Abstract
Variation of volatile organic compound (VOC) concentration and composition in an active landfill were monitored by a developed static chamber for 2 years. The landfill gas from 82 sampling points including 70 points on working face, 8 points on geomembrane (GMB), and 4 points on final cover were analyzed for VOCs by GC-MS. Twenty-eight types of VOCs were detected, including terpenes, sulfur compounds, aromatics, hydrocarbon, oxygenated compounds, aldehyde compounds, and halogenated compounds. Terpenes were the dominant VOCs recorded in the spring, autumn, and winter seasons, whereas sulfur compounds dominated in the summer season. Limonene, ethyl alcohol, and acetone were identified as the main VOCs emitted from the waste working face of the landfill. Limonene dominated the terpenes with a maximum concentration of 43.29 μg m−3 in the autumn season. Limonene was also the dominant VOC escaping from the defects of geomembrane temporary cover reaching an average concentration 38 μg m−3. The defects of geomembranes can be a great emission source of VOCs. Emission rate of limonene was 2.24 times higher than that on the working face. VOC concentrations on the final cover can be 166 times less than those obtained on the working face. VOC emitted from the landfill did not represent a health threat for human health. However, concentrations of methyl mercaptan and ethanethiol on the working face were 3.4–22.8 times greater than their odor threshold, which were the main compounds responsible for odor nuisance. Results obtained from CALPUFF model indicated that methyl mercaptan and ethanethiol would not be a nuisance for the residents around the landfill. However, these compounds are harmful to the workers on the landfill.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfill gas generated by wastes decomposition is generally composed of 50–60% methane, 40% carbon dioxide, and less than 1% volatile organic compounds (VOCs) (Kreith 1995). VOCs have become a growing environmental concern due to their possible impact on human health and contribution to odor formation (Fielder et al. 2001; Shin et al. 2002; Dincer et al. 2006; Hamoda 2006; Xie et al. 2015a, 2015b; Avaliani et al. 2016). Guo et al. (2017) detected 71 volatile compounds in a landfill site in China through collecting air samples and GC-MS analysis. The main VOCs released from landfills are oxygenated compounds, hydrocarbons, halogenated compounds, aromatics, sulfur compounds, and terpenes (Keller 1988; Shin et al. 2002; Shafi et al. 2006; Duan et al. 2014; González et al. 2013; Lu et al. 2015). Duan et al. (2014) reported that limonene, butane, toluene, and trichlorofluoromethane were recognized as the most abundant compounds on the waste working surface of a landfill in Beijing, China. The samples were collected with a specially designed sampler (SOC-01; National Key Laboratory of Odor Pollution Control of EPA-China) at a height of approximately 1.5 m above the working surface of the landfill. Ethyl alcohol, α-piene, hydrogen sulfide, dimethyl sulfide, limonene, methyl mercaptan, dimethyl disulfide, and diethyl sulfide were reported as the major odor-causing compounds on the waste working face of a typical flat sanitary landfill in northern China by Lu et al. (2015) in a 2-year-long case study by collecting air samples at a height of 1.5 m above the surface. The samples were analyzed by GC-MS. Furthermore, their results showed that odor pollution was the most serious in the spring and autumn seasons, whereas lower odor concentrations were detected in the summer and winter seasons. Di et al. (2013) found that the odorous gases emitted from municipal solid waste were mainly composed of aromatic gases, which accounted for 49% of the total VOC concentrations by using the human sensing technologies and GC-MS. González et al. (2013) identified 42 VOCs following the European Standard EN 13725 (EC 2003) for the determination of odor concentrations, which included alkanes (19–62 μg m−3), aldehydes (65–98 μg m−3), ketones (78–129 μg m−3), alcohols (67–78 μg m−3), esters (25–33 μg m−3), BTEX (83–106 μg m−3), halogenated compounds (16–39 μg m−3), terpenes (1.4–2.4 μg m−3), and reduced sulfur compounds (2.6–4.2 μg m−3) from a landfill located on the island of Mallorca (Balearic Islands, Spain). Liu et al. (2016) used a wind tunnel system to investigate the VOC emission rates from the working face of a landfill in Beijing, China. They showed that oxygenated compounds appeared to be the major compounds. Saldarriaga et al. (2014) investigated the VOC concentration in the air inside a composting pile and found that monoaromatic hydrocarbons represented a higher risk to health because of their concentrations and persistence. In addition, waste bins are also an important source of VOCs. Controlled field experiments were carried out by Agapiou et al. (2016) to monitor the emissions of three plastic commercial household waste bins. It is found that VOC emissions are strongly dependent on the waste material. Terpenes and di-limonene play a prominent role in the “pre-compost” odor. Statheropoulos et al. (2005) investigated VOC evolution in urban waste disposal bins in different situations. The results indicated that the most prominent VOCs were decane, acetic acid ethyl ester, limonene, nonane, ethanol, benzene, 1,2,4-trimethyl, and undecane. High levels of alkanes, alkylbenzene, and terpenes are responsible for undesirable odors.
Toxicological relevance of VOCs is related to their high volatility and solubility. VOCs enter the organism through inhalation and absorption through the skin and affect primarily the central and peripheral nervous systems (Vallejo and Baena 2007). The effect of VOCs on the human organ is not a simple sum of the single compound but can sometimes include synergy or antagonism of the VOC compounds (Lazarus et al. 2008). VOC exposure can not only cause acute irritation of human organs, headache, and difficulty focusing (Blount et al. 2006) but also can lead to carcinogenicity and damage to the liver, kidneys, and central nervous system in the long term. In particular, VOC emissions from landfill can increase the levels of carcinogenic benzene to be greater than the air quality limits (5 μg m−3 for the annual average concentration) established for human health protection (EEA—European Environment Agency 2008). Besides, some types of VOCs such as cyclic alkenes and sulfur-containing VOC are responsible for the unpleasant smell and odor nuisance (Zou et al. 2003).
Static chamber techniques have been most widely used to measure concentration and emission rate of landfill gas (Gallego et al. 2014; Obersky et al. 2018). Obersky et al. (2018) used the static chamber method to measure the flux of the molecular and isotope species at the landfill cover surface to determine emission rates of CH4 and CO2. Gallego et al. (2014) used a self-designed cylindrical flux chamber to measure VOC surface emissions in a closed industrial landfill in Spain. The static chamber measurement approach is inexpensive, simple, and highly sensitive at detecting even small fluxes (Schroth et al. 2012). The drawback of static flux chambers is that it may be influenced by the increase in pressure due to chamber installation, leading to the underestimation of pollutant’s emission rates (Hudson and Ayoko 2008).
