Abstract
This study investigated temporal heavy metal characteristics over the past 20 years and the spatial variation of heavy metal characteristics at the Manwan Reservoir tail, center, and head in the Lancang River, Yunnan Province, China. The following heavy metals were selected for analysis: Hg, Cd, Pb, Cu, Zn, As, Cr, Fe, and Mn. Multivariate statistical methods, including principal component analysis (PCA) and the Pearson correlation matrix were used to distinguish the main pollution factors and sources before and after dam construction. The results indicate that the average annual values of heavy metals were far lower than the recommended values of the surface water quality standards in China with the exception of Hg. The concentration of metals showed considerable variation by season, where higher levels of metals generally occurred in high flow periods. The results from PCA show that three significant components were extracted, explaining 76.25% of the total variance. The ANOVA results indicate that Pb, Fe, Mn, and Cr had significant spatial variation (P < 0.05) at the reservoir tail, center and head. The enrichment factor (EF) used to compare the accumulation effect of heavy metals indicated that there was no enrichment (EF < 1) of heavy metals with the exception of Mn, which showed minor enrichment (1 < EF < 3) between the reservoir center and head. The spatiotemporal variation and the accumulation effects of water heavy metals were not distinct due to the construction of the Manwan Reservoir.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dams are constructed for diverse purposes and are significantly important to human economic development (e.g., hydropower generation, seasonal flood control, irrigation and drinking water supplies, recreation, and navigation). Approximately 70% of the world’s rivers are intercepted by large reservoirs and 2.2% of the world’s primary energy is generated by hydropower (Kummu and Varis 2007). Although dams provide various economic benefits, they are also considered to have negative impacts on the natural environment (World Commission on Dams 2000; Coker 2003; Bombino et al. 2006). Impoundments can change not only the hydrologic but also the chemical, biological, and physical characteristics of a water body, alter the basin connectivity, and modify the thermal, hydrologic, and sediment regimes (Ligon et al. 1995; Poff and Hart 2002; Hu et al. 2008). For instance, due to the construction of dams and reservoirs, the water renewal time of rivers has significantly increased from 20 to 100 days (Kummu and Varis 2007), and the changes in flow patterns and water temperature potentially changed the spawning habitat of fish (Tiffan et al. 2002). These changes pose large negative impacts on the aquatic and riparian ecosystems in both the upstream and downstream areas of a dam. Therefore, dam construction is a major factor contributing to significant modifications of river ecosystems (He et al. 2004).
Heavy metals, or special groups of contaminants in water reservoirs, influence environmental quality by accumulating in reservoirs and lead to serious human health hazards and significant ecological effects throughout the food chains (Loska and Wiechuła 2003). Research on heavy metals has led to increased concerns in recent years (Li and Zhang 2010) and the preservation of highly interesting records that have mainly focused on ecosystems such as forests (Zhou et al. 2010), grasslands (Van Vliet et al. 2005), wetlands (Barba-Brioso et al. 2010), agricultures (Huang et al. 2007), and so on. Heavy metals are introduced naturally in water bodies through the weathering of rocks, soil erosion, or the dissolution of water-soluble salts in addition to a variety of human activities (Shine et al. 1995; Kidd et al. 2007). The natural occurrence of heavy metals in aquatic environments, movement of heavy metals through the hydrologic cycle, and the inputs from anthropogenic activity reflect ubiquity and complexity of heavy metals (Idris 2008).
Human disturbances in river ecosystems have induced increasing concerns over the last few decades (Bockelmann et al. 2004). Salati and Moore (2010) demonstrated that municipal, industrial, and domestic discharges strongly affected heavy metal concentrations in the water and sediment of the Khoshk River. However, concentrations of toxicological concern were not found in the reservoirs of South Sri Lanka, which is well-known for its dam construction (Allinson et al. 2002). In addition, the results of Külahcı and Şen (2008) indicated that suspended clay particles in the Keban Dam Lake had a high capacity to absorb organic residues and metals from anthropogenic (e.g., sewage and industrial) and natural sources, thus keeping the level of dissolved metals reasonably low.
