Abstract
The present paper examines the water quality status of Dimapur city and the adverse effects of surface and groundwater contamination with a view to create a database that would pave the way for future management to facilitate the improvement of the water quality. A systematic field-based study was conducted to survey the quality and possible health hazards associated with drinking water sources. Over the post-monsoon season, 15 samples were collected from different areas of Dimapur. Twenty physico-chemical parameters such as Temperature, pH, Dissolved Oxygen (DO), Conductivity, Total Dissolved Solids, Salinity, Turbidity, Hardness, Aluminum, Chromium, Iron, Manganese, Silver, Zinc, Fluoride, Nitrate, Nitrite, Phosphates and Sulphates and bacteriological experiments were performed in accordance with Bureau of Indian Standards (IS: 10500, 2012) and World Health Organization guidelines. With the exception of heavy metals such as iron and manganese and nutrient phosphate levels, other parameters under analysis were within the permissible limit. Microbiological analysis of water sources demonstrated that 40% of urban samples were polluted. This study reveals the extent of groundwater contamination in the region and that with continuous testing, more contaminated water sources are expected to be identified. It is, therefore, essential to regularly monitor the surface and groundwater to detect any occurrence of other significant contaminants and to recognize any changes or patterns in the water bodies over a period of time.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is available almost everywhere, and its quality is usually very good. However, it remains a shrouded resource. Where to locate water beneath the ground is always known but what really keeps it hidden in the restricted measure of knowledge and accessibility, current state and usage. Groundwater is commonly used in many countries, and about 50% of the world's population relies on groundwater and is, therefore, an important component of the water economy (Margat and Gun 2013). Groundwater is also an important part of the water cycle and is used to retain soil moisture, wetlands, streams (Beyene et al. 2019).
In Nagaland state, the primary sources of water supply for drinking and irrigation purposes are surface water such as rivers, streams, ponds, and natural springs and subsurface water that exists as groundwater. Because of the diminishing resources and expanding contamination of surface water, groundwater has become the most reliable asset of water supply in the region. However, an increasing demand from residential, and agricultural use has resulted in depletion and declining quality of groundwater (Kalhor et al. 2019). This study is important because Dimapur is a commercial capital of Nagaland, where rapid growth of the urban areas and the dense population of the region has impacted groundwater quality due to overexploitation of resources. Relatively, water quality reports are extremely inadequate in Nagaland as there is practically not many documentations on the status of water bodies.
Dimapur district with latitude 25°54′45ʺ North and longitude 93°44′30ʺ East are situated in the south-western region of Nagaland, one of the north-eastern states of India. It is located at the elevation of 154 m above sea level and receives an average annual rainfall of 1504.7 mm with temperature ranging from 3.2–36 °C (Ministry of Water Resources 2013). It has a total geographic area of 927 sq.km and has four subdivisions, namely Medziphema, Dhansiripar, Niuland and Kuhuboto. Dhansiri and Diphu rivers are the two major sources of water for irrigation and drinking purposes. Most of the region is at risk of erosion hazard contributing to land degradation and ultimately affecting the quality of water (Ministry of Environment and Forests 2010). According to 2001 and 2011 Census, Dimapur ranked highest in terms of population with a decadal growth rate of 23.13%. While the quantity of groundwater is associated with the sustainability issues, of equal importance is the quality of water. Groundwater in Nagaland is usually found to be high on the iron and fluoride contents (Singh 2004). Press Information Bureau (PIB) has additionally referenced about the iron contamination in Nagaland zone (PIB 2018). Analysis of water samples gathered from various water sources in Kohima uncovered elevated levels of iron, ammonia and nitrate way above the drinking permissible limit for potable water as per the World Health Organization (WHO) guidelines. The trace elements namely iron, copper, zinc, nickel, manganese lead, cadmium, silver and arsenic have been resolved from five different districts/sub-divisions viz- Wokha, Tuensang, Zunheboto, Ungma in Mokokchung and Tseminyu in Kohima of Nagaland, India by Jamir et al. These results, when compared with the WHO guidelines for domestic water, showed that the concentration of lead, iron and manganese in many samples under investigation were above the permissible values (Jamir et al. 2011). Water is prone to contamination by microorganisms and organic matter among other pollutants irrespective of the nature of the source. Contaminated water serves as one of the transmission tools for diseases (World Health Organization 2012) such as dysentery, diarrheal diseases, parasitic infestation, anemia and most commonly gastrointestinal infection etc. Therefore, the water quality must be tested at regular intervals on the grounds that human population suffers from varying water-borne diseases due to the use of contaminated drinking water. Water quality for the most part includes physical, chemical, biological, radiological and aesthetic characteristics of the water (Alobaidy et al. 2010; Ombaka and Gichumbi 2012). Recognizing the distribution of pH, Dissolved oxygen, Turbidity, Salinity, Electrical Conductivity (EC), Total suspended solids (TDS), Total Hardness, Fluoride, Nutrients such as Nitrates and Nitrites, Phosphates and Sulfates and the presence of metals such as Aluminum, Chromium, Iron, Manganese, Silver, and Zinc are viewed as basic determinants for most improvement investigations of water quality (Beyene et al. 2019) and provides the basis to make wise decisions on drinking water quality protection and management (Li and Wu 2019).
