Abstract
Aquatic organisms might be exposed episodically or continuously to chemicals for long-term periods throughout their life span. Pesticides are one example of widely used chemicals and thus represent a potential hazard to aquatic organisms. In addition, these chemicals may be present simultaneously in the environment or as pulses, being difficult to predict accurately how their joint effects will take place. Therefore, the aim of the present study was to investigate how Daphnia magna (clone k6) exposed throughout generations to a model pesticide (the fungicide carbendazim) would react upon an exposure to another chemical compound (triclosan) and to a mixture of both chemicals (carbendazim and triclosan). Responses of daphnids continuously exposed to carbendazim and kept in clean medium will be compared using immobilization tests and the comet assay (DNA integrity). The results showed that triclosan presented similar toxicity to daphnids exposed for 12 generations (F12) to carbendazim (similar 48-h-LC50 values for immobilization data), when compared with daphnids kept in clean medium. However, at subcellular level, daphnids previously exposed to carbendazim for 12 generations (F12) showed different responses than those from clean medium, presenting a higher toxicity; a general higher percentage of DNA damage was observed, after exposure to a range of concentrations of triclosan and to the binary combination of triclosan + carbendazim. The patterns of toxicity observed for the binary mixture triclosan + carbendazim were generally similar for daphnids in clean medium and daphnids exposed to carbendazim, with a dose level deviation with antagonism observed at low doses of the chemical mixture for the immobilization data and a dose ratio deviation with synergism mainly caused by triclosan for DNA damage. With this study, we contributed to the knowledge on long-term induced effects of carbendazim exposure, while looking at the organismal sensitivity to another chemical (triclosan) and to a mixture of carbendazim and triclosan using lethality as an endpoint at the individual level and DNA damage as a subcellular endpoint.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the environment, aquatic organisms may be exposed not only to one chemical but also to a cocktail of chemicals (as mixture). These chemical exposures, either from similar or different sources, may be short-termed (e.g., as pulses), last for the entire lifetime of an organism, or even for several generations (Kuster et al. 2008; Solomon et al. 1996). Chemical input might be related to pesticide seasonal patterns of application in agriculture to waste water treatment plants (WWTP) (e.g., effluents) or to other accidental or deliberate chemical discharges (McCahon and Pascoe 1990; Solomon et al. 1996). A continuous chemical exposure, even at low concentrations, might have consequences to organisms’ fitness, originating more or less sensitive organisms. These changes in organisms’ sensitivity can be scrutinized by testing their sensitivity towards (other) chemical exposure. Acknowledging these concerns, multigenerational studies can be of high importance as they might provide estimation for population effects and support the risk assessment process of chemicals in the aquatic system. Daphnia magna is a suitable test organism for effects’ evaluation throughout several generations as it reproduces by parthenogenesis (with no recombination), eliminating confounding genetic differences and possibly making this species more susceptible to DNA damage throughout time (Harris et al. 2012; Hebert and Ward 1972; Simon et al. 2003; Sukumaran and Grant 2013). Daphnia sp. standardized tests are often advised for regulatory purposes, and the comet assay, which evaluates the DNA damage in single cells expressed as DNA strand breaks, can be used as an early warning tool (Collins et al. 1997; Singh et al. 1988).
Currently, there is not a complete picture on how organisms behave under long-term exposures, and the majority of tests are carried out throughout two or three generations only (Brausch and Salice 2011; Brennan et al. 2006; Massarin et al. 2010). Therefore, increasing exposure time and adding extra stressors to the testing systems may straighten the knowledge gap that exists.
Considering this, the aim of the present study was to investigate how D. magna exposed throughout generations to a model chemical would react upon an exposure to other chemicals and to the mixture of both chemicals (the pre-exposed and the new chemical). For that, the fungicide carbendazim (CBZ; methyl-2-benzimidazole carbamate), largely used in agriculture (WHO 1993), was chosen as a model chemical for the continuous exposure. CBZ has been used in different crops (e.g., from spring to autumn); therefore, it is likely to be continuously released during some months (EU Pesticide Database 2016). CBZ is expected to appear in aquatic systems due to agricultural fields’ runoffs, and it has already been detected at concentrations of 4.5 μg/L in surface waters (Chatupote and Panapitukkul 2005; Palma et al. 2004). In addition, a predicted environmental concentration of 9.3 μg/L of CBZ for surface waters was derived for Southern Europe, for spring and winter cereals crop areas (EFSA 2010). Therefore, in the experimental design, D. magna organisms were continuously exposed to 5 μg/L of CBZ throughout 12 generations. To test if the sensitivity of daphnids was altered upon the exposure to another chemical or mixtures, triclosan (TCS; 5-chloro-2-(2,4-dichlorophenoxy)phenol), an antimicrobial agent present in many personal care products worldwide (Brausch and Rand 2011), was selected to achieve this goal. Immobilization tests and the comet assay were carried out with TCS and with the mixture of TCS and CBZ. When TCS enters the WWTP, its complete removal can be inefficient, leading to its release into the environment (Bester 2003). Concentrations of TCS of up to 6–14 mg/L were found in a river near a manufacturing plant in the USA (NICNAS 2009). In Portugal, TCS was detected in urban wastewater samples, although at lower concentrations: 0.124 μg/L (Neng and Nogueira 2012). Despite the different sources/origins of CBZ and TCS, there is a possibility of their co-occurrence in surface waters. Therefore, studies predicting joint effects of chemicals from different sources, which are usually disregarded, can provide information to derive more accurately risk assessment. Besides using the Test Guideline 202 from OECD (OECD 2004) on the immobilization of D. magna, the DNA damage was the chosen sublethal toxicity endpoint, since DNA damage was already detected in D. magna exposed to CBZ and TCS in previous studies as one of the most sensitive endpoints (Silva et al. 2015, 2017).
Materials and methods
Test organism
D. magna Straus clone K6 (originally from Antwerp, Belgium) were obtained from laboratory cultures maintained at the University of Aveiro (Portugal). Daphnids (20 organisms) are laboratory-cultured in 1-L glass vessel with ASTM moderated-hard-water medium (American Society for Testing and Materials) (ASTM 1980), at temperature between 19 and 21 °C and 16-hlight–8-hdark photoperiod. Medium was renewed three times per week adding the green algae Raphidocelis subcapitata at a concentration of 3 × 105 cells/mL to feed D. magna and supplemented with an organic extract (Marinure seaweed extract, supplied by Glenside Organics Ltd.). The seaweed extract was added to the cultures at 6 ml/L of ASTM. The seaweed extract was prepared by diluting a concentrated plant extract (rich in minerals and trace elements; 93.5% of dry matter) and finally filtered with a cellulose acetate membrane (0.2-μm pore size).