A number of research projects on the spatiotemporal variation of river water heavy metals have been conducted (Bu et al. 2010), such as studies of the Yangtze River (Chen et al. 2002, 2008; Müller et al. 2008) and Han River (Li and Zhang 2010) in China, the Khoshk River (Salati and Moore 2010) in Southwest Iran, the downstream area of the Mekong (Lancang) River (Buschmann et al. 2008) in Southeast Asia, the Waal River (Van Vliet et al. 2005) in the Netherlands, 12 important rivers in central–northern Chile (Pizarro et al. 2010), and Qattieneh Lake in Syria (Hassan et al. 2010). These investigations primarily focused on river reaches in rural, urban, and industrial areas, showing the impacts of land use and landscape pattern change (Paul et al. 2002), urbanization (Friese et al. 2010), and mining (Oyarzun et al. 2007; Pizarro et al. 2010), and were generally focused on global or regional scale (Krishna et al. 2009; Zhang et al. 2009; Paul et al. 2002; Pizarro et al. 2010). Very few studies have centered on river systems that were planned or constructed with cascade hydropower dams in which the concentrations of heavy metals might be affected by the formative reservoirs and prolonged water renewal time (Kummu and Varis 2007). Thus, it is important to investigate the intensity of pollution in reservoir water by inventorying the heavy metal concentrations and their spatiotemporal variation.
As the largest international river in Asia, the Lancang–Mekong River originates from the eastern Tibetan Plateau in China and has a total length of 4,880 km from the headwater to the mouth, entering the South China Sea at the southern part of Vietnam (Zhang et al. 2007). The maximum and minimum annual flows are 1,590 and 923 m3/s, respectively, and the annual variation of floods is relatively regular (Kite 2001; Fu et al. 2008), so the Lancang River has great potential as a source of hydropower. Cascade hydropower dams have been planned and some have already been constructed on the main stream of the Upper Mekong (Lancang) River. The development of cascade hydropower dams may alter the structure and function of river ecosystems, which has led to increased concerned in recent years.
With it completed and put into use in 1993, the Manwan Dam has posed substantial ecological effects on the reservoir and the area downstream from the reservoir. By 1996, 3 years after the Manwan Dam was completed, siltation had increased the elevation of the reservoir bottom to a level of 30 m higher than that before dam construction (He et al. 2004) and the surface water temperature in the reservoir was also higher than that before dam construction (Yao et al. 2006). Consequently, the negatives impacts of hydroplants have attracted great interest from researchers and society as a whole (Chen et al. 2003; Feng et al. 2005, 2006; Maingi and Marsh 2002; Brainwood et al. 2004; Okadera et al. 2006), so it is necessary to investigate the water heavy metals of the Manwan Reservoir for revealing the effect of the Manwan Reservoir on water environment.
The purpose of this study is to describe the magnitude and spatiotemporal variation of metallic pollutants in the Manwan Reservoir in order to quantify the intensity of heavy metal pollution in comparison with the Environmental Qualitative Standards of Surface Water in China (B3838-2002 2002). This study also aims to reassess the impact of the Manwan Dam construction by referring to the historical change of heavy metal concentrations in the reservoir water.
Materials and methods
Study area
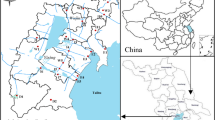
The Manwan Dam, located in the middle reach of the Lancang River in Yunnan Province, is the first dam of the Lancang mainstream cascade development project (Fig. 1), as well as the first multimillion kilowatt hydropower station in Yunnan Province (Zhao et al. 2000). The dam is 132 m high with a crest length of 418 m and a backwater of 70 km near the site of the Xiaowan Dam, with a total installed capacity of 150 × 104 kW. The Manwan project involved two stages. The installed capacity of the first stage is 125 × 104 kW and began to generate power in June 1993 (Wang and Zhang 2000; Zhao et al. 2000). The reservoir is located in the gorge area with high mountains and steep valleys. Most of the mountain peaks near the reservoir are higher than 2,200 m above sea level, and the mainstream valley gradient ratio is over 15‰. The reservoir area is 23.6 km2, which is 2.8 times larger than the area before dam construction. The reservoir water surface is on average 337.1 m wide, which is two times more than the water surface in the natural channel. When stored water reaches the normal pool level of 994 m, the total reservoir capacity is 1,060 × 106 m3, with as effective capacity of 257 × 106 m3, although this is dependent upon seasonal discharge regulation (He and Tang 2000; Wang and Zhang 2000).
Sampling and analytical methods
Data on the temporal characteristic of water heavy metals from 1989 to 2005 were collected from the Gajiu hydrometric station, which is located at the bank of the Lancang River about 2 km downstream from the dam site (Fig. 1). The water heavy metals data collected at this station reflect changes in the Manwan Reservoir before and after dam construction. The time series were divided into three periods, where 1989–1992 represents the period before the Manwan Dam was created, 1993–2001 represents the period after the Manwan Dam was created but before the Xiaowan Dam was constructed (located upstream of Manwan Dam, with its construction possibly affecting the water quality of Manwan Reservoir), and 2002–2005 represents the period after both the Manwan and Xiaowan Dams were created.