This paper presents the outcome of a field investigation conducted to evaluate VOC migration through the waste working face, the geomembrane temporary cover, and final cover system at a landfill in Hangzhou, China. This landfill received much higher amounts of waste than anticipated (e.g., 1,903,408 tons in 2016) and prompted a major investigation on potentially high amount of VOCs generated due to the high contents of food waste present in the waste stream and growing concern of the communities located near or downstream of the landfill about odor pollution. The Gaussian dispersion model was then used to assess the diffusion distance of the VOCs with high emission rates.
Materials and methods
Landfill description
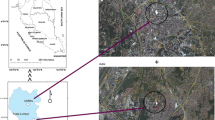
The landfill in Hangzhou is the first standardized valley-type municipal solid waste (MSW) landfill constructed in China. Satellite image of the landfill is shown in Fig. 1. It is located in the city of Hangzhou, east of China (see Fig. 1). The residential areas are 3–5 km away from the site. The landfill site has often been complained of olfactory nuisances. The second stage of the landfill went into service in 2007 after the closure of the previous first landfill. The landfill currently accommodates about 6000 tons of MSW per day. The amount of waste received is increasing every day, reaching a peak of 7200 tons in October 2016. Food waste generally constitutes about 60% of the total weight in the MSW (Zhan et al. 2017). The working face is about 3000 m2. The final cover system consists of a 0.3-m gravel (gas collection layer), 0.4 m compacted clay liner (CCL), 1.5-mm high-density polyethylene (HDPE) geomembrane, geotextile, 0.6 m CCL, and a 0.2-m loamy soil as a vegetative soil layer. The temporary cover of the landfill only consists of a 1.5-mm HDPE geomembrane.
Several measures were taken to control odor and gas emissions from this landfill which included covering the compacted waste with a temporary 1.5-mm HDPE geomembrane liner except for the working face (i.e., the open cell), collecting landfill gas for power generation, and equipping the leachate treatment facilities such as the regulating reservoir and the aeration tank with a gas collection system (Ding et al. 2012).
In Hangzhou, the spring consists of March, April, and May. Summer consists of June, July, and August. Autumn consists of September, October, and November. Winter consists of December, January, and February. The meteorological conditions during sampling are shown in Table 1. Average temperature in Hangzhou in summer is the highest. It can reach about 28 °C. The temperature in winter is the lowest with approximately 7 °C. The total precipitation is 525 mm in winter, which is about 10 times less than that in spring, summer, and autumn. The wind roses for this landfill in four seasons during the year 2015–2016 can be seen in Fig. 2. Wind directions were dominant by east direction in spring and summer. It is indicated from Table 1 that the average wind speeds for the two seasons were 1.68 m/s and 1.78 m/s, respectively. The wind direction in autumn was not fixed. It mainly consisted of northeast wind and southwest wind (see Fig. 2). The average wind speed in autumn was 2.0 m/s (see Table 1). Wind directions in winter were dominant by northeast and northwest direction (see Fig. 2). The average wind speed in winter was 1.9 m/s (see Table 1).
Gas sampling
The static chamber, Tedlar bags, and pump were used to collect landfill gas from selected locations. The volume of the Tedlar bag is 0.5 L. Both the pump (GP-2000) and Tedlar bags were provided by Huibin Instrument Company in Shanghai, China. The chamber used in this study was in-house made, consisting of a glass cylinder and a steel pedestal (see Fig. 3). The chamber is 550 mm high and with a diameter of 400 mm. Once the chamber was placed on the sampling location, the groove in the steel pedestal was filled with water to keep the chamber sealed. All of the valves were then shut off to allow gas to accumulate in the chamber. The landfill gas was sampled every 10 min for the duration of 30 min. Samples were transported to the laboratory and analyzed within 24 h by GC-MS. The 82 sampling points were positioned on the landfill slope, the road side, and the sides of gas collection wells. They are distributed on the waste working face (70 points), the geomembrane temporary cover (8 points), and the final cover system (4 points). The sampling process was conducted sequentially. Two sampling chambers were used at the same time. Sampling on the working face takes 2 days. Sampling on the temporary cover and final cover usually takes 1 day, respectively. Therefore, it takes 4 days for one sampling campaign in a single season when sampling at the three places was conducted at the same season.
The gas emission rate was determined by the following Eqs. (1) and (2) (Senevirathna et al. 2006):
where
where V is the volume of the chamber (m3), t is time (min), △t is the time interval during the two samples, Ct is the gas concentration at time t (mg/m3) in the chamber, Ct + △t is the gas concentration at t + △t (mg/m3), S is the area of the chamber base (m2), and j′ is the gas flux (mg/m2/h).
The gas flux j at temperature T should be corrected according to the environment temperature T (°C):
Take the determination of benzene flux for example. When the chamber was placed and everything is ready, one gas sample was obtained immediately. Concentration of benzene at this time was C0. The gas was sampled every 10 min until four samples were obtained. The concentrations C10, C20, and C30 represent the ones at 10 min, 20 min, and 30 min, respectively. The interval time was chosen to be 10 min to avoid pressure build-up in the chamber. Benzene flux can then be determined by Eq. (2). Temperature during sampling can be obtained by the thermometer. The final benzene emission flux can be obtained through Eq. (3) with the real-time temperature.
