Abstract
Desalination is an important strategy for adapting to the global shortage in safe drinking water. Israel relies heavily on desalinated water (over 50% of supplied drinking water). However, desalinated water may be more corrosive than water from other sources and may cause leaching of heavy metals from materials in contact with water. In this study, we measured heavy metal concentrations (copper, iron, lead) in 1379 drinking water samples in educational institutions in Israel and compared heavy metal concentrations in drinking water from different sources (desalination, groundwater, desalinated and groundwater mixture). 99.9% of the samples met the standard for copper (1400 μg/l), 99.7% for iron (1000 μg/l), and 99.6% for lead (10 μg/l). As expected, heavy metal concentrations were higher in first flush samples compared to flushed samples (significant findings for lead, copper, and iron). Heavy metal concentrations were not higher in desalinated water, or desalinated and groundwater mixture, compared to groundwater. In first flush samples, lead concentrations in groundwater were significantly higher than in desalinated-groundwater mixtures (p = 0.005). In flushed samples, lead concentrations in groundwater were higher than in desalinated-groundwater mixtures but the difference was not significant (p = 0.07). We suggest that regulatory requirements for stabilization of desalinated water and restrictions on lead content of plumbing materials appear to have been effective in preventing increased exposure to lead in desalinated drinking water in Israel. Further study should focus on potential heavy metal leaching in pure desalinated water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global water resources, especially drinking water, are limited. Many countries around the world already suffer from a shortage of safe drinking water. Climate change exacerbates the problem: extreme changes in rainfall and increased frequencies of drought reduce the amount of water in lakes and rivers, and the amount of groundwater. According to the World Health Organization (WHO), by 2025, half of the world’s population will be living in water-stressed areas (WHO 2018). The global water shortage requires the adaptation of a variety of strategies for the production and/or transfer of clean water to populations. These include recycling of treated wastewater, purchasing drinking water from other countries, and water treatment, including desalination.
Israel’s drinking water is unique in that there is a high proportion of desalinated water in the distribution system (over 50% with certain areas reaching 80%). Desalinated water has been used to supplement severely limited natural sources and to help cope with consecutive drought years. Israel’s desalination plants use a two-step reverse osmosis (RO) process. Since desalinated water is used for agricultural irrigation, and a single RO step is not sufficient to decrease boron concentrations, the second step results in a further decrease in boron, and in very low chloride concentrations (20 ppm).
The distribution system includes water from different sources (desalinated, surface, and groundwater), and the mix can fluctuate seasonally, monthly, and hourly. This operational flexibility and the use of a range of drinking water sources allow for a reliable supply system (Israeli Water Authority 2018). However, the extensive usage of desalinated water in the domestic sector may have potential indirect health effects: the desalination process removes essential minerals from the water, such as iodine, fluoride, calcium, and magnesium, and can consequently affect human health (Koren et al. 2017).
Plumbing materials are highly sensitive to the chemical characteristics of water. Chemical interactions occur between the water and the plumbing materials, specifically when the type and chemical content of the water changes (Oldfield 1998). Furthermore, in steel pipes, desalinated water was found to be more corrosive than other drinking water sources (Shams El-Din 2009; WHO 2011). Alam and Sadiq have found increased concentrations of heavy metals (copper, iron, and zinc) in desalinated drinking water after its transportation from the desalination plant to consumers, caused when corrosive water comes into contact with poor quality pipes (Alam and Sadiq 1989). According to a WHO report published in 2004, desalinated and low mineral water poses an increased risk from toxic metals, both due to higher leaching of metals from materials in contact with water and due to the lower protective (antitoxic) capacity of water low in calcium and magnesium (WHO 2004).
In order to prevent infrastructure problems associated with corrosive water, Israel’s drinking water standards require large water suppliers that desalinate seawater to stabilize the water before it is supplied to the public. The required stabilization values include pH level of 7.5–8.3, dissolved calcium level of 80–120 mg/L as CaCO3, alkalinity of above 80 mg/L as CaCO3, Calcium Carbonate Precipitation Potential (CCPP) of 3–10 mg/L as CaCO3, and a positive Langelier Saturation Index. The Ministry of Health considers an additional stabilization parameter, the Larson Index, which takes negative ions into consideration. Of note, water treatment plants in Israel do not add polyphosphates to control chemical corrosion.
In addition, in order to control potential leaching of heavy metals into drinking water, products in contact with drinking water, such as pipes, faucets, and fixtures, must meet requirements of a standard restricting heavy metals including lead in leachate, though these regulatory requirements are relatively new (since 2004). In addition, drinking water suppliers are required by the drinking water standards to routinely monitor heavy metals (lead, iron, and copper) in the supply system. Of note, historically, there was no use of lead pipes in plumbing systems in Israel. However, there has been use of lead in plumbing components (for example, solder joints), and there is import of faucets, coolers, joints, and other plumbing components (excluding pipes).
