Abstract
Daphnia magna were exposed under semistatic conditions (i.e., conditions taking natural degradation into account) to a pesticide mixture consisting of a pyrethroid insecticide (cyfluthrin) and a pre-emergent herbicide (diuron) as well as pesticides individually using a full life cycle exposure (21 days). Subsequently, offspring from the second reproductive brood were used to continue exposure for a second generation. Survival, time to first brood, total number of offspring produced, number of broods produced, growth rate, and population growth rate were recorded for each generation and concentration. Significant differences existed between F0 and F1 D. magna for survival, in which F1 were less sensitive to pesticide mixtures than F0. In addition, F1 D. magna were significantly smaller than F0, which resulted in longer time to first brood. There were no differences in any end point examined between D. magna exposed to the pesticide mixture and diuron alone, although differences existed in survival, total number of offspring, total number of broods, and population growth rate when F0 D. magna were exposed to cyfluthrin alone. This study illustrates the utility of a two-generation study design that may more fully reflect, and more accurately predict, population level effects of pesticide exposures to short-lived aquatic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Acute and chronic toxicity testing using Daphnia magna has been used extensively to study pesticide effects in nontarget aquatic organisms (Day and Kaushik 1987). Although these studies show important information on lethal and sublethal effects, they frequently fail to replicate the environmental exposure that may occur over multiple generations, particularly for organisms with short generation times or persistent compounds. Traditionally, acute and chronic toxicity tests are initiated with D. magna neonates derived from unexposed mothers, thus ignoring potential transfer of contaminants from mothers to offspring during oogenesis and embryogenesis (Sanchez et al. 1999) or other maternal effects. Therefore, these previous studies may not reflect actual environmental exposure. Extending chronic toxicity testing for two generations can yield additional insight into more ecologically relevant scenarios for pesticide exposure and effects (Van Leeuwen et al. 1985; Sanchez et al. 1999; Villarroel et al. 2000; Brausch and Smith 2009; Salice et al. 2009).
Daphnia magna have been used extensively for identification of pesticide effects on nontarget aquatic invertebrates due to their high sensitivity and fast growth and reproductive rates (Organisation for Economic Co-operation and Development 1984; Munzinger and Monicelli 1992). Rapid generation times can subject multiple generations of D. magna to contaminants, even xenobiotics, which are not considered environmentally persistent. Moreover, these characteristics of D. magna allow for calculation of lethal (i.e., LC50s) and sublethal end points, such as reproduction, growth rate, and population growth rate (λ), during a short period of time. Population growth rate has been widely recommended as a useful ecologic end point because it incorporates sublethal end points, such as fecundity, as well as lethal end points that can illustrate population-level effects (Stark et al. 1997; Forbes and Calow 1999).
The objective of the present study was to evaluate the effects of an environmentally relevant pesticide mixture on D. magna using a two-generational approach. Under the Federal Insecticide, Fungicide, and Rodenticide Act, effects of pesticides are typically investigated by single-chemical toxicity testing (Lichtenstein et al. 1973); however, aquatic organisms are likely subject to multiple pesticide stressors simultaneously due the use of multiple pesticides in a season and subsequent runoff and/or spray drift (Thurman et al. 2000). These chemicals can have complex effects on growth, reproduction, and survival, and the effects of multiple pesticides can be antagonistic, additive, or synergistic (Lichtenstein et al. 1973; Cairns 1983; Hoegland et al. 1993). For this study, D. magna were exposed to a postemergent herbicide (diuron), a pyrethroid insecticide (cyfluthrin), or a mixture of both compounds for two generations. We hypothesized that pesticide mixtures would be more toxic to D. magna than individual pesticides, similar to what has been observed in other studies (Lichtenstein et al. 1973; Kungolos et al. 1999; Munn et al. 2006). In addition, we hypothesized D. magna from mothers exposed to pesticide mixtures would result in increased sensitivity and decreased fitness of neonates (F1 generation). Individual parameters, such as survival, growth, brood time, and fecundity, were monitored and used to calculate λ. By extending the typical 21-day life cycle assessment to a second generation, important information regarding pesticide effects on D. magna populations could be determined.
