Abstract
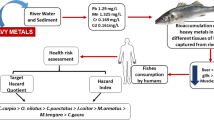
The purposes of this research are to quantify the concentration of heavy metals (Zn, Cu, As, Pb, Cd, and Hg) in the water and fish tissues of common carp (Cyprinus carpio) in the upper Mekong River and to thereby elucidate the potential dietary health risks from fish consumption of local residents. Surface water and fish tissues (gill, muscle, liver, and intestine) from four representative sample areas (influence by a cascade of four dams) along the river were analyzed for heavy metal concentrations. Results revealed that the levels of heavy metals in fish were tissue-dependent. The highest Cu and As levels were found in the liver; the highest Zn and Pb levels occurred in the intestine, and the highest Hg level was found in the muscle. The total target hazard quotient (THQ) value for residents is > 1 for long-term fish consumption, and local residents are, therefore, exposed to a significant health risk. Results from the current study provide an overall understanding of the spatial and tissue distribution of heavy metals in water and fish body along the upper Mekong River under the influence of cascade dams and highlight the potential health risk of As for the local residents of long-term fish consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination in aquatic ecosystems can have significant adverse health effects (Uysal 2011; Alhashemi et al. 2012; Mohan et al. 2012). Heavy metals cannot degrade, so they can accumulate in water, sediments, and fish through aquatic food chains and are toxic to aquatic organisms (Dehn et al. 2006; Bibi et al. 2016). Heavy metals can also be transferred and accumulated in human tissues through long-term food consumption, and this can cause toxic effects to the liver (hepatotoxicity), kidney (nephrotoxicity), central nervous system (neurotoxicity), and deoxyribonucleic acid (genotoxicity) (Sharma et al. 2014; Gupta et al. 2015). Different heavy metals possess unique physicochemical characteristics and have distinctive tissue distributions and accumulation in fish (Arnot and Gobas 2006; Uysal et al. 2008). For example, lead (Pb) is easily absorbed in fish blood and skeleton since it is lipophilic (Hodson 1976; Larsson et al. 1985). Different tissues vary in the metabolism and storage of heavy metals from the environment due to their distinct physiological functions (Ashraf 2005). Fish’s gills and digestive tract have significant potential for heavy metal accumulation (Dural et al. 2007). Their heavy metal contents change with variation of heavy metal levels in the water and food. Both gills and intestine are directly exposed to environmental pollutants by respiration and food ingestion (Uysal et al. 2008). Muscle tissue accumulates fewer heavy metals, and this reflects the long-term heavy metal levels in the environment (Yi et al. 2008). Therefore, heavy metal concentrations in muscle are often used as an indicator to assess the pollution of aquatic environment and health risks of fish consumption (Islam et al. 2015).
Heavy metal levels in fish tissues are significantly correlated with heavy metal levels in the aquatic environment (Wagner and Boman 2003; Jezierska and Witeska 2006). This establishes a quantitative relationship between water pollutant parameters and fish tissue levels (Karatas 2008; Uysal et al. 2009; Danabas and Ural 2012; Ural et al. 2012). Most research has focused on the concentration of heavy metals in fish tissues and monitoring the spatial and temporal distribution of pollutants in reservoirs, lakes, and rivers (Yi and Zhang 2012; Zeng et al. 2012; Dhanakumar et al. 2015). Less information is available on the migration, transport, and transformation of heavy metals in the aquatic ecosystem along rivers by cascade dams (Müller et al. 2008; Käiro et al. 2011). Dam construction may restrict the transport of heavy metals in water column along rivers and affect the distribution and accumulation of heavy metals in the aquatic ecosystem.
Mekong River is the longest international river in Asia and the largest freshwater fishery base in the world. It has been divided into several huge reservoirs by cascade dams (Zhang et al. 2013). The safety of water environment and aquatic products from the Mekong affects the health of > 100 million local residents from six riparian countries (Cenci and Martin 2004). There are high levels of heavy metals in the water and sediment of the upper Mekong basin, and these are derived from mining, domestic wastewater, and agricultural drainage (Chen et al. 2011; Fu et al. 2012; Zhao et al. 2012). In the lower Mekong River basin, heavy metal pollution has been documented in the rice, vegetables, and fish sampled from Cambodia and Vietnam, indicating the potential health risks from long-term consumption of these foods (Phan et al. 2013b; Chanpiwat et al. 2016). Despite above mentioned studies and to the best of our knowledge, there appears to be no information available on the accumulation of heavy metals in fish in the upper reaches of the Mekong River.