Test chemicals
For the multigenerational test, a stock solution of CBZ (CAS No. 10605-21-7, 99.4% purity, Bayer) was prepared in ASTM medium. For the toxicity testing (immobilization and comet assays), stock solutions of TCS and CBZ were prepared in ASTM and acetone as solvent due to the low solubility of TCS and CBZ at higher concentrations; in these toxicity tests, a solvent control of 100-μL acetone/L was also included in all experiments as recommended by the OECD guideline 23 (OECD 2000).
Chemical analyses were performed for TCS and CBZ in the test medium (by the Marchwood Scientific Services, Southampton, UK). For that, extra beakers were run simultaneously to the toxicity tests with TCS and CBZ dissolved in ASTM media. The analyses for TCS ((Irgasan, CAS No. 3380-34-5, 97% purity, Sigma-Aldrich) were performed by gas chromatography-mass spectrometry (GCMS-MS). A representative portion of the samples (200–300 mL) were extracted with 20 mL of acetonitrile (containing 1% acetic acid). Samples were then subjected to a solid phase extraction stage (200-mg cartridge). Then, a methanol wash followed, and a final injection volume (10 μL) applied. Standards were prepared in solvents at seven levels with recoveries in the range 70–120%. The chemical analyses for CBZ were performed by liquid chromatography-mass spectrometry (LCMS-MS) using the QuEChERs method. A representative portion of the sample (200–300 mL) was extracted with 20 mL of acetonitrile (with 1% acetic acid). Afterwards, a partitioning step with magnesium sulfate followed and a subsequent buffering step with sodium acetate. An aliquot was mixed with methanol, and the extract was injected directly into the LCMS-MS system (instrument Agilent 6410 Triple Quad LCMs-MS) without any clean-up. A 10-μL injection volume was utilized. Standards were prepared in solvents at seven levels with recoveries (range 70–120%). In order to determine chemical decay in time, the degradation constant (k0) was calculated by the following equation:
In the equation, C0 corresponds to the initial external concentration (μg/L), k0 represents the constant of degradation of the chemical in the medium (/h), and t corresponds to time (h) (Widianarko and Van Straalen 1996).
Multigenerational experimental setup
An isoclonal population of D. magna was exposed continuously to 5 μg/L of CBZ (Dph_CBZ) for 12 generations. This concentration (5 μg CBZ/L) was based on previous results where a no-observed effect concentration (NOEC) for reproduction of 5 μg/L was derived (Silva et al. 2015) and also representing an environmentally relevant concentration (Palma et al. 2004). An isoclonal population of daphnids in clean medium (Dph_Clean) was kept simultaneously as a control. Both populations were kept in ASTM hard water, fed with R. subcapitata, and supplemented with organic extract (Marinure seaweed extract, supplied by Glenside Organics Ltd.), differing only on the presence/absence of CBZ. Medium renewal was performed as described for the culture procedures. Daphnids were exposed in one vessel per population with 25 daphnids each as it was aimed to increase efforts on the generations’ number and neonates’ fitness testing (see beneath) in detriment of replicates. Since daphnids are clonal organisms, the genetic variation within replicates may be lower than for other organisms and this mimics also what happens in laboratorial cultures, where neonates from the same culture vessels are used to test.
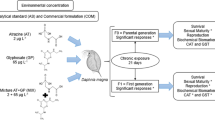
In the multigenerational experiment, neonates from the third to fifth brood (< 24 h old) were used to start the next generation for both populations, using always the same brood for Dph_Clean and Dph_CBZ populations. To control differences in daphnids’ responses due to variation in their sensitivity in each testing generation, both immobilization tests and comet assays were performed simultaneously using Dph_Clean and Dph_CBZ populations (Loureiro et al. 2010). In both tests, neonates spent < 24 h in the same environment as the mothers. The experimental design is schematized in Fig. 1.
Experimental design of the multigenerational setup with two isoclonal populations of Daphnia magna (continuously exposed to carbendazim and daphnids in clean medium), and the generations where toxicity tests were carried out (F8 and F12). Each box represents the generations (F) and the respective tests carried out in both populations (Dph_Clean and Dph_CBZ)
Ecotoxicity of triclosan and the binary mixture of carbendazim and triclosan
During the multigenerational exposure to CBZ, neonates were collected and toxicity tests were run to infer on their changes of sensitivity towards TCS and a set of binary mixtures of CBZ and TCS. Immobilization tests with TCS and the binary mixture with CBZ and TCS were run at F8 and F12 generations, in both neonates originating from both populations Dph_Clean and Dph_CBZ (Fig. 1). In addition, the sensitivity to CBZ was also tested in F8 and F12, as this was part of the mixture toxicity setup.
Immobilization tests were carried out according to the OECD 202 guidelines (OECD 2004), with few adaptations. Daphnids with less than 24 h were used to initiate the test, and after 24 and 48 h, daphnids’ immobilization after gentle agitation of the test beaker was recorded. Neonates were exposed to test solutions of CBZ, TCS, and binary mixtures for 48 h in 50-mL beakers with no food (16:8-h light-dark photoperiod and 20 ± 1 °C). The experimental setup consisted in a full factorial design (Fig. 2a) with three replicates of five neonates each, for every treatment and controls. Replicates were reduced in order to allow simultaneous testing (reducing effort) and to allow a reliable coverage of the exposure/response surface in the mixture trials by increasing the number of treatments. This procedure increases both reliability and power of the analysis, as the response surface analysis is based on a regression model (Loureiro et al. 2010). For the single and mixture experiments with TCS and CBZ, in the F8 and F12 generation, neonates were exposed to concentrations ranging from 200 to 1000 μg/L of TCS and from 20 to 220 μg/L of CBZ corresponding to a minimum of 0.5 toxic units (TUs) and a maximum of 3 TUs of the mixture (where 1 TU = LC50) (Fig. 2a). Single chemical exposures referred above were performed simultaneously in each mixture test not only to account for the sensitivity variations throughout generations but also to predict results that will be latter compared with the observed output (experimental data from the mixture exposure) (Loureiro et al. 2010).