Diuron is the active ingredient in Karmex DF (Dupont) and is used in production of numerous agricultural crops (Brausch et al. 2006). Diuron has been detected in surface water at relatively high concentrations throughout the United States as a result of agricultural runoff and spray drift (Thurman et al. 1998, 2000). Cyfluthrin, the active ingredient in Tempo SC Ultra, is a pyrethroid insecticide registered for use on cotton and many food crops (United States Environmental Protection Agency [USEPA] 1997). We chose to use diuron and cyfluthrin in this study because they are commonly applied at similar times in the production of many crops (Coupe et al. 1998). In addition, Lichtenstein et al. (1973) demonstrated that herbicides have the propensity to synergize toxic effects of insecticides when administered simultaneously to Drosophila melanogaster, Musca domestica, and Aedes aegypti; therefore, the common practice of coapplying insecticides and herbicides could have greater-than-expected effects. Based on Lichtensetin et al.’s (1973) results, an additional objective of this research program was to determine whether diuron could synergize the toxic effects of cyfluthrin when applied as a pesticide mixture.
Materials and Methods
Chemicals
Cyfluthrin, a pyrethroid insecticide, and diuron, a pre-emergent herbicide, were selected for this study based on use, toxicity, and potential to enter aquatic ecosystems (Brausch et al. 2006; Thurman et al. 1998, 2000). These pesticides were purchased from a pesticide supplier as their formulated product, Tempo SC Ultra (11.8% cyfluthrin) and Karmex DF (80% diuron). The concentrations of diuron used in this study are environmentally relevant because concentrations of diuron in agricultural runoff have been observed at concentrations >1 mg/l (Rupp et al. 2006). Concentrations for cyfluthrin were chosen to be equivalent percentages of the established LC50 as diuron.
Daphnia magna
Daphnia magna used in this study were derived from a single individual in March 2009 according to the USEPA guideline EPA-821-R-02-012 (USEPA 2002). D. magna were maintained in moderately hard synthetic freshwater (hardness 89 mg/L as CaCO3; pH 8.1; alkalinity 60 as CaCo3; temperature 24 ± 0.1°C) under a 16:8-hour light-to-dark cycle. Complete water changes occurred three times per week. D. magna were fed daily with 0.1 mL Selenastrum capricornutum (3.0 × 107 cells/ml) and every other day with a 0.1 mL YCT (yeast, cerophyll, and trout chow) mixture as defined by the USEPA (2002). These conditions were maintained for the study duration.
Multigeneration Toxicity Tests
Twenty-one–day chronic toxicity life cycle tests were performed according to USEPA guidelines (1986) using second brood (<24 h old) D. magna. Toxicity tests were conducted in 30-mL glass jars with one D. magna/jar. Jars were randomly assigned to 1 of 256 positions in an incubator (Altair Plant Growth Chamber; Altair Refrigeration, Stafford, TX). D. magna were exposed to diuron or cyfluthrin (8 replicates/treatment, 4 different treatments) or to a mixture of both pesticides (16 replicates/treatment, 5 treatments and 1 control). Each concentration was prepared using a serial dilution and were normalized based on reported LC50 concentrations (USEPA ECOTOX database). The concentrations chosen for both mixtures and individual compound toxicity testing were based on a preliminary study and corresponded to 11.8, 7.1, 4.2, 2.6, and 1.5% of the established 48-hour LC50 value for the active ingredient. These percentages corresponded to nominal concentrations of 1.0, 0.6, 0.36, 0.216, and 0.1296 mg/l diuron and 0.02, 0.012, 0.0072, 0.0043 and 0.0026 μg/l cyfluthrin, respectively. The 4.2% concentration data are not be presented because numerous D. magna died. A preliminary experiment was conducted with concentrations of 10, 5, 2.5, 1.25, and 0.6125 mg/l diuron; 0.2, 0.1, 0.05, 0.025, and 0.0125 μg/l cyfluthrin; or a mixture of both compounds. The preliminary study was conducted to determine concentrations resulting in sublethal effects for the pesticide mixture. For the definitive study, sufficient amounts of each concentration were prepared at experiment initiation to last the entire study duration for use in water changes. D. magna were exposed to freshly made mixture or to individual pesticide concentration at experiment initiation, and the compounds were then allowed to degrade naturally under conditions identical (temperature and light) to those of the study. For complete water changes, water was replaced from the reserves that were made initially to replicate a single field application with natural degradation. This design was intended to simulate an application or runoff event in which pesticides enter the system and degrade somewhat before a second exposure event. D. magna were monitored for survival, growth, total number of offspring, time to first brood, and