GC-MS analysis
Process of GC-MS analysis
The gas samples collected from the static chamber were first pre-concentrated by cryogenic liquid nitrogen to 1 mL in ventilation, in accordance with EPA TO14 (US-EPA 1999). The gas composition was determined by gas chromatography–mass spectrometry (GC-MS). The gas samples were analyzed using a gas chromatography (GC) (Agilent 7890N; Agilent, USA) equipped with a mass selective detector (Agilent 5975 inert MSD; Agilent, USA) and a thermal desorber (Tekmar, Aerotrap 6000, USA). The cryo trap capillary was a 22-cm part of a 0.53-mm id, AT-Q, Q-PLOT column (Alltech Associates), which was used to enhance trapping of VOCs and consequently the chromatographic resolution. Liquid nitrogen was used as cryogen. A 20-s heating pulse was adopted for flash desorption of trapped analytes in the GC column. The heating time was short enough to prevent extensive deterioration of the cryo trap column. The carrier gas is helium, which is provided at 1.0 mL/min for a purity of 99.999%. The odor samples were desorbed for 5 min at 250 °C using helium with a flow rate of 35 mL/min. Helium was carried through a 100-mg Tenax™ TA cryogenic internal trap. The standard sorbent tubes are made of glass, which are based on 2,6-diphenylene oxide. The trap was desorbed for 5 min at 250 °C. The split ratio was 1:50. The inlet temperature was 250 °C. Ion source temperature was 230 °C. Quadrupole temperature was 150 °C. The temperature program for odors was initial oven temperature 30 °C, hold for 2 min; and then increase the temperature from 30 °C to 125 °C at 10 °C/min, hold for 30 min. The GC column HP-5 (30 m × 0.32 mm × 0.25 μm) was set for three different temperature ranges, from 28 °C to 60 °C at an increasing rate of 5 °C/min, from 60 °C to 200 °C at an increasing rate of 5 °C/min, and at 200 °C for 5 min. Electron ionization (EI) was selected as ionization mode at 70 eV. The source temperature was 180 °C. The collection electric current was 200 μA. The source and analyzer vacuum pressure were 1 × 10−4 Pa and 1 × 10−6 Pa, respectively.
Internal standard method was used to quantify the concentration of VOCs. Chromatographic peaks were identified with the help of NIST98 library and enhanced by G170BA substance database. The limit of detection (LOD) is three times the signal-to-noise ratio (SNR). Limit of quantity (LOQ) is 10 times of the SNR. The linear equation of the method was obtained through the relation between concentration of each VOCs and its peak area. R2 of each VOCs was greater than 0.998.
Chemicals and consumables
The chemicals used in this study included diethyl ether, methylbenzene, benzene, and n-hexadecane (Sinopharm Chemical Reagent Co., Ltd., China). Purity of the used chemicals was analytical reagent (AR). The standard solution was obtained by adding 0.2 μL n-hexadecane into 1 mL diethyl ether. Then, methylbenzene was added into the solution by microsyringe. The standard solution was then stored in the refrigerator below 4 °C.
Gaussian dispersion model
The Gaussian dispersion model is used internationally as the core model to analyze landfill gas diffusion (Tagaris et al. 2003; Guarriello 2007; Úbeda et al. 2010):
where C is the gas concentration; y and z are the horizontal and vertical distance, respectively; q is the emission intensity of odor gas (g/s); u is the speed of wind (m/s); z is the vertical distance above the ground (m); and H is the effective stack height (H = h + Δh, where h = physical stack height and Δh = plume rise, m). σy and σz are functions of distance and atmospheric stability, which are the Pasquill–Gifford plume spread parameters based on stability class (Pasquill 1961). Six classes of atmospheric stability or turbulence, known as the Pasquill Stability or Turbulence Classes, A through F, have been described. This is dependent on five classes of wind speed, three daytime levels of insolation, and two classes of night-time cloudiness (Pasquill 1961).
Since VOC emissions from landfills are ground-level sources, which is z = 0 and H = 0 in Eq. (5). Therefore, Eq. (5) can be reduced to Eq. (6)
CALPUFF model
The dispersion of the odor emission from landfill can also be evaluated by the CALPUFF model (California Puff Model). CALPUFF is an air quality model utilized to process chemical transportation and the dispersion of air pollutants emitted from stacks, landfills, vehicles, and ponds. CALPUFF Model is a multi-layer, multi-species non-steady-state puff dispersion model, which considers the effect of time, space variations, and meteorological conditions on pollutant transport, transformation, and removal. It is able to treat stagnation, multiple-hour pollutant build-up, recirculation, and causality effects which are beyond the capabilities of steady-state models. CALPUFF can be applied on scales of tens to hundreds of kilometers. The algorithms for subgrid scale effects (such as terrain impingement) and longer-range effects, such as pollutant removal due to wet scavenging and dry deposition, chemical transformation, and visibility effects of particulate matter concentrations, are included in the model (US EPA 2015). CALPUFF model is one of the three models recommended by the national guidelines of China (HJ2.2-2008). Therefore, dispersion of methyl mercaptan and ethanethiol was also calculated by the CALPUFF model.
CALPUFF includes three major components: CALMET, CALPUFF, and CALPOST (Scire et al. 2000). CALMET is a meteorological model that develops hourly wind and temperature fields on a three-dimensional gridded modeling domain. The associated two-dimensional fields such as mixing height, surface characteristics, and dispersion properties are also included in the file produced by CALMET. Proceeding with an analysis through CALPUFF requires CALMET’s processed output data files. CALPOST processes the generated output data files from CALPUFF and provides the simulation results. The results obtained from the model were compared with that from Gaussian dispersion model. Meteorological data was provided by meteorological station on the landfill.
Results and discussions
Composition and concentration of VOCs on working face
A total of 28 VOCs from 50 points and 134 gas samples were identified during July 2015 to April 2017. The experimental data over four seasons are listed in Tables 2 and 3 and shown in Fig. 4. The detected compounds include hydrocarbon (e.g., butane, methylheptane, and heptane), aromatics (e.g., benzene, toluene, ethylbenzene, and xylene), terpenes (e.g., limonene and pinene), halogenated compounds (e.g., dichloropropane, trichloroethylene, and tetrachloroethylene), oxygenated compounds (e.g., acetone, methyl acetate, and ethyl alcohol), aldehyde compounds (e.g., nonanal, propanal, and heptanal), and sulfur compounds (e.g., methyl mercaptan, dimethyl sulfide, ethanethiol, dimethyl disulfide, and carbon disulfide). The relative abundance of different chemical groups exhibited a different trend, with terpenes > sulfur compounds > oxygenated compounds > aromatics > hydrocarbon > aldehyde compounds > halogenated compounds in spring and winter, sulfur compounds > oxygenated compounds > aromatics > hydrocarbon > terpenes > aldehyde compounds > halogenated compounds in summer, terpenes > oxygenated compounds > sulfur compounds > hydrocarbon > aromatics > aldehyde compounds > halogenated compounds in autumn. Terpenes, sulfur compounds, and oxygenated compounds dominated VOC concentration in spring, autumn, and winter. However, the results slightly changed in summer with sulfur compounds, oxygenated compounds, and aromatics dominating VOC concentration. Concentrations of oxygenated and sulfur compounds are mostly affected by the degradation stages of waste (Chiriac et al. 2011; Fang et al. 2012). In the summer, degradation of waste is faster than that in other seasons. Therefore, concentration of oxygenated and sulfur compounds increases in summer. Greater concentrations of VOCs were detected in summer. Temperature in summer is the highest and atmospheric pressure is the lowest among the four seasons, which makes faster decomposition of wastes and higher emission rate of sulfur compounds.