The purpose of this study was to investigate the heavy metal contents in different tissues (gill, liver, intestine, and muscle) of the common carp (Cyprinus carpio) and to use these data to estimate the health risk of heavy metal pollution in the section of the upper Mekong River under the influence of the cascade dams. Target hazard quotient (THQ) based risk assessment method, widely used to evaluate health risks of environmental pollutants (Chien et al. 2002; Wang et al. 2005; Yi et al. 2011), was applied to estimate the health risk of consuming fish with heavy metal contamination.
Materials and methods
Study area description and sample collection
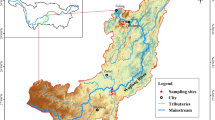
All sampling sites for the surface water and fish tissue collection in different segments of the upper Mekong River were located in western of Yunnan province, China (Fig. 1). Large reservoirs are present behind five dams (Miaowei, Dachaoshan, Gongguo, Xiaowan, and Dachaoshan) along the upper Mekong River. Two of the upper reservoirs (Gongguo Dam and Xiaowan Dam) receive local runoff from nearby mine tailings, such as the Pb, As, and Zn mines. The lower two reservoirs are mainly polluted by human activities such as agricultural drainage, industrial wastewater, and urban domestic sewage (Zhang et al. 2014).
All the water samples and fish samples were collected according to the standard methods from previous literature (Yi et al. 2011). Briefly, the surface water was sampled twice a year at the same time with the fish sampled. Surface water samples were collected in clean 500-mL polyethylene bottles, to which concentrated nitric acid was added (1% of the total water volume), and then frozen for storage. C. carpio fish were sampled from August 2013 to February 2015 by fishermen at different sample sites along the upper Mekong River. The body mass and standard length of each individual fish were measured and recorded. Then, the fish were dissected, and samples of the gill, liver, intestine, and muscle were removed and rinsed two times with pure water. Tissue samples were placed in polyethylene plastic bags and stored at − 20 °C immediately upon laboratory receipt.
Sample digestion and testing
Fish tissues (0.5 ± 0.01 g) of gills, muscle, liver, and intestine were weighed and transferred into acid-washed Teflon microwave digestion vessels. Ultrapure nitric acid (4 mL) and deionized water (4 mL) were added to the vessels, and they were heated with a microwave digestion system for 2-h digestion. Digested samples were transferred to an electric hot plate (180 °C) to evaporate excess acid. Once cooled, the digested samples were transferred to a 25-mL volumetric flask and the volume was increased to 25 mL with deionized water. The water samples were passed through filter paper to remove sediments and diluted with 2% nitric acid. Concentrations of arsenic (As), cadmium (Cd), mercury (Hg), copper (Cu), lead (Pb), and zinc (Zn) in water and fish tissues were measured using an inductively coupled plasma mass spectrometer (ICP-MS) (Agilent 7800, USA).
THQ determination
The health risk assessment for local residents in consuming fish was assessed based on the basis of the THQ (Wang et al. 2005). The method used to estimate the THQ of each heavy metal is described as follows:
where the standard frequency exposure is EF (365 days/year) and the exposure duration is ED (70 years) as a standard for the average human life expectancy. FIR is the fish ingestion rate, considered for local people to be 36 g/person/day (Storelli 2008). WAB represents the average weight (60 kg) of the study population. C is the mean metal concentration in the fish muscles. RFD indicates the oral reference dose (Cu = 0.04 mg/kg/day, Zn = 0.3 mg/kg/day, As = 0.0003 mg/kg/day, Cd = 0.001 mg/kg/day, Hg = 5 × 10−4 mg/kg/day, Pb = 0.004 mg/kg/day) (USEPA 2000, 2009). TA represents the average exposure time for non-carcinogens (365 days/year numbers of exposure years, assuming 70 years in this study). A THQ value above one indicates an exposure level that is bigger than the reference dose; a daily exposure at this level is believed to be likely to induce negative effects during a human’s life time period.
When the organism’s exposure is due to more than one pollutant, it may result in additive and/or interactive effects (Wang et al. 2005). The total THQ (TTHQ) of heavy metals for fish is the sum of the following compositions, derived from the method of (Yi et al. 2011):
Statistical analysis
Results are shown by mean value ± standard deviation, and IBM SPSS 20.0 software was used for statistical analyses. One-way ANOVA was applied to study regional differences in heavy metal content in water and fish. A significant difference was established at p < 0.05.