total number of broods. To continue the experiment for a second generation, offspring from the second brood were removed and placed in clean exposure chambers. One randomly selected neonate from each parent was then exposed to a freshly prepared concentration for 21 days corresponding to the concentration of its parent. Growth was measured from digital images that were captured using a digital camera attached to a LEICA EC3 microscope. Length was measured from eyespot to posterior end of the carapace using Image J software (http://rsbweb.nih.gov/ij/). Instantaneous growth rate (IGR) was calculated using the following equation: IGR = ln(final length) − ln(initial length)/d, where initial length is the mean length of 20 randomly selected neonates at the beginning of the exposure, final length is the mean length of D. magna for each replicate, and d is the number of exposure days (Lampert and Trubetskova 1996). Population growth rate (λ) was estimated for each individual using an age-based matrix model, and data on survival and reproduction was incorporated. The time step was a single day, and survival per day was entered on the subdiagonal, whereas fecundity was included on the first row. The model was a 21 × 21 matrix representing each day of the 21-day experimental generation. Population growth rate was obtained as the dominant eigenvalue of the resulting matrix model (Caswell 2001).
Statistical Analysis
Data analysis was performed using the statistical software R (version 2.5.1; R Development Core Team, Boston, MA, USA). Data were analyzed using analysis of variance; where appropriate, post-hoc Dunnett’s test was used. Controls for the F0 and F1 generations were compared using Student t test. Significance was set at p < 0.05.
Results
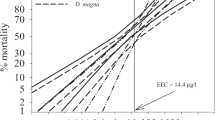
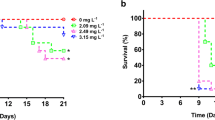
F1 D. magna appeared to be less sensitive than F0 D. magna for all mixture concentrations examined (except for the 7.1%) based on comparison of survival (Tables 1, 2; Fig. 1a), although there was a slight increase in control survival as well. Although differences in survival and other end points occurred between F0 and F1 control D. magna, none of these differences were observed to be significant. Survival for cyfluthrin-exposed D. magna was greater than those exposed to the pesticide mixture for the F0 generation; however, survival was not different among pesticide mixture and diuron alone.
Time to first reproductive brood was significantly increased in response to both generation and concentration (Tables 1, 2; 1c). Increasing mixture concentrations resulted in increased time to first reproductive event for all concentrations studied for the F0 generation, and this trend was also observed for the F1 generation. F1 D. magna generally took longer to reproduce than F0 D. magna when exposed to all pesticide mixture concentrations, but this difference was not significant. When comparing individual pesticides with the pesticide mixture, there were no statistical differences in time to first brood for F0 D. magna.
All pesticide exposure concentrations significantly decreased total number of offspring produced per individual for F0 D. magna (Table 1), although for F1 D. magna only the highest mixture concentration decreased the total number of offspring (Table 2; Fig. 1d). No statistical difference between number of offspring produced between F0 and F1 D. magna existed, although F0 D. magna produced slightly more offspring overall. Offspring produced per individual was not significantly different between exposed D. magna for the mixture or diuron but was significantly decreased compared with cyfluthrin only–exposed D. magna (Table 3).
Number of broods produced per individual D. magna was not affected by mixture concentration (Tables 1, 2). F0 control D. magna had more reproductive events than only the 1.5% exposed D. magna, but F1 D. magna did not have differences in number of reproductive events regardless of exposure to the pesticide mixture. F0 D. magna had more broods than F1 D. magna for two mixture concentrations (2.6 and 7.1%), whereas F1 D. magna had more broods for the 1.5% concentration, although the differences were not significant (Fig. 1e). No statistical difference in total number of broods existed between mixture- and diuron-exposed D. magna; however, cyfluthrin-exposed D. magna had statistically more broods than both (Table 3).
No significant differences in λ between concentrations existed for the pesticide mixture,, although λ decreased with increasing concentrations. A similar trend was also observed for F1 D. magna. No statistical differences existed between generations, although λ approached significance between F0 and F1 generations (Fig. 1f). Population growth rate was not significantly different between diuron and the pesticide mixture but was significantly different than cyfluthrin alone (Table 3).