Composition and concentration of VOCs are mainly function of the waste composition and the process of waste degradation on the working face (Chiriac et al. 2011). Limonene and ethyl alcohol are the indicators of fresh waste. Besides, limonene is the major component of compost process (Agapiou et al. 2016). The emission of aromatics and halogenated compounds is mainly influenced by waste composition such as plastic packaging and aerosol propellants because these compositions are usually inherent substances of MSWs. In contrast, oxygenated and sulfur compounds are the intermediates and end products of waste in degradation processes (Chiriac et al. 2011; Fang et al. 2012). It is indicated in Table 3 that limonene dominated VOCs throughout the four seasons followed by ethyl alcohol and acetone.
Terpenes
Limonene dominated the terpenes with a maximum concentration of 43.29 μg m−3 in the autumn season. Limonene is one of the major causes of odor nuisance (Pierucci et al. 2005; Sadowska-Rociek et al. 2009; Fang et al. 2012). The average concentrations for limonene were 17.78 μg m−3, 2.90 μg m−3, and 19.56 μg m−3 in the spring, the summer, and the winter, respectively. Limonene concentration range detected on other landfills is 0.1–162 μg m−3 (Zou et al. 2003; Davoli et al. 2003).
Limonene is a compound representative of the emissions from fresh or green waste (Davoli et al. 2003; Sadowska-Rociek et al. 2009) or the intermediate products of aerobic reaction (Eitzer 1995). Apart from fresh food and green waste, air fresheners and fragrant household detergents are other sources of direct terpene emissions (Zou et al. 2003). Limonene can be produced by linalyl acetate and neryl acetate. Linalyl acetate is found in cocoa, celery, grape, kelp, peach, linalyl, and jasmine. Neryl acetate is edible essence specified by GB 2760-2011 in China (MHPRC 2011) and is widely used in essence preparation of fruits. Therefore, limonene can be generated during the process of food waste degradation. The high content of food waste (i.e., about 60%) in Hangzhou landfill is the main cause of the high concentration of limonene. The significant high level of limonene detected in the autumn can be attributed to the large amount of waste generated from the traditionally large consumption of citrus fruits in Hangzhou during that period of the year. Wang and Wu (2008) and Wu and Wang (2015) indicated that even small amounts of citrus fruits can contribute to high limonene emission.
Sulfur compounds
Composition and concentration of sulfur compounds are shown in Tables 2 and 3 and Fig. 4. Sulfur compounds comprise 10.7–30.2% of VOCs detected on the working face. The highest concentration of sulfur compounds was found in the summer, which was 1.22 and 2.13 times greater than that recorded in spring and autumn, respectively. Sulfur compounds were dominated by methyl mercaptan and dimethyl sulfide in spring, summer, and winter, whereas dimethyl disulfide and carbon disulfide tend to be the most abundant compounds in autumn. Methyl mercaptan and dimethyl sulfide are commonly known as odor-causing pollutants in landfills due to the fact that they usually have extremely low odor thresholds. The mean concentration of dimethyl sulfide and methyl mercaptan is 0.01–6.32 μg m−3 and 0.47–3.19 μg m−3, respectively, which is 3.4–22.8 times greater than its odor threshold (Talaiekhozani et al. 2016). Concentrations of dimethyl sulfide and methyl mercaptan in Hangzhou landfill detected by Ding et al. (2012) were 1.56–5.30 μg m−3 and 4.64–18.52 μg m−3, respectively. It is indicated that the concentration of VOCs decreased after 5 years of attempt to reduce odor nuisance caused by VOCs, but there is still a need to take some new measures to reduce VOCs emission due to the fact that concentration of methyl mercaptan and dimethyl sulfide are still several times greater than the odor threshold. Sulfur compounds in the working face were mostly emitted during the process of food waste degradation and fermentation for more than 1 year (Wu et al. 2010; Zhang et al. 2013; Zhan et al. 2017). The emission of methyl mercaptan, dimethyl sulfide, dimethyl disulfide, and carbon disulfide from industry or waste-treating facilities is limited by the Ministry of Environmental Protection of China under GB 14554-1993 (Emission Standards for Odor Pollutants) (NEPAC 1996).
Oxygenated compounds
Oxygenated compounds are one of the major compounds in landfill gas, comprising 12.77–25.90% of the total concentration for all samples. The average concentrations of oxygenated compounds in the four seasons were 10.03 μg m−3, 10.10 μg m−3, 9.39 μg m−3, and 10.29 μg m−3 in the spring, summer, autumn, and winter, respectively.
Ethyl alcohol and acetone were dominant VOCs in this group (Table 3). The emission of oxygenated compounds was mainly influenced by the temperature and the degree of waste degradation. Ethyl alcohol dominated oxygenated compounds with mean concentration 5.03 μg m−3 in the spring, 5.33 μg m−3 in the summer, 4.43 μg m−3 in the autumn, and 5.22 μg m−3 in the winter. High ambient temperature and fermentation state were responsible for the highest average concentration of ethyl alcohol in the summer. The presence of ethyl alcohol is due to the consequence of microbial alcohol formation from waste substrate during the storage period under nearly anaerobic conditions at low pH (Staley et al. 2006). Significant amounts of acetone were also observed in the four seasons. Acetone may come from fruit wastes at the early stage of its aerobic decomposition (Wu and Wang 2015). According to Allen et al. (1997) and Scaglia et al. (2011), ethyl alcohol and acetone are representative compounds of fresh waste.
Hydrocarbons
Hydrocarbons were found to be important fractions of the VOC components with the following average concentrations in the four seasons: 4.83 μg m−3 in the spring, 4.84 μg m−3 in the summer, 4.73 μg m−3 in the autumn, and 4.97 μg m−3 in the winter (Table 2). Alkanes usually predominate during the early phases of landfill activity when aerobic processes are still dominant and methane production is limited (Parker et al. 2002). 3-Methylheptane dominated hydrocarbon with average concentrations of 1.59–1.73 μg m−3, followed by 2-methylheptane with average concentration of 1.27–1.41 μg m−3 and butane with average concentration of 1.12–1.27 μg m−3. The detected hydrocarbons are shorter chain alkanes (< C10). This is because wastes are mostly under aerobic conditions on the working face. Therefore, alkanes with low molecular weight and low solubility tend to dominate this period (Duan et al. 2014).