The present paper solely focus on the data accumulation and analysis as more data sets are necessary to comprehend the processes and mechanisms of drinking water quality variation. To understand the status of the water quality, surface and groundwater assessment has been carried out in selected areas of Dimapur Sadar and Chumukedima Circles of the district during post-monsoon season (October and November 2018). A total of fifteen samples were randomly collected from rivers, tube-wells and ring-wells at different sites from seven locations of Dimapur. Out of 75 parameters outlined by the Central Pollution Control Board (Ministry of Environment and Forests 2010) for determining water quality, 20 physico-chemical parameters and bacteriological studies were examined in correlation with BIS (IS: 10500, 2012) and WHO standards.
Materials and methods
Study area
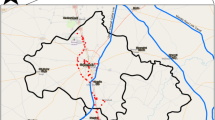
Water samples were collected from seven different sites of Dimapur district, Nagaland, India. The geographical coordinates of the study area are between the latitude 25.7864° N to 25.921° N and longitude 93.735° E to 93.7988° E.
Sampling
The sampling points were confined to rivers, bore-wells and ring-wells used for drinking and domestic purposes (Fig. 1). The depth of the wells varies from 32–200 ft. A total of 15 samples were collected from seven different locations of Dimapur during post-monsoon season. Prior to the day of sample collection, all sample bottles were thoroughly washed, sterilized and dried. The bottles were rinsed with samples multiple times at the time of collection with proper labeling. The latitude and longitude of all the sampling sites were recorded using a GPS model (Model: Garmin GPS 72H) during the time of sample collection (Table 1) and a sampling location map is made by ArcGIS. Standard methods were followed for sample collection and preservation. The collected samples were tested for 20 physico-chemical parameters such as Temperature, pH, Dissolved Oxygen (DO), Conductivity, Total Dissolved Solids (TDS), Salinity, Turbidity, Hardness, Aluminum, Chromium, Iron, Manganese, Silver, Zinc, Fluoride, Nitrate, Nitrite, Phosphates and Sulphates. The sample bottles were taken to the laboratory in an icebox to avoid external contamination and unusual change in physical and chemical properties in water quality and then stored at 4 °C for further analysis of other parameters.
Methods
AR grade reagents, deionized water and borosil glasswares were used for the preparation of solutions. pH was estimated by digital pH meter (EuTech pH 610). TDS, Electrical conductivity and Salinity were measured using Multiparameter EuTech CD 650. Turbidity was determined using turbidity meter (EuTech TN 100). Total hardness was determined by complexometric titration using Erichrome Black-T as an indicator (EDTA method). Presence of cations such as Aluminum, Chromium, Iron, Manganese, Silver and Zinc and anions such as Fluoride, Nitrate, Nitrite, Phosphate and Sulfate were estimated using Hannah Instruments, HI-83200 Multiparameter Photometer. Bacteriological analysis was performed using the Bacteriological Field Test Kit (H2S strip method developed by DRL, DRDO) using a standard protocol. Analytical methods and the instruments used for carrying out the studies of different physico-chemical properties are listed in Table 2.