To investigate the spatial variation of water heavy metals, the data were collected in June 2010. A total of 17 cross-sectional samples were set for the spatial analysis (Fig. 1), in which the first four cross-sectional samples (S1–S4) were chosen to reveal changes at the reservoir tail; the next six cross-sectional samples (S5–S10) were located at the reservoir center (S6–S9 were located at the major tributaries of Manwan Reservoir); and the last seven cross-sectional samples (S11–S17) were located at the reservoir head (S12–S15 were located at the major tributaries of Manwan Reservoir) near Manwan Dam. Each sample in a cross-section was 300 ml of a mixture of three collections taken from the left, middle, and right cross-sections of the reservoir. Samples were immediately filtered in situ through 0.45 μm pore size and 47 mm diameter acid-washed millipore nitrocellulose membrane filters. Before being transferred to the clean polyethylene bottles, filtrates for analyses were acidified with ultra-pure concentrated 6 M nitric acid to adjust the pH (<2) and prevent the absorption of metals in the walls of the bottles, and stored at 4°C in clean high-density polyethylene bottles until treatment and analysis. Bottles were cleaned in a two-step preliminary treatment procedure including washing in a neutral detergent and a 24 h bath in a 10% nitric acid solution. Between the two steps and at the end of the procedure, the bottles were rinsed with distilled, deionised water. After samples were transported to the laboratory, the Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn concentrations were investigated through inductively coupled plasma-mass spectrometry (IRIS Advantages, USA). As and Hg were determined using atomic fluorescence spectrum with a standard addition method. The detection limits of Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn, As, and Hg were 0.2, 2, 1, 2, 0.5, 0.6, 3, 2, 0.5, and 0.05 μg/L, respectively. The relative errors for the analysis of standard solution were less than 5%. Data below the analytical detection limits were set to a value of half of the detection limit to construct all plots and conduct statistical calculations.
Statistical analyses
Evaluation of reservoir contamination sources and identification of temporal variation in water heavy metals were carried out through principal component analysis (PCA) to find and interpret the structure of the underlying data set. This was done through a reduced set of orthogonal (noncorrelated) variables (principal components, PCs) arranged in decreasing order of importance, retaining the PCs accounting for approximately 75% of the total variance and with an eigenvalue >1 (Singh et al. 2004). Kaiser–Meyer–Olkin (KMO) and Bartlett’s sphericity tests were conducted to examine the suitability of the data. Heavy metal concentrations at different sites were subjected to an analysis of variance (ANOVA) in order to classify and test for significant differences (P < 0.05, least significance difference). Relationships among the considered heavy metal variables were tested using Pearson’s coefficient as a nonparametric measure with statistical significance set at P < 0.05 (Li et al. 2008a; Alkarkhi et al. 2008). All data analyses were performed using SPSS 15.0.
Enrichment factor
Comparisons of water heavy metal concentrations among the reservoir tail, center, and head may give us a better understanding of enrichment levels due to dam construction. The concept of enrichment factor (EF) was developed in the 1970s to evaluate the anthropogenic contribution. The EF was calculated using the following equation (Abrahim and Parker 2008):
where, the numerator and denominator is the ratio of the concentration of the examined element (C EE) to the reference element (C RE) in the sample and reference material, respectively. A reference element is often a conservative one, such as Al, Fe, or Mn, as these are the most abundant elements on the earth, moreover, these elements are commonly used as reference element in the assessment of EF (Bergamaschi et al. 2002; Mishra et al. 2004; Han et al. 2006). In this study, Fe was used as the reference element because it mainly originates from natural lithogenic sources and its distribution is not related to other heavy metals (Deely and Fergusson 1994; Ravichandran et al. 1995; Samara and Vousta 2005; Abrahim and Parker 2008).