Results
Distribution of heavy metals in fish tissues
Table 1 shows that the concentration of the different metals is associated with fish tissue types. Significant metal concentrations were observed in different tissues: These were Cu and As in the liver, Zn and Pb in the intestine, and Hg in the muscle. Most of fish tissues had significant high Zn and Cu levels, which may be due to the fact that these two metals are the essential elements for many fish physiological activities. The accumulation order of the Zn in fish tissue was intestine > gill > liver > muscle (Fig. 2a). The highest concentration of Zn (405.62 ± 274.18 mg/kg) was found in the intestine. The next tissue possessing a high level of Zn was gill, with an average content ranging from 175.78 to 263.82 mg/kg. The lowest level of Zn occurred in the muscle where the average concentration ranged from 7.86 to 9.10 mg/kg. The order of Cu accumulation in the fish tissue ranked as liver > intestine > gill > muscle (Fig. 2b). The content of Cu in the liver was extremely high, being almost 20-fold higher than the level in other tissues. For instance, the Cu content in the liver was up to 36.84 ± 19.59 mg/kg in the section of S1, while the concentrations of Cu in intestine, gill, and muscle were only 2.60 mg/kg, 1.72 mg/kg, and 1.22 mg/kg, respectively.
The spatial differentiation of total heavy metals accumulated in different tissues showed distinct distribution patterns along the upper Mekong River (Fig. 3). In muscle, the spatial differentiation of various elements among different segments was minor and showed a similar distribution. It is found that Zn was the most abundant element in muscle, followed by Cu, As, Pb, Hg, and Cd (Fig. 3a). This result indicated that the content of heavy metals in the muscle was quite stable, which can reflect the long-term contents of metals in the water environment. In the liver, the total heavy metal content of each segment varied greatly and no consistent distribution pattern was identified. Regardless of the spatial distribution, Zn and Cu were two of the most abundant elements accumulated in the liver (Fig. 3b). In the intestine and gills, the total metal content increased from upstream to downstream, and all fish tissues showed higher concentration of Zn (Fig. 3c, d).
Heavy metal concentrations in the muscles of fish based on the present work and from other rivers are listed in Table 2. Data from literatures showed that heavy metal concentrations in muscles of fish varied widely from different rivers. The As concentration in the muscle of upper Mekong River fish was the highest documented. In contrast, the Cu, Zn, Pb, and Hg concentrations in muscles of fish collected from Yellow River were significantly higher than levels found in the current study. Interestingly, most of the heavy metal contents in the muscle of the upper Mekong were higher than those in the lower Mekong, indicating that heavy metal contamination in the upstream portion of the Mekong was more serious than downstream.
Target hazard quotients from fish consumption
The average concentration of heavy metals in fish muscle was used to calculate the THQs of heavy metals through fish consumption for local residents in the upper Mekong River. The estimated THQ for individual metal decreased in the following sequence: As > Zn > Hg > Pb > Cu > Cd. The THQ values of As were > 1 in most of the sections and accounted for about 85% of the total THQ. This indicates that people are subject to significant health risk from consumption of As-contaminated fish (Table 3). The next higher risk contributor element was Zn, contributing about 9% to the total THQ. The risk contributions of other four elements were relatively low, and together, they contributed no more than 6% of the total THQ. These data demonstrate that the dominant risk contribution was As and relatively minor risk contributions were made by Hg, Pb, Cu, and Cd for the inhabitants of the study areas. The total THQ for the local residents ranged from 1.205 to 1.933, indicating that they may experience adverse health effect due to consumption of fish from the upper Mekong River.
Heavy metal concentrations in surface water
The spatial and temporal distributions of heavy metals in the surface water of four sites along the upper Mekong River are shown in Fig. 4. The spatial distribution of total heavy metal contents decreased from upper to lower reaches with an exception of S4 segment. The levels of Cu, As, Zn, and Hg were decreased from S1 to S3 (Fig. 4a–e). The relatively higher heavy metal levels in S4 can be ascribed to the tributary influx. There is a large city located upstream of this tributary and the frequent sediment dredging activities in the downstream, resulting in the spiking of heavy metals. There was no significant difference in the spatial distribution of individual heavy metals along the river (Fig. 4f). Water levels of As and Cu in February 2015 were significantly higher than those in other three periods and exceed the limit of the Water Quality Standard for Fisheries of China (GB11607-89) (Fig. 4a, d). The average water concentration of heavy metals followed the order Cu > As > Zn > Hg > Cd > Pb. It is noteworthy that the concentration of Hg consistently failed to meet the Water Quality Standard for Fisheries of China, but Pb was not detected at any of the sampling sites.