For the Comet assay, F12 neonates (< 24 h) from both population Dph_Clean and Dph_CBZ were exposed to CBZ, TCS, and a mixture of both chemicals for 24 h (Fig. 1). Three replicates, with 15 daphnids each, were used for the control and all concentrations used. As this control included a 24-h exposure to clean medium of the CBZ daphnids, this helped to understand their recovery was already starting at a lower organizational level (subcellular level). The Comet assay was based on the methodology developed by Nogueira et al. (2006) and is explained below. Positive controls consisted of daphnid’s cells previously exposed to hydrogen peroxide (H2O2). To prevent UV-induced DNA damage, the comet assay procedures were conducted under yellow light. Briefly, organisms were placed in Eppendorfs (of 1.5 mL) with 1 mL of phosphate-buffered saline (PBS), 10% dimethyl sulfoxide (DMSO), and 20-μM ethylenediamine tetra-acetic acid (EDTA). Organisms were disintegrated mechanically by gently stirring with a pestle. Samples were centrifuged (200 g, 10 min, 4 °C), and most of the supernatant was carefully removed. Ten microliters of the pellet (containing cells) was transferred to Eppendorfs containing 0.5% low melting point agarose (at 37 °C). To uniformly distribute this mixture on the microscope glass slides, containing 1% normal melting agarose, the coverslips were applied. Slides were placed on ice for 10 min. The coverslips were removed and the slides were placed, in a solution of 10-mM Tris-HCl, 100-mM EDTA, 2.5-M NaCl, 10% DMSO, and 1% Triton X-100, pH 10, for at least 1 h for cell lysis. Electrophoresis occurred in a 10-M NaOH, 200-mM Na2-EDTA solution. Slides were placed in the electrophoresis tank for 15 min before starting the electrophoresis, to allow DNA unwinding and denaturation. Then, an electric current of 300 mA (30 Volts) was applied for 10 min. Slides were washed with 0.4-M Tris-HCl (pH = 7.5) for neutralization and further dehydrated with absolute ethanol 100%, for 10 s and left to dry for 1 day in the dark.
For the image analysis, 100 μL of ethidium bromide (20 μL/mL) was used to stain the slides and were then analyzed using a fluorescence microscope (Olympus BX41TF, China) at ×400 magnification. Per slide, 100 cells were examined, and when reading the slides, the scorer was unaware of the treatment condition. DNA damage was visually scored: each cell was scored on a 0 to 4 scale, as described by Duthie and Collins (1997). Type 0 represents no DNA damage, types 1 and 2 mild to moderate damage, respectively, and types 3 and 4 represents extensive DNA damage. The total comet score was determined according to the method of Duthie and Collins (1997): (number of cells in type 0 × (type) 0) + (number of cells in type 1 × (type) 1) + (number of cells in type 2 × (type) 2) + (number of cells in type 3 × (type) 3) + (number of cells in type 4 × (type) 4). Consequently, the total score for 100 cells could range between 0 (all comets with type 0 damage) to 400 arbitrary units (all comets with type 4 damage). Afterwards, the percentage of DNA damage was calculated. Figure 1 SD represents a comet type scale in daphnid cells.
Neonates were exposed to 5, 20, and 25 μg/L of CBZ, representing a NOEC, lowest-observed-effect concentration (LOEC), and EC50 (50% effective concentration) for daphnids’ reproduction (Silva et al. 2015). For TCS exposure, daphnids were exposed to 120, 160, and 206 μg/L of TCS, where the extremes represent the NOEC and EC50 for daphnids’ reproduction, and 160 μg/L was chosen as a mean value concentration between the extremes (Silva et al. 2015). Binary mixtures consisted on combinations of 120 μg/L of TCS and 5 μg/L of CBZ; 160 μg/L of TCS and 20 μg/L of CBZ; and 206 μg/L of TCS and 25 μg/L of CBZ (Fig. 2b), corresponding to a minimum of 1 TU and a maximum of 3 TUs (ΣTUs), respectively (Fig. 2b).
Statistical analysis
Normality of the data was assessed using the Shapiro-Wilk test, and homoscedascity was assessed using Levene’s equal variance test (Systat Software Inc. 2008). In the immobilization tests and the comet assays, differences between the negative control and the solvent control were checked using a t test (Systat Software Inc. 2014). For the immobilization data, the 48-h-LC50 values were calculated using a nonlinear regression (four parameter logistic curve) (Systat Software Inc. 2014). LC50 values were statistically compared according to Sprague and Fogels (1976). For DNA damage, when possible, EC50 was calculated using a nonlinear regression with a logistic function (Systat Software Inc. 2014). To detect differences between Dph_Clean and Dph_CBZ populations, for the DNA damage endpoint, a two-way ANOVA with multiple comparisons examined by Holm-Sidak post hoc method was performed using SigmaPlot (Systat Software Inc. 2008), with chemical exposure and populations as fixed factors. To evaluate the percentage of variance for each factor in the two-way ANOVAs, the r-squared was determined (dividing the sum of squares of each factor and of their interaction by the total sum of squares) (Hullett and Levine 2003).
Data obtained in the mixture experimental setup were analyzed by comparing the obtained effects with the expected mixture effects based on the two conceptual models: concentration addition (CA) and independent action (IA). For that, the MIXTOX tool described by Jonker et al. (2005) was used. The CA model assumes that compounds have the same mode of action (MoA) and they can be seen as dilution of one another, and the IA model assumes that chemicals possess different MoA; therefore, single chemical-induced effects are independent. In the present study, the IA model was the main conceptual model used because it is expected that from both chemical structure and MoA, they will behave differently inside the organism although potentially inducing specific similar effects. Notwithstanding, recently EFSA has released a report from the Scientific Colloquium 21 on the “Harmonisation of human and ecological risk assessment of combined exposure to multiple chemicals,” which states that the mixture patterns should be predicted using the CA model as a precautionary approach in ecological approaches (EFSA 2015); accordingly, the CA model was used as well and was presented in supplementary data. Deviations from the IA or CA models were attained for synergism (more severe effect) or antagonism (less severe effect) or more complex deviations: dose ratio (DR: deviations depending on the composition of the mixture) and dose level (DL: deviations differing at low or high doses of the chemicals) dependencies by adding two additional parameters, a and b. The biological interpretation of parameters a and b is described on Table 1 (additional details can be found in Jonker et al. (2005)). The method of maximum likelihood was utilized to fit the data. Then, the fundamental procedure to minimize the sum of squared residuals (SS) was used by running the Solver Function in Microsoft® Excel. The best fit was chosen using the significance level α = 0.05. The TU approach was used, providing the contribution of each chemical to the toxicity mixture. TUs were calculated using the quotient ci/ECxi, where ci represents the individual concentrations of substances in the mixture and ECxi denotes the effect concentration (Bliss 1939; Jonker et al. 2005; Loewe and Muischnek 1926; Loureiro et al. 2010).