Despite regulatory requirements, there is a concern that the rapid increase in the use of desalinated water may increase leaching of heavy metals from the supply system and plumbing materials in buildings. Of note, there are considerable challenges in enforcing regulatory requirements rearding lead in plumbing materials. In addition, since drinking water sources in Israel may fluctuate very rapidly, there is a concern that drastic changes in source water can impact water chemistry and lead to heavy metal leaching.
In this study, we measured heavy metal concentrations in drinking water in educational institutions in Israel and studied whether desalinated water had higher levels of the following heavy metals: copper, iron, and lead, in comparison to drinking water from other sources (predominantly groundwater). We focused on educational institutions because of known vulnerability of children to heavy metals in drinking water.
Methods
Sampling and laboratory analysis
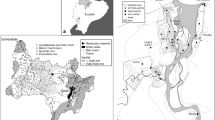
1379 drinking water samples were collected from faucets and coolers, in 900 educational institutions in Israel. For 573 educational institutions, there was a single sample (one outlet only); for 266, there were two samples, while for the other institutions more outlets were sampled (three to nine outlets). Sampling was conducted in the Israeli winter (19/11/2017 to 26/3/2018). Three hundred and three samples were first flush (after non-use overnight) and an additional 1069 samples were random grab samples collected after 2 min of flushing. Samples were collected from a variety of institutions with wide geographical distribution (Fig. 1).
The samples were collected in accordance with the Ministry of Health’s Water Sampling Guidelines (Ministry of Health 2016). In brief, 500 ml of water was placed in high-density polypropylene sample container and acidified with 1.5 mL nitric acid (1:1). Before acidification, turbidity of each water sample was measured using portable turbidity meters (HACH 2100Q (Loveland, Colorado, USA), Merck Turbiquant (Burlington, Massachusetts, USA)). Samples with turbidity < 1 NTU were labelled “for direct analysis” and samples with turbidity ≥ 1 NTU were labelled “for digestion”. The samples were transferred to the Ministry of Health Public Health Laboratories and stored at room temperature until analysis. Concentrations of copper, lead, and iron were measured for each sample within 30 days from the sampling.
Analytical measurements of metals in water were performed according to US EPA Method 200.7 or 200.8 (U.S. EPA 1994a, b) using Varian Vista-Pro ICP-OES (Palo Alto, CA, USA) or Perkin-Elmer NexION 300X ICP-MS (Waltham, Massachusetts, USA). Samples with low turbidity samples (< 1 NTU) were tested by direct injection to analytical instrument; samples with turbidity ≥ 1 NTU were digested before instrumental analysis. Sample preparation and digestion, calibration, standardization, and quality control were done according to the above-mentioned EPA methods. Limits of quantification (LOQ) were as follows: Pb: 2 μg/L, Fe and Cu: 10 μg/L.
Statistical methods
Concentrations below the level of quantification (LOQ) were replaced by 0. Due to the non-normal distributions of the metal contents, we used non-parametric statistical tests. Kruskal-Wallis statistical tests were used to compare the metal distribution between water from all sources. We compared heavy metal concentrations between water from different sources in all samples and separately for first flush samples and flushed samples. Wilcoxon rank-sum test was then used to compare metal distributions between two sources of water (e.g., desalinated water vs. groundwater). To confirm our results, we repeated all analyses when replacing values below the LOQ with LOQ/2.
Results
99.9% of the samples met the standard for copper (1400 μg/l), 99.7% for iron (1000 μg/l), and 99.6% for lead (10 μg/l). In most samples, the metal concentrations were below the level of quantification (92.5% of the samples for lead, 65.8% for iron, and 80.5% for copper). As expected, heavy metal concentrations were higher in first flush samples compared to flushed samples (significant findings for lead, copper, and iron).
Information regarding the sources of water was collected for 1337 samples: 981 samples originated from groundwater diluted with desalinated water, 222 from groundwater only, 24 from desalinated water only, and 110 samples from other sources (surface water or surface water mixed with groundwater and desalinated water).
We compared the metal concentrations between the four sources of water (groundwater, desalinated water, mixture of desalinated and groundwater, and other sources, Fig. 2). All against all comparison between the four distributions yielded statistically significant results (p = 0.009 for lead, p = 0.0015 for iron, and p < 0.0001 for copper). However, pairwise comparisons suggest that metal content in desalinated water is similar to that in groundwater (p > 0.05, for each metal).