Spatial and temporal distribution of individual heavy metals in surface water. Dash line indicates the limit of the water quality standard for fisheries in China (GB11607-89). Asterisk in graph e indicates that the contents of Zn in the sites of S2, S3, and S4 were not checked out, below the limit of detection. Each asterisk represents one data
Discussion
We studied the distribution of heavy metals in surface water and fish tissues in the upper Mekong River and elucidated the potential dietary health risks to local residents from fish consumption. The accumulation of heavy metals in fish had a tissue-dependent distribution pattern, whereas the highest Cu and As levels were found in the liver, the highest Zn and Pb levels occurred in the intestine, and the highest Hg level was found in the muscle. Most importantly, the data showed that there are risks to local residents exposed to high levels of As due to long-term fish consumption. Previous studies have documented the accumulation of heavy metals in the Yangtze River (Yi and Zhang 2012), Yellow River (Lü et al. 2011), and Pearl River (Cheng et al. 2013), but this is the first study to determine heavy metal levels, their distribution in fish, and their health risk for local residents of the upper Mekong River. The Mekong River basin has a very large human population (> 100 million), and there are high levels of heavy metals in the water, soil, and vegetable food of this area (Fu et al. 2012; Phan et al. 2013a, b). Our results highlight the environmental risks of heavy metals in the region and their implication for human health.
Different fish organs possess variable capacity for storage and metabolism of heavy metals from aquatic environment due to the different migration ability of metals in the organ functions (Ashraf 2005). We found that the liver and intestine were likely to accumulate more heavy metals (Cu, As, Cd, and Pb) than the gill and muscle (Table 1), which is consistent with the previous study of Begum et al. 2013. The liver and intestine are the major organs for food digestion, and this may lead to greater heavy metal concentrations (Farkas et al. 2003; Chowdhury et al. 2005). Relatively higher concentrations of heavy metals in the liver and intestine could stem from exposure to bottom food and sediment, because C. carpio is a demersal fish, obtaining food in the sediment environment (Bordajandi et al. 2003; Yi et al. 2008). High background levels of Cu, As, Cd, and Pb in the sediments occur in the upper Mekong River basin (Fu et al. 2012; Liu et al. 2014), and these metals may transfer into fish via the zoobenthic food chain (Begum et al. 2013). In addition to the liver and intestine, the concentration of heavy metals in fish muscles was relatively low and stable, suggesting that the upper Mekong River may be exposed to long-term heavy metal contamination (Gbem et al. 2001). We found that a high As content occurred in fish muscle suggested that fish can accumulate and store a large amount of As from the aquatic environment. This has been confirmed by the higher As content in the water (Castro-Gonzáleza and Méndez-Armenta 2008). To clarify the As resources, it is also necessary to simultaneously measure the metal concentrations in sediment and foods (Cheng et al. 2013).
The THQ-based assessment method has been successfully used to estimate heavy metal health risks for local residents (Chien et al. 2002; Wang et al. 2005; Yi et al. 2011). In the Yangtze River basin, the total THQ value was estimated at 1.13. This value is lower than the THQ value of the present work, and the Pb, Cd, and Hg were the three greatest contributors to the total THQ (Yi et al. 2011). We found that heavy metal accumulation in fish muscle resulted in high total THQ values that ranged from 1.205 to 1.933 (Table 3). This suggests that residents may already be experiencing adverse health effects from the heavy metal contamination. The single THQ value of As exceeds one in three of the reservoirs (1.634, 1.126, and 1.443 for S1, S2, and S4, respectively), indicating that the primary component of health risks is As. There is no quality standard limit for total arsenic in fish tissues, but the negative health effects of arsenic exposure are of great concern (Islam et al. 2015). Numerous studies have been conducted to assess the accumulation of As in human beings through food consumption and its effects on human health including melanosis, leuco-melanosis, keratosis, dorsum, gangrene, and skin cancer (Sharma et al. 2014; Vilizzi and Tarkan 2016). The potential adverse health effect of As exposure is the highest in the present work. This may be ascribed to its lower oral reference dose because the toxicity of As depends on the percentage of the inorganic As and the activity of arsenic in food (Sevcikova et al. 2011; Cheng et al. 2013; Saha and Zaman 2013). Higher As contamination in groundwater and surface soil of agriculture fields in the Mekong Delta in Vietnam (Huang et al. 2016) and higher health risk of As from daily food consumption (rice, vegetable, and fish) in the lower Mekong River basin of Cambodia have been reported (Phan et al. 2013b). These findings indicate the potential health threat from As pollution in the entire Mekong River basin. Our results also highlight the potential health risk of As from long-term fish consumption by residents in the upper Mekong River basin.