Aromatics
Aromatics were also detected and comprised an important part of the VOCs. The percentages of aromatics were 10.77% in the spring, 14.1% in the summer, 5.81% in the autumn, and 10.25% in the winter. The highest percentage of aromatics was found in summer. This result indicates that temperature is the main variable affecting aromatic concentration since high temperature tends to cause rapid biological degradation of organic matter (Zou et al. 2003). Xylene dominated VOCs with an average concentration ranging from 2.53 to 2.82 μg m−3, whereas Ding et al. (2012) showed that toluene dominated VOCs with an average concentration 1.92–60.04 μg m−3 at the landfill in 2009. Benzene is a known carcinogen. When the human body is exposed to 1 μg/m3 of benzene, there is a lifetime risk of 4 × 10−6 for leukemia (WHO 1987). However, the detected average benzene concentration was 25 times less than the carcinogen standard. This indicates that benzene is not a threat for human health on the landfill.
Variation of VOC concentration on final landfill cover
The comparison of VOC concentration on the working face and the final cover system recorded in July 2015 and August 2016 is shown in Fig. 5a and b, respectively. The July 2015 sampling campaign included collection of 6 gas samples from 2 sampling points on the final cover system and 36 gas samples from 12 sampling points on the working face. The August 2016 sampling campaign included 8 gas samples from 2 sampling points on the final cover system and 32 gas samples from 8 sampling points on the working face. The average concentration of limonene on the working face is 20 and 86 times greater than that on the final cover in July 2015 and August 2016, respectively. The average concentration for ethyl alcohol on the working face is 120 and 166 times greater than that on the final cover in July 2015 and August 2016, respectively. The VOC concentration on the working face was 2–166 times greater than that on the final cover. In July 2015, the concentrations of limonene and ethyl alcohol on the working face were 20 and 120 times greater than that recorded on the final cover system. In August 2016, the concentrations of limonene and ethyl alcohol on the working face were 85 and 166 times greater than that on the final cover. Toluene and xylene were not detected in both July 2015 and August 2016. It is indicated that methyl acetate dominated VOCs on the final cover with average concentration of 0.24 μg m−3, followed by enanthaldehyde with an average concentration of 0.12 μg m−3 in July 2015. 3-Methylheptane and butane dominated VOCs on the final cover with average concentration of 0.058 μg m−3 and 0.053 μg m−3, followed by methyl acetate and enanthaldehyde with average concentration of 0.046 μg m−3 and 0.042 μg m−3. This indicated that there are still VOCs emitted from the final cover. This is due to the fact that gas collection efficiency in China’s landfill systems is relatively low, usually around 20% (Chen et al. 2010; Sun et al. 2015). Furthermore, VOCs can transport through geomembrane by diffusion (McWatters and Rowe 2009, 2010; Jones and Rowe 2016).
VOC composition and concentration on holes of temporary GMB cover
A 1.5-mm HDPE geomembrane was used to prevent direct exposure of fresh waste to the ambient air and prevent landfill gas emission to the atmosphere. HDPE geomembranes can be an efficient barrier against landfill gas emission if they are void of defects (Bouazza and Vangpaisal 2006, 2007; Bouazza et al. 2008; Abuel-Naga and Bouazza 2009). However, the geomembrane used in the landfill had several holes caused during its placement on the waste and during landfill activities (Fig. 6). Eight sampling points were located on the positions where defects on the geomembrane were identified. Twenty-four gas samples were collected from these locations. Concentrations of VOCs emitted from holes in the HDPE geomembrane cover are shown in Fig. 7. It can be seen that limonene dominated VOC emissions with an average concentration of 38 μg m−3, which is 6.3 times greater than that of ethyl alcohol. The emission rate of limonene on the holes of temporary HDPE cover was 2.2 times greater than that on the working face. Comparison between Fig. 5b and Fig. 7 indicates that concentration of limonene emanating from the holes on the geomembrane cover is 5 times greater than that on the working face, while concentration of ethyl alcohol is 1.5 times greater than that on the working face. This is because limonene is the intermediate products of aerobic reaction (Eitzer 1995). Aerobic bacteria is accumulated in the holes of HDPE cover due to high content of oxygen around the holes, while it is anoxic under the intact HDPE cover without a hole.
Dispersion of methyl mercaptan and ethanethiol
The above results show that concentration of methyl mercaptan and ethanethiol were 3.4–22.8 times greater than their odor threshold values. The odor threshold values of methyl mercaptan and ethanethiol are 1.1 ppb and 0.19 ppb (Talaiekhozani et al. 2016). The diffusion distance of methyl mercaptan and ethanethiol away from the working face can be determined by Eqs. (5)–(6). The diffusion distance here is the distance between the working face and the point where odor concentration is lower than its odor threshold. Table 4 shows wind speed and emission rates of methyl mercaptan and ethanethiol in the four seasons. The diffusion distance of methyl mercaptan and ethanethiol in the four seasons for different atmospheric stability determined by Gaussian model is shown in Fig. 8. The concentration profiles of methyl mercaptan and ethanethiol in the four seasons with class F are shown in Fig. 9 and Fig. 10, respectively. The origins of the coordinates in both Fig. 9 and Fig.10 stand for the working face on the landfill. Class F represents the most stable condition for atmosphere. It is indicated from Fig. 8 that diffusion distance of methyl mercaptan and ethanethiol varies for different atmospheric stability classes. The diffusion distance increases with the increase of the class of the atmospheric stability. In spring, the diffusion distances of methyl mercaptan and ethanethiol for the cases with class A–B are 2.0 and 1.7 times less than that for class E–F, respectively. It is also indicated from Chemel et al. (2012) that clear sky and calm weather conditions favor odor pollution events. The emission rate and metrological conditions in the four seasons also have effects on the diffusion of VOCs. For methyl mercaptan, the diffusion distance is the longest in autumn for all atmospheric class stability class. This is due to the fact that the emission rate of methyl mercaptan is the greatest in autumn, which is almost 10 times greater than that in winter. It demonstrates that emission rate is an important factor that influences the diffusion distance of VOCs. Odor nuisance can be reduced by reduce VOC emission and increase landfill gas recovery. It is reported that metrological conditions with high temperature, low air temperature, and high humidity will result in high landfill gas emission rate (Duan et al. 2014; Liu et al. 2015). For ethanethiol, its diffusion distance is the shortest in autumn for all atmospheric class stability class. This is due to the fact that the wind speed in autumn is the greatest. Besides, the difference among the emission rate of ethanethiol in four seasons is small. Diffusion distance of ethanethiol is approximately 1.3 times greater than that of methyl mercaptan of all atmospheric stability class in spring, summer, and winter. This is due to the extremely low odor threshold of ethanethiol. Odor threshold of methyl mercaptan is 5.8 times greater than that of ethanethiol. Diffusion distance of both methyl mercaptan and ethanethiol are within 500 m. The results indicate that methyl mercaptan and ethanethiol would be a nuisance for the people working on the landfill but not be a nuisance for the residents living around the landfill.