Results and discussions
Physico-chemical analysis of all the samples from different locations was carried out and the results are shown in Tables 3, 4 and 5. The results obtained from this study are compared to standard BIS and WHO values. The detailed assessment of each parameter is discussed below one by one:
Physical nature analysis
Temperature
Temperature is an important factor to consider while evaluating water quality because it can alter the physical and chemical properties of water (Fondriest Environmental Inc. 2014). Biological activities in the water are also dependent upon temperature. If the temperature increases, these activities will also increase. The optimal water supply temperature should be between 10 °C and 25 °C. The collected samples have a temperature range of 26–30 °C.
Turbidity
Turbidity is an optical determination of the clarity of water based on light dispersion. It is generally measured in units called a Nephelometric Turbidity Unit (NTU) and should ideally be below 5 NTU. More turbidity implies more particles in suspension. These suspended particulates can assist in attaching heavy metals, micro-organisms and many other organic toxic compounds. As a result, turbid waters can be microbiologically contaminated and indirectly pose a health problem. The turbidity of the collected samples was found between 0.50 to 3.91 NTU which is within the recommended limit by BIS and WHO standard.
Chemical nature analysis
pH
pH level in water determines the acid–base equilibrium in most water and is one of the most significant operational water quality parameters. The pH of water is controlled primarily by the rock type from which the well originates. Groundwater with low pH values can cause gastrointestinal disorders, and corrosion of metal pipelines, leading to the lease of toxic metals such as Zn, Pb, Cd, Cu etc. (Vijayakumar et al. 2016). In the present study, the highest value of pH is found to be 8.41 (M-2) and the lowest value is 5.31 (M-11). Of the samples, 53.3% dropped outside the BIS recommended pH range and the majority of the sources had a pH lower than neutrality.
EC
Electrical Conductivity of water is measured in micro-mhos per cm (µmho/cm) at 25 °C. It quantifies water salinity and is directly associated with the concentration of dissolved ionized solids in water. Major positively charged ions that affect the conductivity of water are sodium, calcium, potassium and magnesium and major negatively charged ions are chloride, sulfate, carbonate and bicarbonate. Nitrates and phosphates are less likely to contribute to conductivity, but they are biologically very important. The maximum and minimum values of EC are found in the sample (M-14) and (M-11) and it varies between 470.1 µs/cm and 81.43 µs/cm, respectively. Despite this variation, the electrical conductivity remains low.
Total dissolved solids (TDS)
Total Dissolved Solids are composed of dissolved inorganic salts and a small amount of organic matter. It is expressed in milligram per unit volume (mg/L). The acceptable range of TDS is 500 mg/L as suggested by BIS standards (BIS 2012). In the present study, the range of TDS of analyzed water samples varied between 77.93–411.1 mg/L as shown in Table 3. Samples M-2 and M-11 recorded the highest and lowest TDS value. However, all the values were within the standard limit of BIS and WHO (500 mg/L). Therefore, the drinking water is safe in terms of TDS.
DO
Dissolved oxygen analysis estimates the amount of gaseous oxygen dissolved in an aqueous solution. The value of DO ranges from 5.02 to 9.14 mg/L. About most of the sampling locations are up to permissible limit according to drinking water quality standards (BIS 2006) i.e. > 5 mg/L (tolerance level).
Salinity
Salinity is the dissolved salt concentration in a given volume of water. Depending on the salinity concentration, groundwater quality falls into three categories- freshwater (0–1%), salinized water (1–3.5%) and saline water (3.5–35.7%). The maximum and minimum salinity value was recorded as 410.0 mg/L for M-2 and 78.70 mg/L for M-11.
Hardness
The degree of hardness of drinking-water is important for aesthetic acceptability by consumers. No health-based guideline value is proposed for hardness in drinking-water (World Health Organization 2011). According to BIS, desirable and maximum permissible limits of Total hardness are 300 mg/L and 600 mg/L. The total hardness of the samples ranged 37.5 to 300 mg/L and is relatively low in all samples.