IGR decreased significantly with increasing pesticide concentration for F0 D. magna but not for F1 D. magna offspring exposed to the pesticide mixture. F0 D. magna had higher IGR than corresponding F1 D. magna for all concentrations, except for the 7.1% concentration (Fig. 1g). In addition, F0 D. magna were significantly larger than F1 D. magna. Diuron- and pesticide mixture–exposed D. magna were significantly smaller than cyfluthrin-exposed D. magna (Table 3). In addition, a positive relation between size of F0 D. magna and number and size of offspring for all pesticide concentrations for mixtures and individual chemicals was observed.
Discussion
Results of our multigenerational exposure with a naturally degrading exposure regimen indicated differences in sensitivity of D. magna between generations. D. magna exposed to the pesticide mixture while still in the brood pouch (F1) were less sensitive in terms of mortality than non–previously exposed (F0) D. magna. Decreased pesticide sensitivity in F1 D. magna was possibly due to previous exposure and hence the induction of detoxifying mechanisms. Tolerance is a decreased response to a toxicant due to previous exposure and causes previously exposed individuals to survive xenobiotic concentrations that would otherwise cause mortality (Eaton and Klaassen 2001). Development of tolerance in D. magna has previously been demonstrated in response to cadmium (Ward and Robinson 2005) and diazanon exposure (Sanchez et al. 1999), although other studies (Villarroel et al. 2000) have demonstrated that D. magna exposed while still in the brood pouch were significantly more sensitive than non–previously exposed neonates (Fig. 1).
Numerous studies have examined pesticide effects on multiple generations of D. magna (Sanchez et al. 1999; Villarroel et al. 2000); however, these studies have had conflicting results. Sanchez et al. (1999) found that F0 D. magna were strongly affected by diazinon, whereas offspring demonstrated normal survival with only minimal effects on growth and fecundity. Using a slightly different study design, Villarroel et al. (2000) observed F1 offspring that were significantly more sensitive to tetradifon than F0 D. magna. Results of our study coincide with results observed by Sanchez et al. (1999) in that F1 offspring were significantly less sensitive than F0 D. magna, parsimoniously due to exposure of neonates in the brood pouch and the development of tolerance; however, we observed decreases in reproductive parameters in the F1 generation. Interestingly, in our range-finding study, F1 D. magna exposed to higher concentrations of the pesticide mixture were significantly more sensitive than mixture-exposed F0 D. magna, contrary to what was observed in the definitive study. F0 D. magna exposed to pesticide mixture survived only at the lowest concentration; however, offspring of the surviving D. magna only survived an average of 9 days, and no reproduction occurred. Although replication was low (only 8 D. magna/concentration), these results suggest that there is an upper limit of exposure because neonates that can convey tolerance and maintain offspring viability rather than too high of exposure that can compromise the biologic integrity of offspring. One explanation proposed by Villarroel et al. (2000) was maternal transfer, in which offspring accumulate sublethal concentrations of pesticide, and additional exposures overwhelm detoxifying mechanisms, thus leading to mortality. Tsui and Wang (2004) demonstrated the capacity of D. magna adults to offload contaminants to both neonates and developing eggs in the brood pouch. These investigators demonstrate that depuration into offspring was a significant elimination method of methylmercury in D. magna. Previous studies indicate that diuron has the potential for accumulation and transfer in D. magna (Isensee 1976); thus, maternal offloading is likely at high levels of diuron exposure. This could explain the increased sensitivity in offspring in our preliminary study due to high levels of offloading, but our definitive study suggests that at lower pesticide concentrations tolerance can develop and not negatively affect the biologic integrity of offspring. Additional research must be conducted to determine the extent of pesticide offloading that is beneficial for adults without compromising offspring fitness.