Results and discussion
Chemical analysis
The results on the chemical analysis have already been described elsewhere (Silva et al. 2015). CBZ concentration in the ASTM medium decreased over time; a decay rate (k0) of 0.03/h (SE = 0.005) was found, showing that only 18% of the initial concentration (7.2 μg/L) remained after 48 h. Regarding TCS, the obtained decay rate (k0) was 0.06/h (SE = 0.010), which means that after 48 h of the initial concentration (165 μg/L), only 1.3% of TCS concentration remained.
Multigenerational effects
Upon the multigenerational exposure to CBZ and after 12 generations, daphnids after being in a short 24-h period in clean medium were not able to recover the acquired DNA damage (when compared with those always kept in clean medium (Dph_Clean); p < 0.05, two-way ANOVA, Holm-Sidak method) (Fig. 5). The ability of daphnids to repair DNA damage has been reported (e.g., after exposure to benzo(a)pyrene and after a 9-day recovery period), being considered an important protection against genotoxic compounds (Atienzar and Jha 2004). Therefore, the extension of the recovery time (increasing the period of time of daphnids in clean medium) could allow excretion and elimination of the chemical, thus providing additional information (Villarroel et al. 1999).
Immobilization tests
Multigenerational experiments have recently been brought into focus in many studies investigating the long-term effects of compounds in D. magna (Brausch and Salice 2011; Massarin et al. 2010), though the study of changes in sensitivity towards other chemicals’ exposure is less common. Some authors reported that Daphnia sp. adapts to new environments in few generations (Ward and Robinson 2005); however, what ensues after these generations is not usually investigated and changes in daphnids’ sensitivity might actually occur throughout the generations. Therefore, in the present study, these effects were assessed.
In all the performed tests, no significant differences were observed between the solvent control and the negative control (t test, p > 0.05). In the statistical analysis, all comparisons between chemical treatments and the control group were carried out with the solvent control. In the F8 generation, the 48-h-LC50s for TCS were similar between the two populations, presenting values of 595.5 (SE 9.5) and 605.3 μg/L (SE 0.2), respectively, for Dph_Clean and Dph_CBZ (Fig. 3). For the F12 generation, the 48-h-LC50 values were 693.2 (SE n.d.) and 1040.3 μg/L (SE 423.4), respectively, for Dph_Clean and Dph_CBZ (Fig. 3). The 48-h-LC50 value for TCS was slightly higher for the Dph_CBZ comparing with the Dph_Clean population; but as no confidence intervals were possible to be derived, the statistical comparison using the Sprague and Foguels formula was not carried out; yet, both r2 values derived were close to 1 (1 and 0.99, respectively). The pre-exposure to CBZ during some generations apparently did not affect the organisms’ response to another chemical; in this case, TCS, in terms of survival, shows an overall similar sensitivity between Dph_Clean and Dph_CBZ populations throughout the generations. Although a pulse exposure to another chemical may provide some information regarding cross-tolerance in the present work, this did not seem to occur. In fact, no clear pattern towards tolerance was found also regarding the continuous exposure of daphnids to CBZ in previous multigenerational experiments (Silva et al. 2017; Silva et al. 2018). Cross-tolerance, also referred as cross-resistance by some authors, occurs when one organism becomes tolerant to one chemical and afterwards can become tolerant to another chemical due to, for instance, detoxification mechanisms that might confer tolerance against several chemicals (Georghiou 1972). In the literature, it is demonstrated that cross-resistance usually occurs when compounds have similar chemical structures and/or MoA (Oppenoorth 1985). The chemical structures of CBZ and TCS are different; yet, the exact mode of action in daphnids is not clear. Another factor contributing to a faster development of resistance is the selective pressure: the greater the pressure, the greater the degree of resistance (Crow 1954). In the present study, the pressure might be considered low, since the concentration of CBZ in the continuous exposure throughout the generations of D. magna was low (5-μg CBZ/L). On the opposite, Brausch and Smith (2009) observed that cross-tolerance (referred by the authors as cross-resistance) occurred for both cyfluthrin and naphthalene using D. magna. The authors observed that daphnids resistant to cyfluthrin were also resistant to two additional insecticides: DDT (1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane) (compound with similar MoA) and methyl parathion (compound with different MoA). In their study, cross-tolerance was evaluated by checking changes in 48-h-LC50 values between F0 and F13 generation, representing a 2.1 and 2.6 times higher LC50 values, respectively, for DDT and methyl parathion (Brausch and Smith 2009).
48-h-LC50 values after exposure to triclosan (TCS) derived from immobilization tests with neonates from population in clean medium—Dph_Clean (white dots) and from population continuously exposed to carbendazim—Dph_CBZ (black dots), at generations F8 and F12. Data are expressed as 48-h-LC50 value (μg/L) with standard error.
Silva et al. (2017) describe a multigenerational experiment with D. magna exposed to CBZ; this study evaluates individual endpoints, namely survival, reproduction, growth, and feeding activity. In Silva et al. (2017) study , daphnids pre-exposed to CBZ presented an increase in the feeding activity; however, reproduction, growth, and immobilization were similar throughout the generations. Silva et al. (2018) describe also a multigenerational experiment with D. magna exposed to CBZ. Low effects were found for the individual endpoints; for instance, for the intrinsic rate of natural increase, no differences were found, except for longevity, which decreased at the 12th generation compared with that of control daphnids (Silva et al. 2018).
Besides the effects of a single chemical or mixture of chemicals to an organism, the duration of the exposure is of paramount relevance (Paumen et al. 2008). This highlights the importance to study the toxicity effects in different generations and with different exposure times, since responses to chemicals might vary over time/generations.
Regarding MoA, CBZ acts on cell division, inhibiting the reproduction capacity (Canton 1976) and increasing the number of aborted eggs (observed already in D. magna), which is, in turn, probably related with mitosis inhibition during eggs division in the brood pouch (Ribeiro et al. 2011; Silva et al. 2015). In addition, CBZ also causes DNA damage in daphnids (Silva et al. 2015). TCS has been reported to cause general oxidative stress, to induce the antioxidant enzyme glutathione S-transferase (GST) in D. magna, and to inhibit the superoxide dismutase (SOD) activity, which may indicate damage in the cell membranes (Kovacevic et al. 2016; Peng et al. 2013). Additionally, TCS has the ability to induce DNA damage at concentrations starting in 120 μg/L in D. magna (Silva et al. 2015). Bearing in mind that the specific mechanism of action of CBZ and TCS in daphnids is still vague but assuming a potential difference between them in terms of chemical structure, to predict mixture toxicity effects, the IA model was the main model used as a starting point. All CA modeling was also carried out and is included in the Supplementary data (Table 1 SD).