Cation concentration analysis
Iron
Iron concentration depends on the sampled water table. It is generally observed that the concentration of iron increases as the depth of the water increases. Since, most of the sampled sources have the depth between 32 to 200 m, 20% of the collected samples are found to have a high concentration of iron making it not suitable for drinking purpose without prior treatment due to aesthetic reason. Based on the extensive research carried out in the North Eastern Region, Singh et al. (Singh et al. 2008) confirmed that the amount of iron is relatively high in almost all the north-eastern states. While the highest quantity of iron has been observed in Assam, Arunachal Pradesh, Meghalaya, Mizoram and Tripura, contamination rates are anticipated to increase in neighboring states, therefore, timely intervention is recommended to prevent or limit the damage. The amount of Iron that is above the permissible limit (0.3 mg/L) are shown in Fig. 2 and Table 3.
Manganese
Manganese (Mn) in drinking water originates naturally within the bedrock, particularly in deep well water. Manganese concentrations below 0.1 mg/L are usually acceptable to consumers and above this limit, it can cause unpleasant taste and raise aesthetic concerns. Mn is mainly referred to as a parameter related to the aesthetic evaluation of drinking rather than human health, however, there has been an indication of the possible link of manganese with cancer caused by over-exposure (Spangler and Reid 2010). Chemically, it tends to be viewed as a close relative of iron because of its occurrence in much the same form as iron. In this study, the elevated concentration of Mn and Fe showed comparable characteristics by samples—M-7 and M-13. Overall, more than 50% of the sampled water have higher Mn concentration thus rendering it unhealthy for consumption. Different concentration levels of Mn in different oxidation states are shown in Fig. 3.
Other metals
The concentration of heavy metals viz. Aluminum, Chromium, Silver and Zinc were analyzed and results showed that all the samples which have had some values of these metals were under the permissible limit of Indian standards IS:10500 and WHO (Figs. 4, 5).
Anion concentration analysis
Fluoride
In India, fluoride related health issues have been reported in the works of Saxena and Ahmed (2003), Jacks et al. (2005), Reddy et al. (2010), Ali et al. (2019) and Yadav et al.(2019) among others and have very high occurrences of dental, and in some cases, skeletal fluorosis primarily due to the excess fluoride in drinking water. Water with elevated levels of fluoride is not appropriate for drinking purposes. However, it is found that the concentration of fluoride in the region is generally lower than the desirable limit set by both Indian standards and WHO (Singh et al. 2008; Puzari et al. 2015). Out of 15 samples, four samples have detectable fluorides ion and their values are much lower than specified limit making the quality of water unhealthy for consumption.
Nitrate and Nitrite
High-nitrate drinking water is most often associated with privately owned wells (Fewtrell 2004). The factors responsible for elevated nitrate contents in well-water sources include groundwater hydrology, the addition of nitrates naturally and from surface contamination by nitrogenous fertilizers or by the organic waste of human or animal origin (Shearer et al. 1972). Excessive nitrate concentration contributes to bacterial contamination in water and can transform normal hemoglobin to methemoglobin causing blue baby (Methaemoglobinemia) syndrome in children (PEHSU 2014). It is, therefore, vital to determine whether the nitrate concentration is within the acceptable standard. The maximum permissible limit of nitrate in drinking water as per IS: 10500 is 45 mg/L. The nitrate concentrations in this study are well within the permitted limit and secure for use.
The presence of significant amounts of nitrite indicates recent contamination resulting from sewage seepage and oxygen deficiency (Aghzar et al. 2002) and based on the results, Nitrite was not detected in all sources of water. Therefore, it can be concluded that there is no recent contamination by seepage water in the samples.
Phosphate
Phosphate is an essential plant nutrient and is present in most fertilizers as a major component. Phosphates are non-toxic except in elevated concentrations. There are essentially three phosphate forms: phosphorus, organic phosphate, and inorganic phosphate. The main issue with phosphates in water is the causation of Eutrophication. The minimum phosphate concentration that triggers algae blooms is only 0.05 mg/L. It is also routinely added in drinking water supplies to prevent the entry of lead and copper from the corrosion of old pipes (ILO 2011). There is no limit set by BIS standard for phosphate however WHO recommends the permissible limit to be 0.1 mg/L. The present investigation found phosphate concentration ranging from 0.5 to 7.5 mg/L. All the samples exceed the limit fixed by WHO and are not fit for consumption without filtration.
