prevention and treatment for diseases. Transworld Research Network, India, pp 187–256
Anderson DM (2009) Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast Manag 52(7):342
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Busch CJ, Binder CJ (2017) Malondialdehyde epitopes as mediators of sterile inflammation. Biochim Biophys Acta 1862(4):398–406
Chen S, Chen M, Wang Z, Qiu W, Wang J, Shen Y, Wang Y, Ge S (2016) Toxicological effects of chlorpyrifos on growth, enzyme activity and chlorophyll a synthesis of freshwater microalgae. Environ Toxicol Pharmacol 45:179–186
Davey MW, Stals E, Panis B, Keulemans J, Swennen RL (2005) High-throughput determination of malondialdehyde in plant tissues. Anal Biochem 347(2):201–207
Fischer UA, Carle R, Kammerer DR (2011) Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS n. Food Chem 127(2):807–821
Glibert PM (2017) Eutrophication, harmful algae and biodiversity—challenging paradigms in a world of complex nutrient changes. Mar Pollut Bull 124(2):591–606
Haroon AM, Abdel-Aal EI (2016) Chemical composition and in vitro anti-algal activity of Potamogeton crispus and Myriophyllum spicatum extracts. Egypt J Aquat Res 42(4):393–404
Hassan HM, Scandalios JG (1990) Superoxide dismutases in aerobic organisms. In: Alscher RG, Cumming JR (eds) Stress responses in plants: adaptation and acclimatation mechanisms. Wiley-Liss, New York, pp 175–199
Hong Y, Hu HY, Xie X, Sakoda A, Sagehashi M, Li FM (2009) Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat Toxicol 91(3):262–269
Huang HM, Xiao X, Ghadouani A, Wu JP, Nie ZY, Peng C, Xu X, Shi J (2015) Effects of natural flavonoids on photosynthetic activity and cell integrity in Microcystis aeruginosa. Toxins 7(1):66–80
Huang H, Xiao X, Lin F, Grossart HP, Nie Z, Sun L, Xu C, Shi J (2016) Continuous-release beads of natural allelochemicals for the long-term control of cyanobacterial growth: preparation, release dynamics and inhibitory effects. Water Res 95:113–123
Hung SM, Hsu BD, Lee S (2014) Modelling of isothermal chlorophyll extraction from herbaceous plants. J Food Eng 128:17–23
Kazemi M, Karim R, Mirhosseini H, Abdul HA (2016) Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: punicalagin and hydroxybenzoic acids. Food Chem 206:156–166
Ken CF, Hsiung TM, Huang ZX, Juang RH, Lin CT (2005) Characterization of Fe/Mn-superoxide dismutase from diatom Thallassiosira weissflogii: cloning, expression, and property. J Agric Food Chem 53(5):1470–1474
Laue P, Bährs H, Chakrabarti S, Steinberg CEW (2014) Natural xenobiotics to prevent cyanobacterial and algal growth in freshwater: contrasting efficacy of tannic acid, gallic acid, and gramine. Chemosphere 104(3):212–220
Li J, He X, Li M, Zhao W, Liu L, Kong X (2015) Chemical fingerprint and quantitative analysis for quality control of polyphenols extracted from pomegranate peel by HPLC. Food Chem 176:7–11
Li J, Liu Y, Zhang P, Zeng G, Cai X, Liu S, Yin Y, Hu X, Hu X, Tan X (2016) Growth inhibition and oxidative damage of Microcystis aeruginosa induced by crude extract of Sagittaria trifolia tubers. J Environ Sci (China) 43:40–47
Magdaleno A, Velez CG, Wenzel MT, Tell G (2014) Effects of cadmium, copper and zinc on growth of four isolated algae from a highly polluted Argentina river. Bull Environ Contam Toxicol 92(2):202–207
Mallick N, Mohn FH, Rai LC, Soeder CJ (2000) Evidence for the non-involvement of nitric oxide synthase in nitric oxide production by the green alga Scenedesmus obliquus. J Plant Physiol 156(3):423–426
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86(1):271–278
Mu D, Ruan R, Addy M, Mack S, Chen P, Zhou Y (2017) Life cycle assessment and nutrient analysis of various processing pathways in algal biofuel production. Bioresour Technol 230:33–42
Ni L, Jie X, Wang P, Li S, Wang G, Li Y, Li Y, Acharya K (2015) Effect of linoleic acid sustained-release microspheres on Microcystis aeruginosa antioxidant enzymes activity and microcystins production and release. Chemosphere 121:110–116
Pakdel FM, Sim L, Beardall J, Davis J (2013) Allelopathic inhibition of microalgae by the freshwater stonewort, Chara australis, and a submerged angiosperm, Potamogeton crispus. Aquat Bot 110:24–30