Our results did not corroborate the results of Lichtenstein et al. (1973) in which herbicides synergized insecticide toxicity because diuron did not alter cyfluthrin toxicity; in fact, diuron was likely responsible for the overall toxicity of the mixture (Table 3). Toxicity of the pesticide mixture to D. magna differed compared with cyfluthrin alone, but did not differ from that of diuron (Table 3). This suggests that diuron was largely responsible for the toxicity of the pesticide mixture, possibly due to diuron’s slow degradation rates compared with cyfluthrin. This belief is supported by time-to-death data (not shown) indicating that the majority of mortality occurred at 4 days in the mixture study; however, studies of individual pesticides demonstrate that cyfluthrin-sensitive D. magna died from pesticide effects within the first 48 h, whereas mortality in diuron-susceptible D. magna was delayed (96 h [unpublished data]). Furthermore, cyfluthrin degrades much more rapidly than diuron and based on its half-life of approximately 1 day (USEPA 1992) would be expected to be at low concentrations by the time mortality was occurring. Contrary to cyfluthrin, diuron is relatively stable in surface water (Howard 1991), and thus higher concentrations would have remained throughout. Furthermore, diuron’s major metabolite 3,4-dichloroaniline (DCA) has also been observed to be toxic to aquatic invertebrates (Kersting 1975).
Based on study design, which allowed for natural degradation of diuron, it is likely that D. magna would have been exposed to DCA. Concentrations of DCA would have been increasing throughout the study based on the half-life and degradation rates of diuron in water (DPR Pesticide Chemistry Database 2003) but would have remained low throughout due to its short half-life. Low-level DCA exposure has been implicated in increased developmental time and decrease in offspring production (Baird et al. 1990). These effects would not have been observed during a short-term toxicity test with diuron due to its relatively long half-life. This further illustrates the need for long-term life cycle testing for compounds with toxic metabolites such as diuron.
Numerous studies have identified decreased reproduction as a result of contaminant exposure. Villarroel et al. (2000) observed decreased offspring production in F1 D. magna as a result of multigenerational tetradifon exposure. Bervoets et al. (1993) and Baldwin et al. (1995) both observed decreased reproduction in second-generation offspring exposed to wastewater and diethylstilbestrol, respectively. Similar to these studies, we identified decreased reproduction in D. magna in response to pesticide mixture; furthermore, we observed decreases, although not statistically significant, in D. magna reproduction in the F1 generation that appear to manifest as a decrease in overall λ. This could have deleterious effects on D. magna populations exposed to persistent or continuously applied chemicals because it could decrease reproductive rates to a point where population growth stalls, leading to local extinctions or to possible indirect effects on other taxa.
Population growth rate (λ) and intrinsic rate of natural increase (r) are important end points to examine for long-term studies because they provide the most environmentally relevant measures of toxic effects. Because they incorporate both lethal and sublethal effects, realistic assessment of effects from pesticide exposure on populations can be derived. Our study indicates that population level effects could occur based on low-level pesticide mixture exposure as a result of slight decreases in λ and IGR. Furthermore, our study indicates that diuron alone is likely to decrease population growth at environmentally relevant concentrations.
Previous studies have also indicated that exposure to xenobiotics causes decreased growth and overall size in D. magna (Flickinger et al. 1982; Hammers-Wirtz and Ratte 2000). Exposure to numerous compounds, such as carbaryl (Hanazato 1998), tetradifon (Villarroel et al. 2000), and cadmium (Ward and Robinson 2005), have been implicated in decreased growth and overall size in D. magna. Hanazato (1998) observed larger adult D. magna produce larger and a greater number of offspring compared with smaller D. magna. Furthermore, it was identified that size was inversely related to sensitivity, with smaller neonates being more sensitive to carbaryl exposure than larger neonates (Hanazato 1998), and similar results were observed by Enserink et al. (1990) resultant of Cd exposure. We found similar effects in our study in that larger F0 D. magna produced more offspring, but due to the lack of mortality in the F1 generation we did not observe increased sensitivity in smaller neonates.
How the development of tolerance, and thus decreased sensitivity, versus decreased size of offspring, resulting in increased sensitivity, influences pesticide sensitivity of D. magna populations remains unclear. Our studies indicate that tolerance has the ability to overcome decreased size, but this may be influenced by compounds ability for maternal transfer, capability of D. magna to develop tolerance, chemical mode of action, among others.
Currently, fitness of offspring exposed to pesticides is not incorporated or considered in ecologic risk assessment. As a result of this study, in which relevant concentrations of pesticide mixtures decreased fitness in D. magna, it is vital to evaluate fitness of offspring, either through multiple-generation life cycle assessments or through population growth experiments. Other studies have identified similar effects in F1 D. magna, and these investigators have also recommended replacing Daphnia reproduction tests with population growth experiments to produce the most relevant information for ecologic risk assessments (Hammers-Wirtz and Ratte 2000).