Considering the exposure to the binary mixtures of TCS and CBZ in the immobilization test, it was found that data for Dph_Clean population in the F8 generation was significantly adjusted to the IA model (Table 2). Along the framework to assess potential deviations, antagonism was the best data fit detected (Table 2). The selection of this deviation is supported by the r2 value, by the lowest SS value comparing with all the others deviations in the IA model and supported by the isobologram as well (Fig. 4a1). Antagonism means that the effect of a mixture is less pronounced than the predicted based on individual chemical effects. For the F8 generation of neonates from Dph_CBZ population, the IA model fitted our data significantly with no further improvement by adding parameters for deviations (Table 2 and Fig. 4b1). This means that, for daphnids previously exposed to CBZ (Dph_CBZ), the relative effect of one chemical remains unchanged in the presence of other chemical (Bliss 1939), and an additivity of responses is achieved.
Concentration-response relationship for the binary mixture of triclosan and carbendazim for the immobilization data in the F8 and F12 generations of Daphnia magna (2D isobolic surfaces)): F8 Dph_Clean a observed data, a1 modeled data showing antagonistic; F8 Dph_CBZ (eight generations of pre-exposure to carbendazim) b observed data, b1 IA modeled data; F12 Dph_Clean c observed data, c1 modeled data showing a dose level (DL) deviation; F12 Dph_CBZ (twelve generations of pre-exposure to carbendazim) d observed data, d1 modeled data showing a dose level (DL) deviation
In the F12 generation, immobilization data for Dph_Clean population was significantly adjusted to the IA model (Table 2). Continuing the nested framework for assessing potential deviations, the DL deviation showed the best fit (Table 2). For the DL deviation, the derived parameter a was positive meaning that there was an antagonism at low doses of both chemicals and synergism at high doses; parameter b was lower than one, providing the information that the alteration from antagonism to synergism would occur at higher concentrations than the ones used in this experiment (Table 1). Therefore, synergism was not observed in the concentration range tested, and the main pattern for this endpoint was antagonism (Fig. 4c1). In the F12 generation, the same deviation was observed for Dph_CBZ population, with a DL deviation with antagonism at low doses and synergism at high doses (a > 0). The parameter b was again negative (Fig. 4d1). Therefore, the pattern obtained and observed for F12 was also antagonistic (Table 2 and Fig. 4d1). Although it has been reported that sometimes the reproducibility of mixture experiments is difficult to accomplish (Cedergreen et al. 2007), results for the immobilization data were similar in F8 and F12, with antagonism observed as the main pattern.
Differences in the toxicity prediction between both conceptual models, IA and CA models, are small and distinctions regarding MoA used to decide which reference model to choose is difficult (Cedergreen et al. 2008; EFSA 2015), although the EFSA report advises to have as starting point for ecotoxicity data the CA model (EFSA 2015). Even though the CA model is generally a more conservative and protective for the risk assessment of pesticide mixtures (Schell et al. 2018); in the present study, similar findings were derived from the IA model. Patterns derived from the CA model for the binary mixture with TCS and CBZ are presented in the Supplementary data (Table 1 SD and Fig. 2 SD), supporting similar findings (towards antagonism).
Comet assay
The comet assay was performed with the purpose of evaluating the genotoxic effects of TCS, CBZ, and its binary mixture in daphnids previously exposed to CBZ during several generations. In the F12 generation, the percentage of DNA damage increased with increasing concentrations of CBZ and TCS for both Dph_Clean and Dph_CBZ populations (Fig. 5a, b). After exposure to CBZ, significant DNA damages were detected (two-way ANOVA, F3,23 = 56.923, p < 0.001) (Fig. 5a and Table 3), and significant differences were observed between Dph_Clean and Dph_CBZ populations as well (two-way ANOVA, F1,23 = 44.871, p < 0.001) (Fig. 5a and Table 3); this factor explained 13% of the total variation (Table 3). A pre-exposure to CBZ for 12 generations (F12) appeared to have worsened the effect of a new exposure to CBZ, at all the concentrations tested, except for the last one 25 μg/L (Fig. 5a). Both factors interacted, indicating that the populations responded differently to CBZ concentrations (two-way ANOVA, F3,23 = 34.186, p < 0.001).
DNA damage (%) in F12 Daphnia magna cells from populations kept in clean medium- Dph_Clean (white dots) and from populations kept in carbendazim—Dph_CBZ (black dots) during the multigenerational test: a exposure to carbendazim, b exposure to triclosan, and c exposure to the mixture of carbendazim and triclosan (toxic units, where 1 TU = EC50). Data are expressed as mean values and standard error
After exposure to TCS, significant DNA damage was also detected (two-way ANOVA, F3,23 = 30.671, p < 0.001) (Table 3), and significant differences were observed between Dph_Clean and Dph_CBZ populations (two-way ANOVA, F1,23 = 61.346, p < 0.001) (Table 3). Similar to what have occurred after exposure to CBZ, in Dph_CBZ population, a higher percentage of DNA damage was always observed, when compared with Dph_Clean population at all TCS concentrations (Fig. 5b). The population factor explained 35% of the total variation (Table 4). However, both factors did not interact, indicating that the populations responded similarly to the concentrations of TCS (two-way ANOVA, F3,23 = 2.119, p > 0.05), which is clear in Fig. 5b. In the binary mixture of CBZ and TCS, significant effects on DNA damage were detected (two-way ANOVA, F3,23 = 40.657, p < 0.001) (Fig. 5c and Table 3). Significant differences were found between Dph_Clean and Dph_CBZ populations (two-way ANOVA, F1,23 = 21.610, p < 0.001) (Fig. 5c and Table 3) and both factors interacted, indicating that the isoclonal populations responded differently to the mixture exposures (two-way ANOVA, F3,23 = 8.463, p < 0.05) (Fig. 5c). However, the population factor explained only 12% of the total variation (Table 3).
These results are in accordance with Silva et al. (2017), where no to low differences were found for individual endpoints in the multigenerational exposure to CBZ; yet, deleterious effects were more prominent at a subcellular level (DNA damage). In Silva et al. (2018), different subcellular endpoints were assessed as well: biochemical biomarkers (cholinesterase, catalase, and glutathione-S-transferase), lipid peroxidation, and energy-related parameters (carbohydrates, lipids, and proteins along with available energy and energy consumption). In that study, differences between daphnids exposed and non exposed to CBZ throughout the generations were found for cholinesterase, glutathione-S-transferase, and lipid peroxidation (Silva et al. 2018).