Park J, Church J, Son Y, Kim KT, Lee WH (2017) Recent advances in ultrasonic treatment: challenges and field applications for controlling harmful algal blooms (HABs). Ultrason Sonochem 38:326–334
Patino R, Rashel RH, Rubio A, Longing S (2018) Growth-suppressing and algicidal properties of an extract from Arundo donax, an invasive riparian plant, against Prymnesium parvum, an invasive harmful alga. Harmful Algae 71:1–9
Pichierri S, Pezzolesi L, Vanucci S, Totti C, Pistocchi R (2016) Inhibitory effect of polyunsaturated aldehydes (PUAs) on the growth of the toxic benthic dinoflagellate Ostreopsis cf. ovata. Aquat Toxicol 179:125–133
Qu W, Pan Z, Zhang R, Ma HX, Chen ZB, Wang Z, Atungulu GG (2009) Integrated extraction and anaerobic digestion process for recovery of nutraceuticals and biogas from pomegranate marc. Trans ASABE 52(6):1997–2006
Rice EL (1984) Allelopathy, 2nd edn. Academic Press, New York
Shabtay A, Eitam H, Tadmor Y, Orlov A, Meir A, Weinberg P, Weinberg ZG, Chen Y, Brosh A, Izhaki I (2008) Nutritive and antioxidative potential of fresh and stored pomegranate industrial byproduct as a novel beef cattle feed. J Agric Food Chem 56(21):10063–10070
Sun R, Sun P, Zhang J, Esquivel-Elizondo S, Wu Y (2018) Microorganisms-based methods for harmful algal blooms control: a review. Bioresour Technol 248:12–20
Wang R, Wang J, Xue Q, Tan L, Cai J, Wang H (2016a) Preliminary analysis of allelochemicals produced by the diatom Phaeodactylum tricornutum. Chemosphere 165:298–303
Wang R, Hua M, Yu Y, Zhang M, Xian QM, Yin DQ (2016b) Evaluating the effects of allelochemical ferulic acid on Microcystis aeruginosa by pulse-amplitude-modulated (PAM) fluorometry and flow cytometry. Chemosphere 147:264–271
Xi J, He L, Yan LG (2017) Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem 230:354–361
Yalcin B, Artuz ML, Pavlidou A, Cubuk S, Dassenakis M (2017) Nutrient dynamics and eutrophication in the Sea of Marmara: data from recent oceanographic research. Sci Total Environ 601-602:405–424
Yan RW, Ji YH, Ji HL, Fang YM, Kerr PG, Yang LZ (2011) The decoction of radix Astragali inhibits the growth of Microcystis aeruginosa. Ecotoxicol Environ Saf 74:1006–1010
Yang C, Yang Z, Zhang M, Dong Q, Wang X, Lan A, Zeng F, Chen P, Wang C, Feng J (2011) Hydrogen sulfide protects against chemical hypoxia-induced cytotoxicity and inflammation in HaCaT cells through inhibition of ROS/NF-kappaB/COX-2 pathway. PLoS One 6(7):e21971
Yang Z, Kong F, Shi X, Yu Y, Zhang M (2015) Effects of UV-B radiation on microcystin production of a toxic strain of Microcystis aeruginosa and its competitiveness against a non-toxic strain. J Hazard Mater 283:447–453
Zak L, Kosakowska A (2015) The influence of extracellular compounds produced by selected Baltic cyanobacteria, diatoms and dinoflagellates on growth of green algae Chlorella vulgaris. Estuar Coast Shelf Sci 167:113–118
Zamocky M, Jakopitsch C, Furtmuller PG, Dunand C, Obinger C (2008) The peroxidase-cyclooxygenase superfamily: reconstructed evolution of critical enzymes of the innate immune system. Proteins 72(2):589–605
Zheng H, Sun C, Hou X, Wu M, Yao Y, Li F (2018) Pyrolysis of Arundo donax L. to produce pyrolytic vinegar and its effect on the growth of dinoflagellate Karenia brevis. Bioresour Technol 247:273–281
Acknowledgments
The authors acknowledge Associate Professor Li Tizheng for his selfless help in the statistical analysis of the data.
Funding
This work was funded by the National Natural Science Foundation of China (Grant number 21677115) and the Shaanxi Provincial Natural Science Foundation Research Key Project (2016JZ019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vitor Manuel Oliveira Vasconcelos
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, L., Wang, Y., Shi, L. et al. Identification of allelochemicals from pomegranate peel and their effects on Microcystis aeruginosa growth. Environ Sci Pollut Res 26, 22389–22399 (2019). https://doi.org/10.1007/s11356-019-05507-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05507-1