Dispersion of ethanethiol and methyl mercaptan simulated by CALPUFF model is shown in Fig. 11 and Fig. 12, respectively. It is indicated that the diffusion distance of both ethanethiol and methyl mercaptan are within the landfill boundary. The concentration of both ethanethiol and methyl mercaptan outside the landfill are lower than the odor threshold. It indicates that people living near the landfill would not be bothered by ethanethiol and methyl mercaptan. Besides, diffusion distance simulated by CALPUFF model is within 100 m, which is smaller than that simulated by Gaussian model. This is due to the simplified treatment of turbulence and meteorology in Gaussian model. Gaussian models have been shown to consistently overpredict concentrations in low wind conditions (Sokhi et al. 1998; Holmes and Morawska 2006). Furthermore, the Gaussian plume equation assumes that there is no interaction between plumes, which can become significant within urban environments (Holmes and Morawska 2006).
Conclusions
The temporal concentrations of VOCs in landfill in Hangzhou, China were monitored by the developed static chamber. The landfill gas from 82 sampling points including 70 points on working face, 8 points on geomembrane, and 4 points on final cover was obtained and analyzed for VOCs by GC-MS. Gaussian dispersion model and the CALPUFF model were used to analyze the diffusion distance of methyl mercaptan and ethanethiol. The main conclusions are drawn as follows:
-
1.
Hydrocarbon, aromatics, terpenes, halogenated compounds, oxygenated compounds, aldehyde compounds, and sulfur compounds were detected in the landfill with average concentration ranging from 0.56 to 43.50 μg m−3. The most abundant compounds were terpenes, sulfur compounds, hydrocarbon, and oxygenated compounds.
-
2.
Terpenes dominated VOCs with percentage of 34.02%, 59.06%, and 37.79% recorded in the spring, autumn, and winter seasons, while sulfur compounds dominated VOCs with percentage of 30.18% in the summer season. Limonene, ethyl alcohol, and acetone were the dominant VOCs recorded on the working face of the landfill. This is due to the fact that the wastes on the working face were fresh since limonene and ethyl alcohol are the indicators of fresh waste and limonene is the major component of compost process. Methyl mercaptan and ethanethiol are the main VOC compounds responsible for odor nuisance. The concentration of methyl mercaptan and ethanethiol were 3.4–22.8 times higher than their odor threshold.
-
3.
Limonene was the main VOC escaping from the holes present in the geomembrane temporary cover with average concentration of 38 μg m−3, which is 2.14 times greater than that on the working face. Emission rate of limonene from defects of the temporary cover was found to be 2.24 times more than that on the working face. This indicates that the construction quality of geomembranes plays an important role in mitigating landfill gas emission.
-
4.
The VOC concentrations on the final cover can be up to 166 times less than those obtained on the working face. This may be due to the degradation of waste below the final cover was nearly completed. Methyl acetate, enanthaldehyde, methylheptane, and butane dominated VOCs emitted from the final cover system.
-
5.
Methyl mercaptan and ethanethiol would be a nuisance for the people working on the landfill. The diffusion distance for both of the gases simulated by CALPUFF model and Gaussian model is within 100 m and 500 m, respectively. This indicates that people living near the landfill would not be bothered by ethanethiol and methyl mercaptan. The correlations between metrological conditions and the emission and diffusion of VOCs should be further investigated.
-
6.
This study provides important information and database in assessing the effect of the landfill gas emission on the atmosphere and the environment in the Hangzhou region
References
Abuel-Naga HM, Bouazza A (2009) Numerical characterization of advective gas flow through GM/GCL composite liners having a circular defect in the geomembrane. J Geotech Geoenviron 135(11):1661–1671
Agapiou A, Vamvakari JP, Andrianopoulos A, Pappa A (2016) Volatile emissions during storing of green food waste under different aeration conditions. Environ Sci Pollut Res 23(9):8890–8901
Allen MR, Braithwaite A, Hills CC (1997) Trace organic compounds in landfill gas at seven UK waste disposal sites. Environ Sci Technol 31(4):1054–1061
Avaliani SL, Balter BM, Balter DB, Faminskaya MV, Revich BA, Stalnaya MV (2016) Air pollution source identification from odor complaint data. Air Qual Atmos Health 9(2):179–192
Blount BC, Kobelski RJ, McElprang DO, Ashley DL, Morrow JC, Chambers DM, Cardinali FL (2006) Quantification of 31 volatile organic compounds in whole blood using solid-phase microextraction and gas chromatography-mass spectrometry. J Chromatogr B 832(2):292–301
Bouazza A, Vangpaisal T (2006) Laboratory investigation of gas leakage rate through a GM/GCL composite liner due to a circular defect in the geomembrane. Geotext Geomembr 24(2):110–115
Bouazza A, Vangpaisal T (2007) Gas transmissivity at the interface of a geomembrane and the geotextile cover of a partially hydrated geosynthetic clay liner. Geosynth Int 14(5):316–319
Bouazza A, Vangpaisal T, Abuel-Naga H, Kodikara J (2008) Analytical modelling of gas leakage rate through a geosynthetic clay liner-geomembrane composite liner due to a circular defect in the geomembrane. Geotext Geomembr 26(2):122–129
Chemel C, Riesenmey C, Batton-Hubert M, Vaillant H (2012) Odour-impact assessment around a landfill site from weather-type classification, complaint inventory and numerical simulation. J Environ Manag 93(1):85–94
Chen Z, Gong H, Jiang R, Jiang Q, Wu W (2010) Overview on LFG projects in China. Waste Manag 30(6):1006–1010