Sulfate
The BIS standard suggests the concentration of Sulfate to be within 100–200 mg/L. The concentration of sulfate in water samples was observed to range from trace amounts to 80 mg/L, which is within the permissible limit.
Bacteriological studies
Bacteriological analyses of the collected water samples are also required in combination with the physical and chemical assessment to test the overall quality of the water because the main threat to human health associated with the consumption of polluted water are microbiological in nature.
As specified by WHO guidelines (Geneva 1997), ideal drinking-water should not contain any micro-organisms that can cause disease (World Health Organization 1997). And among different sources, in terms of bacterial contamination, bore wells were classified as ‘low risk’ by WHO (Obioma et al. 2017; Dhawde et al. 2018) which holds valid in this study. On the basis of the overall results of the bacteriological assessment, 40% samples were found unfit for human consumption as per BIS of drinking water.
Isolation of bacteria from water sample
First, the water sample to be tested for the presence of bacterial contamination was plated on general-purpose medium which supports a huge range of bacteria such as Nutrient Agar. After overnight incubation at 37° for 24 h, the colonies formed were purified by sub-culturing twice using the streaking plate method. Young cultures were used for primary screening which includes tests such as Gram Staining to isolate between the Gram Positive and Gram Negative bacteria, Indirect Staining of Bacteria (CAPSULE STAINING), Hanging Drop Method to determine bacterial motility and Endospore Staining to differentiate genera of bacteria, such as Bacillus and Clostridium which have the ability to produce resistant survival forms termed endospore. Further, secondary screening includes Biochemical tests which are used for characterization of the bacteria. These tests include Catalase test to detect the synthesis of catalase enzyme by bacteria, Oxidase test to determine the presence of cytochrome-c oxidase, an enzyme sometimes called indophenol oxidase, an enzyme of the bacterial electron transport chain, Indole production test, MR-VP test, Citrate utilization to perform citrate utilization test for the detection of fecal coliforms, Triple sugar iron test to determine whether gram-negative bacilli utilize glucose and lactose or sucrose fermentatively and produce hydrogen sulphide (H2S), Carbohydrate Fermentation Test, Nitrate reduction test to check the differentiation between members of Enterobacteriaceae on the basis of their ability to produce nitrate reductase enzyme and Urease test to check the ability of the bacteria to produce urease enzyme. Alternatively, isolation of bacteria can also be done using differential media for different bacteria which support their growth. Some of those media include.
-
i.
Tryptic Soy Agar (TSA) for the cultivation of non-fastidious bacteria, growth indicates non-fastidious bacteria present.
-
ii.
Chocolate Agar for the cultivation of fastidious organisms such as Neisseria or Haemophilus sp.
-
iii.
MacConkey (lactose) Agar which acts as both Selective and differential media. It selects for non-fastidious gram-negatives; red colonies indicate fermentation of lactose, white indicates no fermentation of lactose.
-
iv.
Eosin-methylene Blue Agar (EMB) to differentiates lactose fermenters (E. coli) from non-fermenters (Salmonella, Shigella).
-
v.
Mannitol Salt Agar selects for Staphylococci, which grow at high salt concentrations; differentiates Staphylococcus aureus from other Staphylococci. Staphylococcus aureus is yellow (ferments mannitol), other staphylococci are white.
-
vi.
SS (Salmonella-Shigella) Agar is a selective medium used to isolate Salmonella and Shigella species.
-
vii.
Thiosulphate-Citrate-Bile-Sucrose (TCBS) Agar is a selective medium used to isolate Vibrio cholerae and other Vibrio species.
-
viii.
Crystal violet blood agar is a selective media for Streptococcus pyogenes.
From the above study, it was inferred that only E. coli is present in the samples as mentioned in Table 6 and this has been verified by multiple tests described above.