There is limited information about the effects of cascade dams on the migration, transport, and transformation of heavy metals in the water and fish tissues along the river (Müller et al. 2008; Käiro et al. 2011). The construction of cascade dams in the upper Mekong River disrupted the original migration and accumulation pattern of heavy metals in the river ecosystems (Fu et al. 2012; Liu et al. 2014), which provides an opportunity to study the effects and mechanism of the new pattern. We found a gradual decline of heavy metals in the water from upstream to downstream indicating that cascade dams interfere with the downstream migration of heavy metals. The step-down decline effects of cascade dams on heavy metals in the water were obvious, especially with Pb, Cu, and Zn (Fig. 4a), which are easily absorbed by suspended solids or deposited in sediments (Tealdi et al. 2011). This may be attributed to the dams blocking most of sediments, which then settle down in the front of dams. This significantly decreases the total amounts of heavy metals in the water downstream (Wei et al. 2009; Zhao et al. 2013). Previous research has documented that heavy metal (Pb, As, and Cd) concentrations were reduced in water as it moved down into the lower reaches of Lancang River due to the trapping effects of one large reservoir (Song et al. 2013; Wang et al. 2012). However, we found no decrease in heavy metals in fish muscles suggesting that the cascade dams provide less interference with the transformation and accumulation of heavy metals in the fish body. It is possible that a large amount of the heavy metals associated with sediments has been deposited into the deep bottoms of the reservoirs. These heavy metal deposits cannot be directly absorbed by fish gills or enter fish via the food chain (Gupta et al. 2009).
Conclusion
This study documents heavy metal distribution in the water and in C. carpio fish tissues in a section of the upper Mekong River under the influence of cascade dams. We found that the accumulation of heavy metals in fish exhibited a tissue-dependent distribution pattern, whereas the highest Cu and As were detected in the liver, the highest Zn and Pb were measured in the intestine, and the highest Hg was found in the muscle. In terms of health risk, the total target hazard quotient value for residents ranged from 1.205 to 1.933, suggesting high potential health risk of local residents due to long-term fish consumption in the upper Mekong River. To clarify the impact of cascade dams on heavy metal accumulation and migration in the river system, it will be necessary to also measure the metal concentrations on sediment simultaneously even it is not included as a part of this study. Regular and long-term monitoring of heavy metal pollution in the aquatic ecosystem of the upper Mekong River is highly recommended, and a variety of fish species should be sampled to provide a comprehensive health risk assessment.
References
Alhashemi AH, Karbassi A, Kiabi BH, Monavari SM, Sekhavatjou MS (2012) Bioaccumulation of trace elements in different tissues of three commonly available fish species regarding their gender, gonadosomatic index, and condition factor in a wetland ecosystem. Environ Monit Assess 184:1865–1878. https://doi.org/10.1007/s10661-011-2085-8
Arnot JA, Gobas FA (2006) A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14:257–297. https://doi.org/10.1139/a06-005
Ashraf W (2005) Accumulation of heavy metals in kidney and heart tissues of Epinephelus microdon fish from the Arabian Gulf. Environ Monit Assess 101:311–316. https://doi.org/10.1007/s10661-005-0298-4
Begum A, Mustafa AI, Amin MN, Chowdhury TR, Quraishi SB, Banu N (2013) Levels of heavy metals in tissues of shingi fish (Heteropneustes fossilis) from Buriganga River, Bangladesh. Environ Monit Assess 185:5461–5469. https://doi.org/10.1007/s10661-012-2959-4
Bibi M, Hashmi MZ, Malik RN (2016) The level and distribution of heavy metals and changes in oxidative stress indices in humans from Lahore district, Pakistan. Hum Exp Toxicol 35:78–90. https://doi.org/10.1177/0960327115578063