Conclusion
Substantial differences existed between time to first brood and instantaneous growth rates for the mixture study, although no differences were observed for survival between generations. However, at slightly higher concentrations used in the range-finding study, significant differences in survival between parental and first-generation offspring existed. Furthermore, this study indicates that D. magna were strongly affected by diuron exposure, more so than cyfluthrin, at the concentrations studied. Based on our results, it is possible that diuron is causing sublethal effects to aquatic invertebrates at the current application rates. This study indicates that the use of two-generation toxicity testing can identify the effects of contaminants that are otherwise unseen using a classical chronic-exposure regime.
References
Baird DJ, Barber I, Calow P (1990) Clonal variation in general responses of Daphnia magna Straus to toxic stress. I. Chronic life-history effects. Funct Ecol 4:399–407
Baldwin WS, Milam DL, Leblanc GA (1995) Physiological and biochemical perturbations in Daphnia magna following exposure to the model environmental estrogen diethylstilbestrol. Environ Toxicol Chem 14:945–952
Bervoets L, Baillieul M, Blust R, DeBoeck G, Verheyen R (1993) Impact assessment of industrial effluents on freshwater ecosystems. Sci Total Environ 134 (Suppl 2):1123–1128
Brausch JM, Smith PN (2009) Development of resistance to cyfluthrin and naphthalene among Daphnia magna. Ecotoxicology 18:600–609
Brausch JM, Cox S, Smith PN (2006) Pesticide usage on the Southern High Plains and acute toxicity of four chemicals to the fairy shrimp Thamnocephalus platyurus. Tex J Sci 58:309–324
Cairns J Jr (1983) Are single species toxicity tests alone adequate for estimating environmental hazard? Hydrobiologia 100:4–57
Caswell H (2001) Matrix population models: construction, analysis, and interpretation, 2nd edn. Sinauer Associates, Sunderland
Coupe RH, Thurman EM, Zimmerman LR (1998) Relation of usage to the occurrence of cotton and rice in three streams of the Mississippi delta. Environ Sci Technol 32:3673–3680
DPR Pesticide Chemistry Database (2003) Environmental Monitoring Branch. Department of Pesticide Registration, Sacramento, CA
Day K, Kaushik NK (1987) An assessment of the chronic toxicity of synthetic pyrethroid, fenvalerate, to Daphnia galeata mendotae, using life tables. Environ Pollut 44:13–26
Eaton DL, Klaassen CD (2001) Principles of toxicology. In: Klaassen CD (ed) Casarett & Doull’s toxicology: the basic science of poisons. McGraw-Hill, New York, pp 11–34
Enserink L, Luttner W, Maas-Diepeveen H (1990) Reproductive strategy of Daphnia magna affects the sensitivity of its progeny in acute toxicity tests. Aquat Toxicol 17:15–26
Flickinger AL, Bruins RJF, Winner RW, Skillings JH (1982) Filtration and photoactive behavior as indices of chronic copper stress in Daphnia magna Straus. Arch Environ Contam Toxicol 11:457–463
Forbes VE, Calow P (1999) Is the per capita rate of increase a good measure of population-level effects in ecotoxicology? Environ Toxicol Chem 18:1544–1556
Hammers-Wirtz M, Ratte HT (2000) Offspring fitness in Daphnia: is the Daphnia reproduction test appropriate for extrapolating effects on the population level? Environ Toxicol Chem 19:1856–1866
Hanazato T (1998) Growth analysis of Daphnia early juvenile stages as an alternative method to test the chronic effects of chemicals. Chemosphere 36:1903–1909
Hoegland KD, Drenner RW, Smith JD, Cross DR (1993) Freshwater community response to mixtures of agricultural pesticides: effects of atrazine and bifenthrin. Environ Toxicol Chem 12:627–637
Howard PH (1991) Handbook of environmental fate and exposure data for organic chemicals. Lewis, Chelsea, pp 9–21
Isensee AR (1976) Variability of aquatic model ecosystem-derived data. Int J Environ Stud 10:35–41