Chiriac R, Morais JDA, Carre J, Bayard R, Chovelon JM, Gourdon R (2011) Study of the VOC emissions from a municipal solid waste storage pilot-scale cell: comparison with biogases from municipal waste landfill site. Waste Manag 31(11):2294–2301
Davoli E, Gangai ML, Morselli L, Tonelli D (2003) Characterisation of odorants emissions from landfills by SPME and GC/MS. Chemosphere 51(5):357–368
Di Y, Liu J, Liu J, Liu S, Yan L (2013) Characteristic analysis for odor gas emitted from food waste anaerobic fermentation in the pretreatment workshop. J Air Waste Manage Assoc 63(10):1173–1181
Dincer F, Odabasi M, Muezzinoglu A (2006) Chemical characterization of odorous gases at a landfill site by gas chromatography-mass spectrometry. J Chromatogr A 1122(1):222–229
Ding Y, Cai C, Hu B, Xu Y, Zheng X, Chen Y, Wu W (2012) Characterization and control of odorous gases at a landfill site: a case study in Hangzhou, China. Waste Manag 32(2):317–326
Duan Z, Lu W, Li D, Wang H (2014) Temporal variation of trace compound emission on the working surface of a landfill in Beijing, China. Atmos Environ 88:230–238
EEA-European Environment Agency, 2008. Directive 2008/50/EC. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0050&from=en last access 27/04/2018
Eitzer BD (1995) Emissions of volatile organic chemicals from municipal solid waste composting facilities. Environ Sci Technol 29(4):896–902
European Council BS EN 13725 (2003) Air quality Determination of odour concentration by dynamic olfactometry
Fang JJ, Yang N, Cen DY, Shao LM, He PJ (2012) Odor compounds from different sources of landfill: characterization and source identification. Waste Manag 32(7):1401–1410
Fielder HM, Palmer SR, Poonking C, Moss N, Coleman G (2001) Addressing environmental health concerns near Trecatti landfill site, United Kingdom. Arch Environ Health: Int J 56(6):529–535
Gallego E, Perales JF, Roca FJ, Guardino X (2014) Surface emission determination of volatile organic compounds (VOC) from a closed industrial waste landfill using a self-designed static flux chamber. Sci Total Environ 47:587–599
González CRN, Björklund E, Forteza R, Cerdà V (2013) Volatile organic compounds in landfill odorant emissions on the island of Mallorca. Int J Environ Anal Chem 93(4):434–449
Guarriello NS (2007) Determining emissions from landfills and creating odor buffer distances. Florida State University, Florida
Ministry of Environmental Protection of the People’s Republic of China (2008). Guidelines for environmental impact assessment atmospheric environment. (HJ2.2-2008). Beijing: Standards Press of China
Guo H, Duan Z, Zhao Y, Liu Y, Mustafa MF, Lu W, Wang H (2017) Characteristics of volatile compound emission and odor pollution from municipal solid waste treating/disposal facilities of a city in eastern China. Environ Sci Pollut Res 24(22):18383–18391
Hamoda MF (2006) Air pollutants emissions from waste treatment and disposal facilities. J Environ Sci Health A 41(1):77–85
Holmes NS, Morawska L (2006) A review of dispersion modelling and its application to the dispersion of particles: an overview of different dispersion models available. Atmos Environ 40(30):5902–5928
U S EPA, 2015. CALPUFF Modeling System. http://www.epa.gov/ttn/scram/dispersion_prefrec.htm#calpuff
Hudson N, Ayoko GA (2008) Odour sampling. 2. Comparison of physical and aerodynamic characteristics of sampling devices: a review. Bioresour Technol 99(10):3993–4007
Jones DD, Rowe RK (2016) BTEX migration through various geomembranes and vapor barriers. J Geotech Geoenviron 142(10):04016044
Keller AP (1988) Trace constituents in landfill gas, Task report on inventory and assessment of cleaning technologies. Final report, may 1984–February 1987 (no. PB-88-217021/XAB). SCS Engineers, Inc., Covington, KY (USA)
Kreith F (1995) In: Handbook of solid waste management. McGraw-Hill, New York, pp 1211–1213
Lazarus, S. B., Tsourdos, A., Zbikowski, R., and White, B. A. (2008). Unstructured environmental mapping using low cost sensors. IEEE international conference on networking, sensing and control (pp.1080-1085). IEEE
Liu Y, Lu W, Li D, Guo H, Caicedo L, Wang C, Xu S, Wang H (2015) Estimation of volatile compounds emission rates from the working face of a large anaerobic landfill in China using a wind tunnel system. Atmos Environ 111:213–221
Liu Y, Lu W, Guo H, Ming Z, Wang C, Xu S, Liu Y, Wang H (2016) Aromatic compound emissions from municipal solid waste landfill: emission factors and their impact on air pollution. Atmos Environ 139:205–213
Lu W, Duan Z, Dong L, Jimenez LMC, Liu Y, Guo H, Wang HT (2015) Characterization of odor emission on the working face of landfill and establishing of odorous compounds index. Waste Manag 42:74–81
McWatters RS, Rowe RK (2009) Transport of volatile organic compounds through PVC and LLDPE geomembranes from both aqueous and vapour phases. Geosynth Int 16(6):468–481
McWatters RS, Rowe RK (2010) Diffusive transport of VOCs through LLDPE and two coextruded geomembranes. J Geotech Geoenviron 136(9):1167–1177
Ministry of Health of the People’s Republic of China (MHPRC) (2011) Sanitary standards of using food additives (GB 2760-2011). Standards Press of China, Beijing
National Environmental Protection Agency of China (NEPAC) (1996) Emission standards for odor pollutants (GB 14554-1993). Standards Press of China, Beijing
Obersky L, Rafiee R, Cabral AR, Golding SD, Clarke WP (2018) Methodology to determine the extent of anaerobic digestion, composting and CH4 oxidation in a landfill environment. Waste Manag 76:364–373
Parker, T., Dottridge, J., and Kelly, S. (2002). Investigation of the composition and emissions of trace components in landfill gas. Environment agency, R&D technical report P1-438/TR
Pasquill F (1961) The estimation of the dispersion of windborne material. Aust Meteorol Mag 90:33–49
Pierucci P, Porazzi E, Martinez MP, Adani F, Carati C, Rubino FM, Colombi A, Calcaterra E, Benfenati E (2005) Volatile organic compounds produced during the aerobic biological processing of municipal solid waste in a pilot plant. Chemosphere 59:423–430