Bordajandi LR, Gómez G, Fernández MA, Abad E, Rivera J, González MJ (2003) Study on PCBs, PCDD/Fs, organochlorine pesticides, heavy metals and arsenic content in freshwater fish species from the River Turia (Spain). Chemosphere 53:163–171. https://doi.org/10.1016/s0045-6535(03)00417-x
Castro-Gonzáleza MI, Méndez-Armenta M (2008) Heavy metals: implications associated to fish consumption. Environ Toxicol Pharmacol 26:263–271. https://doi.org/10.1016/j.etap.2008.06.001
Cenci RM, Martin JM (2004) Concentration and fate of trace metals in Mekong River Delta. Sci Total Environ 332:167–182. https://doi.org/10.1016/j.scitotenv.2004.01.018
Chanpiwat P, Sthiannopkao S, Widmer K, Himeno S, Miyataka H, Vu N, Tran V, Pham T (2016) Assessment of metal and bacterial contamination in cultivated fish and impact on human health for residents living in the Mekong Delta. Chemosphere 163:342–350. https://doi.org/10.1016/j.chemosphere.2016.08.003
Chen SQ, Chen B, Su MR (2011) An estimation of ecological risk after dam construction in LRGR, China: changes on heavy metal pollution and plant distribution. Procedia Environ Sci 5:153–159. https://doi.org/10.1016/j.proenv.2011.03.061
Cheng Z, Chen KC, Li KB, Nie XP, Wu SC, Wong CKC, Wong MH (2013) Arsenic contamination in the freshwater fish ponds of Pearl River Delta: bioaccumulation and health risk assessment. Environ Sci Pollut Res 20:4484–4495. https://doi.org/10.1007/s11356-012-1382-2
Chien LC, Hung TC, Choang KY, Yeh CY, Meng PJ, Shieh MJ, Han BC (2002) Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci Total Environ 285:177–185. https://doi.org/10.1016/S0048-9697(01)00916-0
Chowdhury MJ, Baldisserotto B, Wood CM (2005) Tissue-specific cadmium and metallothionein levels in rainbow trout chronically acclimated to waterborne or dietary cadmium. Arch Environ Contam Toxicol 48:381–390. https://doi.org/10.1007/s00244-004-0068-2
Cui BS, Zhang QJ, Zhang KJ, Liu XH, Zhang HG (2011) Analyzing trophic transfer of heavy metals for food webs in the newly-formed wetlands of the Yellow River Delta, China. Environ Pollut 159:1297–1306. https://doi.org/10.1016/j.envpol.2011.01.024
Danabas D, Ural M (2012) Determination of metal (Cu, Zn, Se, Cr and Cd) levels in tissues of the Cyprinid fish, Capoeta trutta (Heckel, 1843) from different regions of Keban Dam Lake (Euphrates-Turkey). Bull Environ Contam Toxicol 89:455–460. https://doi.org/10.1007/s00128-012-0744-2
Dehn LA, Follmann EH, Thomas DL, Sheffield GG, Rosa C, Duffy LK, O’Hara TM (2006) Trophic relationships in an Arctic food web and implications for trace metal transfer. Sci Total Environ 362:103–123. https://doi.org/10.1016/j.scitotenv.2005.11.012
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Saf 113:145–151. https://doi.org/10.1016/j.ecoenv.2014.11.032
Dural M, Göksu MZL, Özak AA (2007) Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem 102:415–421. https://doi.org/10.1016/j.foodchem.2006.03.001
Farkas A, Salánki J, Specziár A (2003) Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res 37:959–964. https://doi.org/10.1007/s00244-004-0068-2
Fu K, Su B, He D, Lu X, Song J, Huang J (2012) Pollution assessment of heavy metals along the Mekong River and dam effects. J Geogr Sci 22:874–884. https://doi.org/10.1007/s11442-012-0969-3
Gbem TT, Balogun JK, Lawal FA, Annune PA (2001) Trace metal accumulation in Clarias gariepinus (Teugels) exposed to sublethal levels of tannery effluent. Sci Total Environ 271:1–9. https://doi.org/10.1016/S0048-9697(00)00773-7
Gupta A, Rai DK, Pandey RS, Sharma B (2009) Analysis of some heavy metals in the riverine water, sediments and fish from river Ganges at Allahabad. Environ Monit Assess 157:449–458. https://doi.org/10.1007/s10661-008-0547-4
Gupta VK, Singh S, Agrawal A, Siddiqi NJ, Sharma B (2015) Phytochemicals mediated remediation of neurotoxicity induced by heavy metals. Biochem Res Int 2015(534769):1–9. https://doi.org/10.1155/2015/534769
Hodson PV (1976) δ-Amino levulinic acid dehydratase activity of fish blood as an indicator of a harmful exposure to lead. J Fish Res Board Can 33:268–271. https://doi.org/10.1139/f76-036
Huang Y, Miyauchi K, Endo G, Don LD, Manh NC, Inoue C (2016) Arsenic contamination of groundwater and agricultural soil irrigated with the groundwater in Mekong Delta, Vietnam. Environ Earth Sci 75:1–7. https://doi.org/10.1007/S12665-016-5535-3