Kersting K (1975) The use of microsystems for the evaluation of the effect of toxicants. Hydrobiol Bull 9:102–108
Kungolos A, Samaras P, Kipopoulou AM, Zoumboulis A, Sakellaropoulos GP (1999) Interactive toxic effects of agrochemicals on aquatic organisms. Wat Sci Tech 40:357–364
Lampert W, Trubetskova I (1996) Juvenile growth rate as a measure of fitness in Daphnia. Funct Ecol 10:631–635
Lichtenstein EP, Liang TT, Anderegg BN (1973) Synergism of insecticides by herbicides. Science 181:847–849
Munn MD, Gilliom RJ, Moran PW, Nowell LH (2006) Pesticide toxicity index for freshwater aquatic organisms, 2nd ed. United States Geological Survey Scientific Investigation Report 2006-5148, Reston, 87 pp
Munzinger A, Monicelli F (1992) Heavy metal co-tolerance in a chromium tolerant strain of Daphnia magna. Aquat Toxicol 23:203–216
Organisation for Economic Co-operation and Development (1984) Guidelines for testing chemicals. Daphnia sp. acute immobilization test and reproduction test. OECD, Paris
Rupp DE, Peachey RE, Warren KL, Selker JS (2006) Diuron in surface runoff and tile drainage from two grass-seed fields. J Environ Qual 35:303–311
Salice CJ, Miller TJ, Roesijadi G (2009) Demographic responses to multigeneration cadmium exposure in two strains of the freshwater gastropod, Biomphalaria glabrata. Arch Environ Contam Toxicol 56:785–795
Sanchez M, Ferrando MD, Sancho E, Andreu E (1999) Assessment of the toxicity of a pesticide with a two-generation reproduction test using Daphnia magna. Comp Biochem Physiol C 124:247–252
Stark JD, Tanigoshi L, Bonfour M, Antonelli A (1997) Reproductive potential: its influence on the susceptibility of a species to pesticides. Ecotoxicol Environ Safe 37:273–279
Thurman EM, Zimmerman L, Scribner E, Coupe R Jr (1998) Occurrence of cotton pesticides in surface water of the Mississippi embayment. USGS Fact Sheet FS-022-098. United States Geological Survey, Washington
Thurman EM, Bastian KC, Mollhagen T (2000) Occurrence of cotton herbicides and insecticides in playa lakes of the High Plains of West Texas. Sci Total Environ 248:189–200
Tsui MTK, Wang W-X (2004) Maternal transfer efficiency and transgenerational toxicity of methylmercury in Daphnia magna. Environ Toxicol Chem 23:1504–1511
United States Environmental Protection Agency (1986) Daphnia magna life-cycle (21-day renewal) chronic toxicity test. USEPA, Washington. EPA 540/9-86-141
United States Environmental Protection Agency (1992) Pesticide environmental fate one line summary: Cyfluthrin. USEPA, Washington
United States Environmental Protection Agency (1997) Pesticide fact sheet number 164: Cyfluthrin. USEPA Office of Pesticide Programs, Washington
United States Environmental Protection Agency (2002) Methods for measuring the acute toxicity of effluent and receiving waters to freshwater and marine organisms, 5th ed. EPA-821-R-02-012. USEPA, Washington
United States Environmental Protection Agency. ECOTOX database system. http://www.epa.gov/med/databases/databases.html#aquire. Accessed 1 April 2009
Van Leeuwen CJ, Luttmer WJ, Griffieon PS (1985) The use of cohorts and populations in chronic toxicity studies with Daphnia magna: a cadmium example. Ecotoxicol Environ Safe 9:26–39
Villarroel MJ, Ferrando MD, Sancho E, Andreu E (2000) Effects of tetradifon on Daphnia magna during chronic exposure and alterations in the toxicity to generations pre-exposed to the pesticide. Aquat Toxicol 49:39–47
Ward TJ, Robinson WE (2005) Evolution of cadmium resistance in Daphnia magna. Environ Toxicol Chem 24:2341–2349
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brausch, J.M., Salice, C.J. Effects of an Environmentally Realistic Pesticide Mixture on Daphnia magna Exposed for Two Generations. Arch Environ Contam Toxicol 61, 272–279 (2011). https://doi.org/10.1007/s00244-010-9617-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9617-z