We calculated the average concentrations of the three metals in different water sources. The average lead levels in all samples (first flush samples and flushed samples) in groundwater (0.55 ppb) were significantly higher than in the groundwater-desalinated mixture (0.36 ppb, p = 0.001). Lead levels in the mixture were higher than the level in desalinated water (0.21 ppb). The maximum lead concentrations were 11.5 ppb in groundwater, 89.2 ppb in groundwater-desalinated water mixture, 5.1 ppb in desalinated water, and 2.2 ppb in water from other sources. As for iron, the groundwater-desalinated mixture average level (46 ppb) was higher than the desalinated (40 ppb) and the groundwater (31 ppb) levels. The maximum iron concentrations were 664, 5700, 894, and 164 ppb in groundwater, groundwater-desalinated water mixture, desalinated water, and water from other sources, respectively. In addition, the copper average level in the groundwater-desalinated mixture (10 ppb) was lower than those in groundwater and in desalinated water (23 ppb in each). The maximum copper concentrations were 255, 1505, 180, and 127 ppb, in groundwater, groundwater-desalinated water mixture, desalinated water, and water from other sources, respectively (see also supplementary Table 1).
In first flush samples (N = 303), average lead levels in groundwater (1.23 ppb) were higher than average lead levels in the groundwater-desalinated mixture (0.59, p = 0.005). As there were only four desalinated water first flush samples, we were unable to evaluate whether heavy metal concentrations in these samples were significantly different than groundwater and groundwater-desalinated mixture samples.
In flushed samples, (N = 1069), there was a trend for higher average lead levels in groundwater (0.33 ppb) compared to the groundwater-desalinated mixture (0.29 ppb, p = 0.07) but not when compared to desalinated water samples (0.26, p = 0.69) (see also supplementary Table 1).
Discussion
Most of the supplied drinking water in Israel is a mixture of groundwater and desalinated water; this explains the relatively small number of samples (n = 24) of pure desalinated water. In all samples (flushed and first flush), groundwater samples had statistically significant higher levels of lead and copper in comparison to a mixture of desalinated water and groundwater. Lead levels were significantly higher in groundwater compared to the groundwater-desalinated mixture in first flush samples (p = 0.005) and were borderline significant in flushed samples (p = 0.07). This finding is in agreement with the findings of Liu et al. that showed lower lead release in mixtures with higher contents of desalinated water (Liu et al. 2010). The researchers showed that maintaining pH of 7.8 and low alkalinity contributes to less release of lead and copper. Indeed, desalinated water in Israel generally has well controlled water chemistry (pH, 7.5–8.3; chloride, 20 μg/L; alkalinity, above 80 μg/L; TDS, 300 μg/L) as opposed to groundwater with a broad range of natural variation (pH, 6–8.7; chloride, 20–450 μg/L; alkalinity, 10–400 μg/L; TDS, 50–1600 μg/L).
In addition, we show that mixtures of desalinated water and groundwater had statistically higher iron levels in comparison to desalinated water. This finding is in agreement with the findings of Taylor et al. who showed an increased release of iron when pipes were exposed to a mixture of desalinated seawater (by RO) and treated surface water, probably due to the alkalinity reduction in the mixture (Taylor et al. 2005).
In our survey of tap water in educational institutions in Israel, only 0.4% of samples had lead concentrations above the standard (10 μg/L). Comparing the lead concentrations found in the current study to similar surveys in US States and in Canada in which little or no desalinated water is used for drinking reveals that the drinking water in Israel in educational institutions, though enriched with desalinated water, has much lower concentrations of lead, compared to educational institutions in New York, California, and in Canada (California Water Boards 2018; New-York State 2018; Doré et al. 2018).
We note that this survey was limited in that it was conducted only in educational institutions; we did not have information on type of plumbing materials; and this survey did not include water samples in municipal water systems. It is not clear to what extent this survey in educational institutions can be used to draw conclusions about lead levels in households and other institutions in Israel—since educational institutions may have unique water use patterns (long periods of non-use or minimal water usage during holiday breaks and long weekends, and fixtures are rarely if ever used for example in classroom faucets) (Burlingame et al. 2018). In addition, only 24 samples of pure desalinated water were included in the current study. As there were only four desalinated water first flush samples, we were unable to evaluate whether heavy metal concentrations in these samples were significantly different than groundwater and groundwater-desalinated mixture samples. Of note, all samples were collected in winter (the coldest season), and therefore, this survey may not fully represent seasonal variations in drinking water in Israel, since temperature plays a role in water chemistry. Finally, there was low statistical power due to use of non-parametric tests. On the other hand, the survey included a large number of samples, with broad geographical and socioeconomic coverage of included cities and smaller communities. We conducted all analysis replacing values below the LOQ with zero, and alternately with LOQ/2, and note that results were similar using both methods.