Islam MS, Ahmed MK, Habibullah-Al-Mamun M, Masunaga S (2015) Assessment of trace metals in fish species of urban rivers in Bangladesh and health implications. Environ Toxicol Pharmacol 39:347–357. https://doi.org/10.1016/j.etap.2014.12.009
Jezierska B, Witeska M (2006) The metal uptake and accumulation in fish living in polluted waters. In: Twardowska I, Allen HE, Häggblom MM, Stefaniak S (eds) Soil and Water Pollution Monitoring, Protection and Remediation. Springer, Netherlands, pp 107–114
Käiro K, Möls T, Timm H, Virro T, Järvekülg R (2011) The effect of damming on biological quality according to macroinvertebrates in some estonian streams, central-baltic Europe: a pilot study. River Res Appl 27:895–907. https://doi.org/10.1002/rra.1406
Karatas M (2008) Evaluation of heavy metals in fishes (Cyprinus carpio and Barbus plebejus) of Bedirkale Dam lake, Turkey. Asian J Chem 20:5741–5744
Larsson Å, Haux C, Sjöbeck M-L (1985) Fish physiology and metal pollution: results and experiences from laboratory and field studies. Ecotoxicol Environ Saf 9:250–281. https://doi.org/10.1016/0147-6513(85)90045-4
Liu SL, Wang C, Yang JJ, Zhao QH (2014) Assessing the heavy metal contamination of soils in the water-level fluctuation zone upstream and downstream of the Manwan Dam, Lancang River. J Soils Sediments 14:1147–1157. https://doi.org/10.1007/s11368-014-0855-y
Lü CW, He J, Fan QY, Xue HX (2011) Accumulation of heavy metals in wild commercial fish from the Baotou Urban Section of the Yellow River, China. Environ Earth Sci 62:679–696. https://doi.org/10.1007/s12665-010-0508-4
Mohan M, Deepa M, Ramasamy EV, Thomas AP (2012) Accumulation of mercury and other heavy metals in edible fishes of Cochin backwaters, Southwest India. Environ Monit Assess 184:4233–4245. https://doi.org/10.1007/s10661-011-2258-5
Müller B, Berg M, Yao ZP, Zhang XF, Wang D, Pfluger A (2008) How polluted is the Yangtze River? Water quality downstream from the Three Gorges Dam. Sci Total Environ 402:232–247. https://doi.org/10.1016/j.scitotenv.2008.04.049
Phan K, Phan S, Huoy L, Suy B, Wong MH, Hashim JH, Yasin MSM, Aljunid SM, Sthiannopkao S Kim K (2013a) Assessing mixed trace elements in groundwater and their health risk of residents living in the Mekong River basin of Cambodia. Environ Pollut 182:111–119. https://doi.org/10.1016/j.envpol.2013.07.002
Phan K, Sthiannopkao S, Heng S, Phan S, Huoy L, Wong MH, Kim KW (2013b) Arsenic contamination in the food chain and its risk assessment of populations residing in the Mekong River basin of Cambodia. J Hazard Mater 262:1064–1071. https://doi.org/10.1016/j.jhazmat.2012.07.005
Saha N, Zaman MR (2013) Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ Monit Assess 185:3867–3878. https://doi.org/10.1007/s10661-012-2835-2
Sevcikova M, Modra H, Slaninova A, Svobodova Z (2011) Metals as a cause of oxidative stress in fish: a review. Vet Med 56:537–546
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int 2014:640754. https://doi.org/10.1155/2014/640754
Song JY, Fu KD, Su B, Huang QS, Hunag JC, Zhang JL (2013) Spatial distribution of heavy metal concentrations and pollution assessment in the bed loads of the Lancang River System. J Geogr Sci 68:389–397 (in Chinese)
Storelli MM (2008) Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem Toxicol 46:2782–2788. https://doi.org/10.1016/j.fct.2008.05.011
Tealdi S, Camporeale C, Ridolfi L (2011) Modeling the impact of river damming on riparian vegetation. J Hydrol 396:302–312. https://doi.org/10.1016/j.jhydrol.2010.11.016
Ural M, Arca S, Örnekçi GN, Demirol F, Yüce S, Uysal K, Çiçek A, Köse E, Koçer MAT (2012) Metal accumulation in sediment, water, and freshwater fish in a Dam Lake. Toxicol Environ Chem 94:49–55. https://doi.org/10.1080/02772248.2011.633912
USEPA (2000) Risk-based concentration table. United States Environment Protection Agency, Washington, DC, Philadelphia
USEPA (2009) Risk-based concentration table. United States Environment Protection Agency, Washington, DC, Philadelphia
Uysal K (2011) Heavy metal in edible portions (muscle and skin) and other organs (gill, liver and intestine) of selected freshwater fish species. Int J Food Prop 14:280–286. https://doi.org/10.1080/10942910903176378