Results and discussion
Temporal variation of heavy metals before and after dam construction
Annual variation of heavy metals in the Manwan Reservoir
The average concentrations of heavy metals recorded during 1989–2005 are shown in Fig. 2, in comparison to the three grade levels (I, II, and III) of the Environmental Qualitative Standards of Surface Water in China (GB3838-2002 2002). Different metals showed different temporal variations. The concentration ranges (mean value) of Hg, Cd, Pb, Cu, Zn, and As in reservoir water from 1989 to 2005 were 0–0.2, 0–3.9, 11–32, 0–80, 0–706, 5.3–21 μg/L, respectively. The concentrations of Hg, Pb, Cu, and Zn showed great changes during 1992–1997, which may be related to dam construction and impoundment of the reservoir as well as the industrial sewage discharged from the upstream lead/zinc mine (located in Lanping and Dali; Zhang et al. 2005). Various types of mercury compounds were absorbed by suspended solid materials deposited in the bottom sediment as suspended solids and lead compounds were the same as the mercury compounds, primarily accumulating in the sediments a result of their insolubility and easy absorption characteristics (Zhang et al. 2005; Salati and Moore 2010). Hg, Pb, and other metal concentrations accumulated continually after impoundment of the Manwan Reservoir, which induced an increase in water depth and a decrease in the flow rate. The annual variation of Cd concentrations presented an increasing trend from 1997 to 2004 because Cd was suspended as a composition of solid material (Zhang et al. 2005; Barba-Brioso et al. 2010), and the concentration of As fluctuated over time. The average concentration of Hg in 1989 was not detected, and the average concentrations during 1990–1992 increased, with average approximate concentrations of 0.1, 0.1, 0.2 μg/L, respectively, which were higher than or equal to the level of 0.1 μg/L for grade III, indicating that concentrations of Hg were at higher levels than before dam construction (Zhang et al. 2005). The concentration of Hg became higher in the 1994–1997 period, with concentrations exceeding 0.1 μg/L. The average concentrations declined during 1998–2005, with concentration ranges of 0–0.04 μg/L, which were lower than the level of 0.05 μg/L for grade I. Although concentrations of Hg were low during 1998–2005, it is notable that the maximum value was 0.04 μg/L, which approximates to 0.05 μg/L. The results indicate that Cd concentrations were not detected in the pre-dam 1989–1992 period or the first 2 years after dam construction (1993–2001), but became severe during 1995–2004 with concentrations of 39.33%, 40%, 16%, 26%, 29.33%, 11.33%, 39.33%, 52.67%, 57.33%, and 77.33%, all higher than the grade II level. The lowest contamination occurred in 2005, with a percentage of 0.8%, meeting the grade I level. For Pb, the worst scenario occurred in 1993, with a percentage of 64% at the grade III level. For Cu and Zn, the worst contamination occurred in 1992 and 1993, respectively, and the reasons may be related to the decreasing flow velocity (Yao et al. 2005). The results show that, Hg, Pb, and Cu were the major pollutants in the Manwan Reservoir before construction of dam (1989–1992), Cd and Pb were the major pollutants during the post-dam period (1993–2005), and Hg was severe in the post-dam period of 1993–2001 but minimal in post-dam period of 2002–2005.
Annual concentration of heavy metals (μg/L) from 1989 to 2005 and the limit value of the Environmental Qualitative Standards of Surface Water in China (GB3838-2002 2002)
Seasonal variation of heavy metals in the Manwan Reservoir
Figures 3, 4, and 5 present the seasonal variation trends of heavy metal concentrations (Hg, Cd, Pb, Cu, Zn, and As) in the Manwan Reservoir during the three periods of dam construction. The concentration of heavy metals showed considerable variation in different seasons.
The highest Hg concentration (0.16 μg/L) after construction of the first dam was observed during the low-flow season in 1993–2001 (Fig. 3). The Hg concentration was below the detection limit in 2002–2005, showing large variation before and after dam construction. The Cd concentration was below the detection limit during the pre-dam period of 1989–1992 as well as during the high flow and mean flow seasons, indicating that Cd accumulation increased after dam construction. The Pb concentration was the highest (26 μg/L) in the pre-dam period of 1989–1992. The maximum Cu concentration values in the Manwan Reservoir were found in the low-flow season (80.3 μg/L) of 1993–2001, while Cu concentrations were at 17 and 0.5 μg/L during the pre-dam 1989–1992 and post-dam 2002–2005 periods, respectively. The Zn concentration was below the detection limit during the 1989–1992 period, increasing to a higher level that stabilized and after dam construction. During the low-flow season, the concentration of As fluctuated over time.
During the high flow season (Fig. 4), the highest Hg concentration in the water was detected before dam construction, and that level was the highest (0.2 μg/L) in all flow seasons. The Hg concentration decreased to 0.11 and 0.01 μg/L after construction of dam, reflecting the self-purification of reservoir water. The Cd concentrations were similar to those during the low-flow season in the period of 1989–1992, and the highest Cd concentration was about 3.3 μg/L during the post-dam 2002–2005 period. The lowest Pb concentration in water was found in the high flow period during the post-dam period of 2002–2005 (2.4 μg/L), which was only 10% of that during the post-dam 1993–2001 period. The Cu concentrations were 35 and 32.6 μg/L in the pre-dam 1989–1992 and post-dam 2002–2005 periods, respectively, decreasing to as low as 9.2 μg/L during the post-dam period of 1993–2001. Similar to Hg, the highest concentration of Zn in the Manwan Reservoir occurred during the high flow season.