Sadowska-Rociek A, Kurdziel M, Szczepaniec-Cięciak E, Riesenmey C, Vaillant H, Batton-Hubert M, Piejko K (2009) Analysis of odorous compounds at municipal landfill sites. Waste Manag Res 27(10):966–975
Saldarriaga JF, Aguado R, Morales GE (2014) Assessment of VOC emissions from municipal solid waste composting. Environ Eng Sci 31(6):300–307
Scaglia B, Orzi V, Artola A, Font X, Davoli E, Sanchez A, Adani F (2011) Odors and volatile organic compounds emitted from municipal solid waste at different stage of decomposition and relationship with biological stability. Bioresour Technol 102(7):4638–4645
Schroth MH, Eugster W, Gómez KE, Gonzalez-Gil G, Niklaus PA, Oester P (2012) Above- and below-ground methane fluxes and methanotrophic activity in a landfill-cover soil. Waste Manag 32(5):879–889
Scire JS, Strimaitis DG, Yamartino RJ (2000) A user’s guide for the CALPUFF dispersion model (version 5.0). Earth Tech Inc., Concord, MA http://www.src.com/calpuff/download/CALPUFF_UsersGuide.pdf
Senevirathna DG, Achari G, Hettiaratchi JP (2006) A laboratory evaluation of errors associated with the determination of landfill gas emissions. Can J Civ Eng 33(3):240–244
Shafi S, Sweetman A, Hough RL, Smith R, Rosevear A, Pollard SJ (2006) Evaluating fugacity models for trace components in landfill gas. Environ Pollut 144(3):1013–1023
Shin HC, Park JW, Park K, Song HC (2002) Removal characteristics of trace compounds of landfill gas by activated carbon adsorption. Environ Pollut 119(2):227–236
Sokhi, R., Fisher, B., Lester, A., McCrae, I., Bualert, S., and Sootornstit, N. (1998). Modelling of air quality around roads. In proc of 5th Int. Conf. On Harmonisation with Atmospheric
Staley BF, Xu F, Cowie SJ, Barlaz MA, Hater GR (2006) Release of trace organic compounds during the decomposition of municipal solid waste components. Environ Sci Technol 40(19):5984–5991
Statheropoulos M, Agapiou A, Pallis G (2005) A study of volatile organic compounds evolved in urban waste disposal bins. Atmos Environ 39(26):4639–4645
Sun Y, Yue D, Li R, Yang T, Liu S (2015) Assessing the performance of gas collection systems in select Chinese landfills according to the LandGEM model: drawbacks and potential direction. Environ Technol 36(23):2912–2918
Tagaris E, Sotiropoulou REP, Pilinis C, Halvadakis CP (2003) A methodology to estimate odors around landfill sites: the use of methane as an odor index and its utility in landfill siting. J Air Waste Manage Assoc 53(5):629–634
Talaiekhozani A, Bagheri M, Goli A, Khoozani MRT (2016) An overview of principles of odor production, emission, and control methods in wastewater collection and treatment systems. J Environ Manag 170:186–206
Úbeda, Y., Ferrer, M., Sanchis, E., Calvet, S., Nicolas, J., Lopez, P.A., (2010). Evaluation of odor impact from a landfill area and a waste treatment facility through the application of two approaches of a Gaussian dispersion model. In: 2010 International congress on environmental modelling and software modelling for environment’s sake. International Environmental Modelling and Software Society (iEMSs), Ottawa, Canada
US EPA (2015) CALPUFF modeling system. http://www.epa.gov/ttn/scram/dispersion_prefrec.htm#calpuff
US-EPA (Environmental Protection Agency). (1999). Compendium method TO-14. determination of volatile organic compounds (VOCs) in ambient air using specially prepared canisters with subsequent analysis by gas chromatography
Vallejo MD, Baena CA (2007) Toxicologı’a Ambiental. Wills Ltd., Colombia
Wang X, Wu T (2008) Release of isoprene and monoterpenes during the aerobic decomposition of orange wastes from laboratory incubation experiments. Environ Sci Technol 42(9):3265–3270
World Health Organization (WHO) (1987) Air quality guidelines for Europe. Copenhagen, Denmark
Wu T, Wang X (2015) Emission of oxygenated volatile organic compounds (OVOCs) during the aerobic decomposition of orange wastes. J Environ Sci 33:69–77
Wu T, Wang X, Li D, Yi Z (2010) Emission of volatile organic sulfur compounds (VOSCs) during aerobic decomposition of food wastes. Atmos Environ 44(39):5065–5071
Xie H, Jiang Y, Zhang C, Feng S (2015a) An analytical model for volatile organic compound transport through a composite liner consisting of a geomembrane, a GCL and a soil liner. Environ Sci Pollut Res 22(4):2824–2836
Xie H, Jiang Y, Zhang C, Feng S (2015b) Steady-state analytical models for performance assessment of landfill composite liners. Environ Sci Pollut Res 22(16):12198–12214
Zhan LT, Xu H, Chen YM, Lü F, Lan JW, Shao LM, Lin WA, He PJ (2017) Biochemical, hydrological and mechanical behaviors of high food waste content MSW landfill: preliminary findings from a large-scale experiment. Waste Manag 63:27–40
Zhang H, Schuchardt F, Li G, Yang J, Yang Q (2013) Emission of volatile sulfur compounds during composting of municipal solid waste (MSW). Waste Manag 33(4):957–963
Zou SC, Lee SC, Chan CY, Ho KF, Wang XM, Chan LY, Zhang ZX (2003) Characterization of ambient volatile organic compounds at a landfill site in Guangzhou, South China. Chemosphere 51(9):1015–1022
Acknowledgements
The financial supports from the National Natural Science Foundation of China (grant nos. 41672288, 51478427, 51278452, and 51008274), the National Key R&D program (grant no. 2018YFC1802303), the Fundamental Research Funds for the Central Universities (grant no. 2017QNA4028), and Zhejiang Provincial Public Industry Research Project (grant no. 2015C31005) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Constantini Samara
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Q., Zuo, X., Xia, M. et al. Field investigation of temporal variation of volatile organic compounds at a landfill in Hangzhou, China. Environ Sci Pollut Res 26, 18162–18180 (2019). https://doi.org/10.1007/s11356-019-04917-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04917-5