Uysal K, Emre Y, Köse E (2008) The determination of heavy metal accumulation ratios in muscle, skin and gills of some migratory fish species by inductively coupled plasma-optical emission spectrometry (ICP-OES) in Beymelek Lagoon (Antalya/Turkey). Microchem J 90:67–70. https://doi.org/10.1016/j.microc.2008.03.005
Uysal K, Köse E, Bülbül M, Dönmez M, Erdoğan Y, Koyun M, Ömeroğlu Ç, Özmal F (2009) The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey). Environ Monit Assess 157:355–362. https://doi.org/10.1007/s10661-008-0540-y
Vilizzi L, Tarkan AS (2016) Bioaccumulation of metals in common carp (Cyprinus carpio L.) from water bodies of Anatolia (Turkey): a review with implications for fisheries and human food consumption. Environ Monit Assess 188:243. https://doi.org/10.1007/s10661-016-5248-9
Wagner A, Boman J (2003) Biomonitoring of trace elements in muscle and liver tissue of freshwater fish. Spectrochim Acta B 58:2215–2226. https://doi.org/10.1016/j.sab.2003.05.003
Wang XL, Sato T, Xing BS, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37. https://doi.org/10.1016/j.scitotenv.2004.09.044
Wang C, Liu SL, Zhao QH, Deng L, Dong SK (2012) Spatial variation and contamination assessment of heavy metals in sediments in the Manwan Reservoir, Lancang River. Ecotoxicol Environ Saf 82:32–39. https://doi.org/10.1016/j.ecoenv.2012.05.006
Wei GL, Yang ZF, Cui BS, Li B, Chen H, Bai JH, Dong SK (2009) Impact of dam construction on water quality and water self-purification capacity of the Lancang River, China. Water Resour Manag 23:1763–1780. https://doi.org/10.1007/s11269-008-9351-8
Xie WP, Chen KC, Zhu XP, Nie XP, Zhen GM, Pan DB, Wang SB (2010) Evaluation on heavy metal contents in water and fishes collected from the waterway in the Pearl River Delta, South China. J Agro-Environ Sci 29:1917–1923 (in Chinese)
Yi YJ, Zhang SH (2012) Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ Sci Pollut Res 19:3989–3996. https://doi.org/10.1007/s11356-012-0840-1
Yi YJ, Wang ZY, Zhang K, Yu G, Duan XH (2008) Sediment pollution and its effect on fish through food chain in the Yangtze River. Int J Sediment Res 23:338–347. https://doi.org/10.1016/S1001-6279(09)60005-6
Yi YJ, Yang ZF, Zhang SH (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585. https://doi.org/10.1016/j.envpol.2011.06.011
Yu Y, Wang YC, Zhou HD, Gao B, Zhao GF (2013) Pollution characteristics and health risk assessment of heavy metals in carp (Cyprinus carpio) from the Three Gorges Reservoir after 175 m impoundment. Acta Sci Circumst 33:2012–2019 (in Chinese)
Zeng J, Yang LY, Wang X, Wang WX, Wu QLL (2012) Metal accumulation in fish from different zones of a large, shallow freshwater lake. Ecotoxicol Environ Saf 86:116–124. https://doi.org/10.1016/j.ecoenv.2012.09.003
Zhang Y, He D, Lu Y, Feng Y, Reznick J (2013) The influence of large dams building on resettlement in the Upper Mekong River. J Geogr Sci 23:947–957. https://doi.org/10.1007/s11442-013-1054-2
Zhang JL, Fu KD, Wang B, Chen LQ, Song JY, Su B (2014) Assessment of heavy metal pollution of bed sediment in the Lancang River. Prog Geogr 33:1136–1144 (in Chinese)
Zhao QH, Liu SL, Deng L, Yang ZF, Dong SK, Wang C, Zhang Z (2012) Spatio-temporal variation of heavy metals in fresh water after dam construction: a case study of the Manwan Reservoir, Lancang River. Environ Monit Assess 184:4253–4266. https://doi.org/10.1007/s10661-011-2260-y
Zhao QH, Liu SL, Deng L, Dong SK, Wang C (2013) Longitudinal distribution of heavy metals in sediments of a canyon reservoir in Southwest China due to dam construction. Environ Monit Assess 185:6101–6110. https://doi.org/10.1007/s10661-012-3010-5
Funding
This work has been financially supported by the National Natural Science of China (Grant Nos. 21567029, 41571032, 41561144012), Yunnan University Graduate Students Fund (YNU2016018), and Outstanding Doctoral Cultivation Fund of Yunnan University (Grant No. ynuy201405).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J.L., Fang, L., Song, J.Y. et al. Health risk assessment of heavy metals in Cyprinus carpio (Cyprinidae) from the upper Mekong River. Environ Sci Pollut Res 26, 9490–9499 (2019). https://doi.org/10.1007/s11356-019-04291-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04291-2