Genotoxic effects have been reported in different organisms for both CBZ (in the plant Hordeum vulgare L. and marine invertebrate Donax faba) and TCS (in the zebra mussel Dreissena polymorpha and algae Closterium ehrenbergii) (Binelli et al. 2009; Ciniglia et al. 2005; JanakiDevi et al. 2013; Singh et al. 2008). Nevertheless, these genotoxic studies usually disregard a possible chemical pre-exposure that might occur in the organisms. Noteworthy is that, in the present work, genotoxicity of CBZ, TCS, or their mixture (expressed as DNA damage) was generally higher in organisms that were exposed to CBZ (Dph_CBZ) for 12 generations (F12), when compared with organisms that were always kept in clean medium (Dph_Clean), showing a higher sensitivity in daphnids pre-exposed to CBZ (Fig. 5a, b, and c). Carry-over effects between chemicals acting on different targets as reported by Ashauer et al. (2017) seem to have occurred. A pre-exposure to one chemical changed the survival over time curve of the other chemical (Ashauer et al. 2017). In the present work, one hypothesis for this increase in genotoxicity could be the transmission of DNA damage from parents to neonates throughout the generations. A possible accumulation and transmission of DNA damage to the offspring were already demonstrated in a multigeneration experiment with D. magna and benzo(a)pyrene (Atienzar and Jha 2004) and also in daphnids exposed to depleted uranium (Plaire et al. 2013). Additionally, genotoxicity is dependent on the efficiency of several repair mechanisms (Jha 2008), and some chemicals might affect those mechanisms of repair, making them less effective (reduced or slower) and contributing to an increase in DNA damage (Collins et al. 1995).
For the mixture analysis of DNA damage, to obtain a dose-response curve with decrease on results when concentrations were increased, the percentage (%) of DNA damage was converted into % of no damage. CBZ and TCS did not induce a full dose-response relationship; for this reason, the EC50 values were overestimated, and further analysis in the MIXTOX tool was undertaken with fixed EC50 values. This alternative was already implemented with success in the study of Loureiro et al. (2010) and Pérez et al. (2013). For the F12 generation, the IA model fitted the data significantly for Dph_Clean population. When adding the additional parameters for deviations, the DR deviation fitted the data significantly, with a negative a value and a positive b value (Table 4 and Fig. 6a1). This indicates that synergism (a < 0) was mainly caused by TCS, except for mixture ratios where antagonism is observed and caused mainly by CBZ (b > 0) (Table 1). For the F12 generation for Dph_CBZ population, data was significantly adjusted to the IA model. Extending the model with parameters a and bDR, a significant decrease in the SS value was observed, showing the best fit to the data (Table 4). Similar to the pattern in the F12 Dph_Clean, the parameter a was negative and parameter b was positive, meaning that synergism was mainly caused by TCS (Table 4 and Fig. 6b1). It should be noted that, in the aquatic environment, these two compounds might appear together and that synergism (predicted in the DR deviation) was observed in both Dph_Clean and Dph_CBZ populations. It is important to realize that synergism, meaning that a combination in the mixture cause a greater (severe) effect, is considered the worst case scenario possible in terms of mixture patterns in the environment (Cedergreen 2014).
Concentration-response relationship for the binary mixture of triclosan on the % of no DNA damage on Daphnia magna cells (2D isobolic surfaces): F12 Dph_Clean a observed data, a1 modeled data showing a Dose Ratio (DR) deviation; F12 Dph_CBZ b observed data, b1 modeled data showing a dose ratio (DR) deviation
Considering the above mentioned, patterns derived from the CA model for the binary mixture with TCS and CBZ for DNA damage are presented in the Supplementary data as well (Table 1 SD and Fig. 3 SD).
Comparing with other study, similar mixture patterns for the combination of TCS and CBZ were found previously for D. magna: DL dependency with antagonism observed at low doses of the chemical mixture for the immobilization data and DR dependency with synergism mainly driven by TCS for the DNA damage (Silva et al. 2015).
As previously demonstrated in other works, the use of different endpoints is of utmost importance when studying the effects of mixtures, since different patterns of toxicity may be found (Schell et al. 2018). This was found in the present study, where antagonism was mainly found for the acute data (immobilization) and mostly synergism was found for the sublethal data (DNA damage). DNA damage might have pernicious consequences in fitness, adaptability, and survival of the organisms, consequently affecting the ecosystem quality (Jha 2008). Additionally, although the high number of generations used in the present study could complicate the results analysis, previous works showed that even using only two generations and the same compound tested in different laboratories across the world, the results in terms of multigenerational effects were not linear (Barata et al. 2016). In any case, either short or long multigenerational experiments combined with mixture effects (even with only two compounds) are worth to perform since they can provide additional information for environmental risk assessment procedures.
Conclusions
The present study demonstrates that the long-term exposure to CBZ, lasting for 12 generations, induced no changes on daphnids’ sensitivity to TCS in terms of acute toxicity (similar 48-h-LC50 values for immobilization). Notwithstanding, daphnids exposed to CBZ throughout 12 generations and then exposed to CBZ, TCS, and the binary combination of these compounds presented generally a higher percentage of DNA damage when compared with those reared always in clean medium. Considering the mixture patterns after the exposure to CBZ and TCS, patterns were generally similar in daphnids in clean medium when compared with daphnids exposed to CBZ for the immobilization and DNA damage data. Data from the present study, mostly DNA damage data, which might be an early sign of effects, can add value towards hazard and risk assessment of CBZ, TCS, and also of their mixtures in the environment. The multigenerational effects triggered by chemicals can bridge information from individuals to populations and highlight the need to develop a standardized protocol for multigeneration experiments with daphnids.