Conclusions
The data set reported here indicates that, with regard to physico-chemical, cationic and anionic properties, all samples obtained from different locations comply with both WHO and Indian Standards except for heavy metals such as Iron and Manganese, and nutrient Phosphate concentrations which are well above the acceptable limit and, therefore, not suitable for drinking and domestic purposes. However, prior to use, proper treatment of the water can improve the water quality. Bacteriological contamination was likewise observed in some sample sources. This study revealed the nature of groundwater metal contamination in the region and that with continuous testing, more contaminated groundwater aquifers are bound to be identified (Bhuyan 2011). And the periodic determination of various water quality parameters may help improve the water treatment process from the acquired analysis results. In addition, it must be understood that water testing is an important strategy to guarantee the accessibility of contamination-free drinking water while, at the same time, raising awareness of sanitation and hygienic conditions among the individuals must be encouraged. Large data sets are, therefore, required to understand the processes and mechanisms of variability in the quality of drinking water. As such, more research needs to be done to attest to the viability of improving water sources by data accumulation.
References
Aghzar, N., Berdai, H., Bellouti, A., & Soudi, B. (2002). Ground water nitrate pollution in Tadla (Morocco). Revue des Science l’Eau, 15, 459–492. https://doi.org/10.7202/705465ar.
Ali, S., Fakhri, Y., Golbini, M., et al. (2019). Concentration of fluoride in groundwater of India: a systematic review, meta-analysis and risk assessment. Groundwater for Sustainable Development, 9, 1–10. https://doi.org/10.1016/j.gsd.2019.100224.
Alobaidy, A. H. M. J., Maulood, B. K., & Kadhem, A. J. (2010). Evaluating raw and treated water quality of tigris river within baghdad by index analysis. Journal of Water Resource and Protection, 02, 629–635. https://doi.org/10.4236/jwarp.2010.27072.
Beyene, G., Aberra, D., & Fufa, F. (2019). Evaluation of the suitability of groundwater for drinking and irrigation purposes in Jimma Zone of Oromia, Ethiopia. Groundwater for Sustainable Development, 9, 1–8. https://doi.org/10.1016/j.gsd.2019.100216.
Bhuyan, B. (2011). Assessment of arsenic and iron contamination of groundwater in four development blocks of Lakhimpur District, Assam, India. Der Chemica Sinica, 2, 316–323.
BIS. (2012). Indian standards drinking water specifications IS 10500:2012. Indian Standard for Drinking Water as per BIS Specifications, 2, 11.
Dhawde, R., Surve, N., Macaden, R., et al. (2018). Physicochemical and bacteriological analysis of water quality in drought prone areas of Pune and Satara Districts of Maharashtra, India. Environments, 5, 1–20. https://doi.org/10.3390/environments5050061.
Fewtrell, L. (2004). Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environmental Health Perspectives, 112, 1371–1374. https://doi.org/10.1289/ehp.7216.
Fondriest Environmental Inc. (2014) Temperature profiling in lakes. Fundamentals of Environmental Measurements
ILO. (2011). Phosphates, inorganic and organic. Encyclopaedia of Occupational Health and Safety, 3, 1–8.
Jacks, G., Bhattacharya, P., Chaudhary, V., & Singh, K. P. (2005). Controls on the genesis of some high-fluoride groundwaters in India. Applied Geochemistry, 20, 221–228. https://doi.org/10.1016/j.apgeochem.2004.07.002.
Jamir, T. T., Devi, W. B., Singh, U. I., & Singh, R. K. B. (2011). Lead, iron and manganese contamination in spring, pond and well water in Nagaland, one of the Seven North-Eastern states of India: a future danger. Journal of Chemical and Pharmaceutical Research, 3, 403–411.
Kalhor, K., Ghasemizadeh, R., Rajic, L., & Alshawabkeh, A. (2019). Assessment of groundwater quality and remediation in karst aquifers: a review. Groundwater for Sustainable Development, 8, 104–121. https://doi.org/10.1016/j.gsd.2018.10.004.
Li, P., & Wu, J. (2019). Drinking water quality and public health. Exposure and Health, 11, 73–79. https://doi.org/10.1007/s12403-019-00299-8.