In summary, although desalinated water is considered corrosive and suspected to promote leaching of heavy metals, we found that desalinated drinking water in Israel has similar and even lower levels of heavy metals in comparison to groundwater. This may be due to strict regulatory requirements to stabilize desalinated water and requirements since 2004 that restrict heavy metal leaching in products in contact with drinking water (these requirements were expanded in 2018–2020 to restricting lead content to 0.25%). However, we note that there has been an increase in “red water” complaints and appearances in different areas in Israel following drastic changes in drinking water sources. In these places, the Ministry of Health instructs water suppliers to conduct frequent heavy metals testing. In light of this increase in “red water” appearances, and uncertainty regarding the water characteristics which cause this phenomenon, there is still uncertainty regarding the long-term impact of desalination and changes in drinking water sources on potential drinking water exposure to lead and other heavy metals.
Conclusion
One of the lessons of the Flint Michigan lead contamination episode was that continued vigilance about lead in drinking water is required, especially when changing drinking water sources. This study explored potential impact of steadily increasing use of desalinated water in Israel and found that, to date, heavy metal concentrations in supplied water do not exceed standards. We will continue to measure lead and other heavy metals in supplied drinking water, with emphasis on drinking water supplied to vulnerable populations.
References
Alam IA, Sadiq M (1989) Metal contamination of drinking water from corrosion of distribution pipes. Environ Pollut 57:167–178
Burlingame GM, Bailey C, Nelson J et al (2018) Lessons learned from helping schools manage lead in drinking water to protect children’s health. J Am Water Works Assoc 110:44–53
California water boards (2018) https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/leadsamplinginschools.html. Accessed October 2018
Doré E, Deshommes E, Andrews RC, Nour S, Prévost M (2018) Sampling in schools and large institutional buildings: implications for regulations, exposure and management of lead and copper. Water Res 140:110–122. https://doi.org/10.1016/j.watres.2018.04.045
Israeli water authority, Desalination (2018) (Hebrew). http://www.water.gov.il/Hebrew/WaterResources/Desalination/Pages/default.aspx. Accessed October 2018
Koren G, Shlezinger M, Katz R, Shalev V, Amitai Y (2017) Seawater desalination and serum magnesium concentrations in Israel. J Water Health 15:296–299. https://doi.org/10.2166/wh.2016.164
Liu H, Schonberger KD, Korshin GV, Ferguson JF, Meyerhofer P, Desormeaux E, Luckenbach H (2010) Effects of blending of desalinated water with treated surface drinking water on copper and lead release. Water Res 44(14):4057–4066. https://doi.org/10.1016/j.watres.2010.05.014
Ministry of Health (2016) Guidelines for water sampling (Hebrew) https://www.health.gov.il/hozer/bsv_140310.pdf. Accessed March 2019
New-York State (2018) Department of Health, Annual Water Quality Report https://www.health.ny.gov/environmental/water/drinking/annual_water_quality_report/ Accessed October 2018
Oldfield JW (1998) Electrochemical theory of galvanic corrosion. In “Galvanic Corrosion”, ASTM STP 979 H.P.Hack Ed. ASTM international, West Conshohocken (PA) https://doi.org/10.1520/STP26188S
Shams El-Din AM (2009) Three strategies for combating the corrosion of steel pipes carrying desalinated potable water. Desalination 238:166–173
Taylor J, Dietz J, Randall A, Hong S (2005) (2005) Impact of RO-desalted water on distribution water qualities. Water Sci Technol 51(6-7):285–291
U.S. EPA. 1994a. “Method 200.7: determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry,” Revision 4.4. Cincinnati, OH
U.S. EPA. 1994b “Method 200.8: determination of trace elements in waters and wastes by inductively coupled plasma-mass spectrometry,” Revision 5.4. Cincinnati, OH
WHO (2004) Health Risks From Drinking Demineralised Water http://www.who.int/water_sanitation_health/dwq/nutrientschap12.pdf Accessed October 2018
WHO (2011) Safe Drinking-water from Desalination http://www.who.int/water_sanitation_health/publications/2011/desalination_guidance_en.pdf. Accessed October 2018
WHO (2018) Drinking water. http://www.who.int/news-room/fact-sheets/detail/drinking-water. Accessed October 2018
Acknowledgments
The work of the first author was supported by the Environment and Health Fund, Jerusalem, Israel. The authors wish to thank Ronnie Levin for helpful feedback on the manuscript. The authors wish to thank Luda Groisman from the National Public Health Laboratory, Ministry Of Health, Tel Aviv, for her dedication and efficient analyses, and to personnel in Ministry of Health District Offices for hard work on sample collection.
Funding
This study was funded by the Israel Ministry of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 26.9 kb)
Rights and permissions
About this article
Cite this article
Barnett-Itzhaki, Z., Eaton, J., Hen, I. et al. Heavy metal concentrations in drinking water in a country heavily reliant on desalination. Environ Sci Pollut Res 26, 19991–19996 (2019). https://doi.org/10.1007/s11356-019-05358-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05358-w