References
Ashauer R, O'Connor I, Escher BI (2017) Toxic mixtures in time-the sequence makes the poison. Environ Sci Technol 51:3084–3092
ASTM (1980) Standard practice for conducting acute toxicity tests with fishes, macroinvertebrates and amphibians. Report E-729-80. American Standards for Testing and Materials, Philadelphia
Atienzar FA, Jha AN (2004) The random amplified polymorphic DNA (RAPD) assay to determine DNA alterations, repair and transgenerational effects in B(a)P exposed Daphnia magna. Mutat Res Fundam Mol Mech Mutagen 552:125–140
Barata C, Campos B, Rivetti C, LeBlanc GA, Eytcheson S, McKnight S, Tobor-Kaplon M, de Vries Buitenweg S, Choi S, Choi J, Sarapultseva EI, Coutellec MA, Coke M, Pandard P, Chaumot A, Queau H, Delorme N, Geffard O, Martinez-Jeronimo F, Watanabe H, Tatarazako N, Lopes I, Pestana JL, Soares AM, Pereira CM, De Schamphelaere K (2016) Validation of a two-generational reproduction test in Daphnia magna: an interlaboratory exercise. Sci Total Environ
Bester K (2003) Triclosan in a sewage treatment process—balances and monitoring data. Water Res 37:3891–3896
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009) Cytotoxic and genotoxic effects of in vitro exposure to triclosan and trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp Biochem Physiol C Toxicol Pharmacol 150:50–56
Bliss C (1939) The toxicity of poisons applied jointly. Ann Appl Biol 26:585–615
Brausch JM, Rand GM (2011) A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82:1518–1532
Brausch JM, Salice CJ (2011) Effects of an environmentally realistic pesticide mixture on Daphnia magna exposed for two generations. Arch Environ Contam Toxicol 61:272–279
Brausch JM, Smith PN (2009) Development of resistance to cyfluthrin and naphthalene among Daphnia magna. Ecotoxicology 18:600–609
Brennan SJ, Brougham CA, Roche JJ, Fogarty AM (2006) Multi-generational effects of four selected environmental oestrogens on Daphnia magna. Chemosphere 64:49–55
Canton J (1976) The toxicity of benomyl, thiophanate-methyl, and BCM to four freshwater organisms. B Environ Contam Toxicol 16:214–218
Cedergreen N (2014) Quantifying synergy: a systematic review of mixture toxicity studies within environmental toxicology. PLoS One 9:e96580
Cedergreen N, Kudsk P, Mathiassen SK, Sorensen H, Streibig JC (2007) Reproducibility of binary-mixture toxicity studies. Environ Toxicol Chem 26:149–156
Cedergreen N, Christensen AM, Kamper A, Kudsk P, Mathiassen SK, Streibig JC (2008) A review of independent action compared to concentration addition as reference models for mixtures of compounds with different molecular target sites. Environ Toxicol Chem 27:1621–1632
Chatupote W, Panapitukkul N (2005) Regional assessment of nutrient and pesticide leaching in the vegetable production area of Rattaphum Catchment, Thailand. Water Air Soil Pollut Focus 5:165–173
Ciniglia C, Cascone C, Giudice RL, Pinto G, Pollio A (2005) Application of methods for assessing the geno- and cytotoxicity of Triclosan to C. ehrenbergii. J Hazard Mater 122:227–232
Collins AR, Ai-guo M, Duthie SJ (1995) The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat Res DNA Repair 336:69–77
Collins AR, Dobson VL, Dusinska M, Kennedy GRS (1997) The comet assay: what can it really tell us? Mutat Res 375:183–193
Crow JF (1954) Analysis of a DDT-resistant strain of Drosophila. J Econ Entomol 47:393–398
Duthie SJ, Collins AR (1997) The influence of cell growth, detoxifying enzymes and DNA repair on hydrogen per oxide-mediated DNA damage (measured using the comet assay) in human cells. Free Radic Biol Med 22:717–724
EFSA (European Food Safety Authority) (2010) Conclusion on the peer review of the pesticide risk assessment of the active substance carbendazim. EFSA J 8(5):1598
EFSA (European Food Safety Authority) (2015) Harmonisation of human and ecological risk assessment of combined exposure to multiple chemicals. EFSA supporting publication 2015: EN-784. 39pp
EU Pesticide Database (2016) EU pesticide database, European Comission. http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.detail&language=EN&selectedID=1080. Accessed Sept 2017
Georghiou GP (1972) The evolution of resistance to pesticides. Annu Rev Ecol Syst 3:133–168
Harris KDM, Bartlett NJ, Lloyd VK (2012) Daphnia as an emerging epigenetic model organism. Genet Res Int 2012:1–8
Hebert PDN, Ward RD (1972) Inheritance during parthenogenesis in Daphnia magna. Genetics 71:639–642
Hullett CR, Levine TR (2003) The overestimation of effect sizes from F values in meta-analysis: the cause of a solution. Commun Monogr 70:52–67
JanakiDevi V, Nagarani N, YokeshBabu M, Kumaraguru AK, Ramakritinan CM (2013) A study of proteotoxicity and genotoxicity induced by the pesticide and fungicide on marine invertebrate (Donax faba). Chemosphere 90:1158–1166
Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221
Jonker MJ, Svendsen C, Bedaux JJM, Bongers M, Kammenga JE (2005) Significance testing of synergistic/antagonistic, dose level-dependent, or dose ratio-dependent effects in mixture dose-response analysis. Environ Toxicol Chem 24:2701–2713
Kovacevic V, Simpson AJ, Simpson MJ (2016) H NMR-based metabolomics of Daphnia magna responses after sub-lethal exposure to triclosan, carbamazepine and ibuprofen. Comparative biochemistry and physiology. Part D. Genomics Proteomics 19:199–210
Kuster M, Lopez de Alda MJ, Barata C, Raldua D, Barcelo D (2008) Analysis of 17 polar to semi-polar pesticides in the Ebro river delta during the main growing season of rice by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry. Talanta 75:390–401
Loewe S, Muischnek H (1926) Combinated effects I announcement—implements to the problem. Naunyn-Schmiedebergs Arch Exp Pathol Pharmakol 114:313–326
Loureiro S, Svendsen C, Ferreira ALG, Pinheiro C, Ribeiro F, Soares AMVM (2010) Toxicity of three binary mixtures to Daphnia magna: comparing chemical modes of action and deviations from conceptual models. Environ Toxicol Chem 29:1716–1726
Massarin S, Alonzo F, Garcia-Sanchez L, Gilbin R, Garnier-Laplace J, Poggiale J-C (2010) Effects of chronic uranium exposure on life history and physiology of Daphnia magna over three successive generations. Aquat Toxicol 99:309–319
McCahon CP, Pascoe D (1990) Episodic pollution: causes, toxicological effects and ecological significance. Funct Ecol 4:375–383
Neng NR, Nogueira JM (2012) Development of a bar adsorptive micro-extraction-large-volume injection-gas chromatography-mass spectrometric method for pharmaceuticals and personal care products in environmental water matrices. Anal Bioanal Chem 402:1355–1364