Margat, J., Gun, J. van der (2013) Groundwater around the world
Ministry of Environment and Forests. (2010). Status of water quality in India- 2010. Monitoring of National Aquatic Resources, 1, 1–141.
Ministry of Water Resources (2013) Ground Water Information Booklet Dimapur District, Nagaland
Obioma, A., Chikanka, A. T., & Loveth, N. W. (2017). Evaluation of bacteriological quality of surface, well, borehole and River Water in Khana local government area of Rivers State, Niger Delta. Annals of Clinical and Laboratory Research, 5, 1–5. https://doi.org/10.21767/2386-5180.1000183.
Ombaka, O., & Gichumbi, J. M. (2012). Water quality assessment of Ruguti River in Meru. International Journal of Water Resources and Environmental Engineering, 4, 404–414. https://doi.org/10.5897/IJWREE12.093.
PEHSU (2014) Nitrates, methemoglobinemia, and drinking water : a factsheet for clinicians nitrates. Pediatric Environental Health Specification Units 1–3
PIB Government of India Ministry of Water Resources (2018) Study on Groundwater Contamination. 1–5
Puzari, A., Khan, P., Thakur, D., & Kumar, M. (2015). Quality assessment of drinking water from Dimapur District of Nagaland and Karbi-Anglong District of Assam for possible related health hazards. Current World Environment, 10, 634–640.
Reddy, A. G. S., Reddy, D. V., Rao, P. N., & Prasad, K. M. (2010). Hydrogeochemical characterization of fluoride rich groundwater of Wailpalli watershed, Nalgonda District, Andhra Pradesh, India. Environmental Monitoring and Assessment, 171, 561–577. https://doi.org/10.1007/s10661-009-1300-3.
Saxena, V. K., & Ahmed, S. (2003). Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environmental Geology, 43, 731–736. https://doi.org/10.1007/s00254-002-0672-2.
Shearer, L. A., Goldsmith, J. R., Young, C., et al. (1972). Methemoglobin levels in infants in an area with high nitrate water supply. American Journal of Public Health, 62, 1174–1180.
Singh, A.K. (2004). Arsenic contamination in groundwater of North Eastern India. National Semin Hydrol with focal theme Water Quality 1–8
Singh, A. K., Bhagowati, S., Das, T. K., et al. (2008). Assessment of arsenic, fluoride, iron, nitrate and heavy metals in drinking water of northeastern India. Bulletin Himalayan Ecology, 16, 20.
Spangler, J. G., & Reid, J. C. (2010). Environmental manganese and cancer mortality rates by County in North Carolina: an ecological study. Biological Trace Element Research, 133, 128–135. https://doi.org/10.1007/s12011-009-8415-9.
Vijayakumar, V., Krishnakumar, S., & Vasudevan, S. (2016). An environmental quality assessment on groundwater geochemistry in Western part of Perambalur District, Tamilnadu, India. Journal of Environmental Science and Pollution Research, 2, 54–56.
World Health Organization (2012) Water quality and health strategy 2013–2020. 1–15
World Health Organization (2011) Guidelines for drinking-water quality: Fourth Edition. 1–518
World Health Organization (1997) Guidelines for drinking-water quality. 3:1–28
Yadav, K. K., Kumar, S., Pham, Q. B., et al. (2019). Fluoride contamination, health problems and remediation methods in Asian groundwater: a comprehensive review. Ecotoxicology and Environmental Safety, 182, 1–23. https://doi.org/10.1016/j.ecoenv.2019.06.045.
Acknowledgements
The authors acknowledged the financial support received from National Mission on Himalayan Studies (NMHS, Project ID: HSF 2017-18 / I-23/11), Ministry of Environmental, Forest and Climate Change, Govt. of India, and for their encouragement in carrying out this work. The authors wish to extend their appreciation to A.Elow, Geological Survey of India and A. Mavani, National Institute of Technology Nagaland for their help in generating location map of the study areas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pamei, M., Naresh, G., Dutta, D. et al. Accessibility of safe drinking water in greater Dimapur area of Nagaland and related health hazards: an analytical study. Int J Energ Water Res 4, 245–255 (2020). https://doi.org/10.1007/s42108-020-00074-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-020-00074-5