NICNAS (2009) National Industrial Chemicals Notification and Assessment Scheme (NICNAS). Australian Government, Department of Health and Ageing. Priority Existing Chemical Assessment Report No. 30, Triclosan. Sydney, Australia
Nogueira PR, Lourenço J, Mendo S, Rotchell JM (2006) Mutation analysis of ras gene in the liver of European eel (Anguilla anguilla L.) exposed to benzo[a]pyrene. Mar Pollut Bull 52:1611–1616
OECD (2000) OECD series on testing and assessment, number 23. Guidance document on aquatic toxicity testing of difficult substances and mixtures
OECD (2004) OECD guidelines for testing of chemicals. Guideline 202: Daphnia sp., acute immobilisation test, adopted April 2004
Oppenoorth FJ (1985) Biochemistry and genetics of insecticide resistance. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. Permagon Press, Oxford, pp 731–773
Palma G, Sánchez A, Olave Y, Encina F, Palma R, Barra R (2004) Pesticide levels in surface waters in an agricultural–forestry basin in Southern Chile. Chemosphere 57:763–770
Paumen ML, Steenbergen E, Kraak MH, Van Straalen NM, Van Gestel CA (2008) Multigeneration exposure of the springtail Folsomia candida to phenanthrone: from dose-response relationships to threshold concentrations. Environ Sci Technol 42:6985–6990
Peng Y, Luo Y, Nie X-P, Liao W, Yang Y-F, Ying G-G (2013) Toxic effects of triclosan on the detoxification system and breeding of Daphnia magna. Ecotoxicology 22:1384–1394
Perez J, Domingues I, Monteiro M, Soares AM, Loureiro S (2013) Synergistic effects caused by atrazine and terbuthylazine on chlorpyrifos toxicity to early-life stages of the zebrafish Danio rerio. Environ Sci Pollut Res Int 20:4671–4680
Plaire D, Bourdineaud J-P, Alonzo A, Camilleri V, Garcia-Sanchez L, Adam-Guillermin C, Alonzo F (2013) Transmission of DNA damage and increasing reprotoxic effects over two generations of Daphnia magna exposed to uranium. Comp Biochem Physiol Part C Toxicol Pharmacol 158:231–243
Ribeiro F, Ferreira NCG, Ferreira A, Soares AMVM, Loureiro S (2011) Is ultraviolet radiation a synergistic stressor in combined exposures? The case study of Daphnia magna exposure to UV and carbendazim. Aquat Toxicol 102:114–122
Schell T, Goedkoop W, Zubrod JP, Feckler A, Luderwald S, Schulz R, Bundschuh M (2018) Assessing the effects of field-relevant pesticide mixtures for their compliance with the concentration addition model—an experimental approach with Daphnia magna. Sci Total Environ 644:342–349
Silva ARR, Cardoso DN, Cruz A, Lourenco J, Mendo S, Soares AMVM, Loureiro S (2015) Ecotoxicity and genotoxicity of a binary combination of triclosan and carbendazim to Daphnia magna. Ecotoxicol Environ Saf 115:279–290
Silva ARR, Cardoso DN, Cruz A, Pestana JL, Mendo S, Soares AMVM, Loureiro S (2017) Multigenerational effects of carbendazim in Daphnia magna. Environ Toxicol Chem:1–12
Silva ARR, Santos C, Ferreira NGC, Morgado R, Cardoso DN, Cruz A, Mendo S, Soares A, Loureiro S (2018) Multigenerational effects of carbendazim in Daphnia magna: from a subcellular to a population level. Environ Toxicol Chem
Simon JC, Delmonte F, Rispe C, Crease T (2003) Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biol J Linn Soc 79:151–163
Singh NP, McCoy MT, Tice RT, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Singh P, Srivastava AK, Singh AK (2008) Cell cycle stage specific application of cypermethrin and carbendazim to assess the genotoxicity in somatic cells of Hordeum vulgare L. Bull Environ Contam Toxicol 81:258–261
Solomon KR, Baker DB, Richards RP, Dixon KR, Klaine SJ, La Point TW, Kendall RJ, Weisskopf CP, Giddings FM, Giesy JP, Hall LWJ, Williams WM (1996) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15:31–76
Sprague JB, Fogels A (1976) Watch the Y in bioassay. In: Proceedings of the 3rd Aquatic Toxicity Workshop, Halifax, Nova Scotia Nov. 2–3, Environment Canada, Tech. Report No. EPS-5AR-77-1, pp 107–18
Sukumaran S, Grant A (2013) Multigenerational demographic responses of sexual and asexual Artemia to chronic genotoxicity by a reference mutagen. Aquat Toxicol 144-145:66–74
Systat Software Inc. (2008) SigmaPlot for Windows (version 11.0). San Jose, California, USA
Systat Software Inc. (2014) SigmaPlot for Windows (version 12.5). San Jose, California, USA
Villarroel MJ, Ferrando MD, Sancho E, Andreu E (1999) Daphnia magna feeding behavior after exposure to tetradifon and recovery from intoxication. Ecotoxicol Environ Saf 44:40–46
Ward TJ, Robinson WE (2005) Evolution of cadmium resistance in Daphnia magna. Environ Toxicol Chem 24:2341–2349
WHO (1993) Environmental health criteria 149. International Programme on Chemical Safety, Geneva Available from: http://www.inchem.org/documents/hsg/hsg/hsg82_e.htm#SectionNumber:1.4. Accessed Sept 2017
Widianarko B, Van Straalen NM (1996) Toxicokinetics-based survival analysis in bioassays using nonpersistent chemicals. Environ Toxicol Chem 15:402–406
Funding
This work was supported by the project RePulse-Responses of Daphnia magna exposed to chemical pulses and mixtures throughout generations (FCOMP-01-0124-FEDER-019321; Refª. FCT PTDC/AAC-AMB/117178/2010), by funding FEDER through COMPETE- Programa Operacional Factores de Competitividade, and by the Portuguese Science Foundation (FCT) through CESAM: UID/AMB/50017/2013. Ana Rita R. Silva was funded by the project “MARPRO—Conservation of marine protected species in Mainland Portugal” through the doctoral fellowship (BD/UI88/5534/2011); Diogo Cardoso was funded by the doctoral grant (PD/BD/52569/2014), and Andreia Cruz was funded by an individual post-doctoral grant (BPD/UI88/2886/2013), within the project “Sustainable Use of Marine Resources”—MARES (CENTRO-07-ST24-FEDER-002033), financed by QREN, Mais Centro-Programa Operacional Regional do Centro e União Europeia/Fundo Europeu de Desenvolvimento Regional. Thanks are also due for the financial support to CESAM (UID/AMB/50017/2019), to FCT/MCTES through national funds.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Cinta Porte
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, A.R.R., Cardoso, D.N., Cruz, A. et al. Long-term exposure of Daphnia magna to carbendazim: how it affects toxicity to another chemical or mixture. Environ Sci Pollut Res 26, 16289–16302 (2019). https://doi.org/10.1007/s11356-019-05040-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05040-1