During the mean flow season of the pre-dam 1989–1992 and post-dam 2002–2005 periods (Fig. 5), the concentration of Hg was below the detection limit. During the post-dam period, Hg was only 0.069 μg/L, indicating there was a low concentration of Hg in the mean flow season. The concentration of Cd was 1.7 and 2 μg/L during the post-dam 1993–2001 and 2002–2005 periods, respectively, while it was below the detection limit during the pre-dam period of 1989–1992, showing an increasing trend of Cd concentration in the mean flow season after dam construction. Cu concentrations showed a decreasing trend before and after dam construction. The concentration of As changed slightly in the three flow seasons, with insignificant seasonal variation.
Generally, higher values of heavy metals occurred in the high flow period both before and after dam construction, which may be a result of the precipitation and discharge of surface water into the reservoir. During the high flow period, the concentration of Hg was no more than 0.01 μg/L during the post-dam 2002–2005 period, and Hg level was below the detection limit during the low and mean flow periods. Seasonal variation of Cd concentrations during the post-dam 2002–2005 and 1993–2001 periods reached 48.3% and 50.6%, respectively, levels were lower than those of other metals. The seasonal variation of Pb concentrations during the three periods reached 78.9%, 91.8%, and 198.0%. Before and after dam construction, the Cu concentrations varied from 17 to 35, 9.2 to 80.3, and 0.5 to 32.6 μg/L, with seasonal variation of 65.0%, 197.2%, and 276.7%, respectively. After dam construction, both the highest (83 μg/L) and lowest (12 μg/L) concentrations of Zn occurred in the post-dam 2002–2005 period. During the post-dam period of 1993–2001, there was insignificant seasonal variation between the highest and lowest concentration of Zn, amounting to 51 and 19 μg/L, respectively. The seasonal dynamics of As in the Manwan Reservoir water show that the concentration was much lower than the grade I level during the period before and after dam construction, with insignificant seasonal variation.
Major pollutants (Hg, Pb, Cu, and Zn) exhibited larger seasonal variation as compared with other elements (Fig. 3, 4, and 5). Pb, Cu, and Zn showed higher values in 1989–2005 and were under the permissible limits. However, Hg surpassed the maximum permissible limits, which represents an anthropogenic signature. Anthropogenic activities, such as mining activities and the use of agrochemicals in the upper stream, played an important role in the content of metals in the reservoir (Huang et al. 2007). Weathering was an important source of other metals in the water (Censi et al. 2006). The Cd and As content had very small variations in the reservoir because they derived primarily from the weathering of parent material and subsequent pedogenesis (Huang et al. 2007). For those metals, the acidification status of the soil in drainage areas was the most important factor regulating the transport process into the reservoir. Cd and As passed through the water body as essential elements but there was evidence of accumulation of trace levels in the reservoir after dam construction. The increasing concentration of heavy metals affected the quality of the reservoir water.
Correlation and PCA of temporal variation
Correlations between the heavy metals were analyzed to assess whether the metals came from similar sources. The Pearson correlation coefficients of heavy metals in water are summarized in Table 1. The results showed significantly positive correlation between Cu and Zn (P < 0.05), suggesting a natural origin and/or a shared contamination source for the two elements. The absence of strong correlations between other metals suggested that the concentrations of these metals were not controlled by a single factor but by a combination of geochemical support phases and their mixed associations (Jain et al. 2005). The possible sources of heavy metals were confirmed by the presence of a relation between heavy metal concentrations. For instance, Cd and As were negatively correlated with all other elements showing positive correlation only with each other. This indicated that Cd and As may have originated from the same source, whereas other elements were positively correlated, reflecting another source. The results in the present study indicated that most of the metals examined had no common sources, or in other words, the heavy metals have originated from various sources, so their behaviors during transport were like to vary.
In order to differentiate the possible origins of the measured elements in the current study (based on the correlation matrix of the variables shown in Table 1), PCA was used to evaluate the impact of dam construction on the Lancang River, focusing on the water quality through the measurement of Hg, Cd, Pb, Cu, Zn, and As concentrations from 1989 to 2005 (Table 2). Three main components with eigenvalues greater than 1 were determined (Kowalkowski et al. 2006), explaining 76.25% of the total variance. The first component, with a variance of 33.83%, was highly correlated with Cd, Cu, and Zn. This component can be called a “natural factor” because its metal concentrations seemed to be controlled by the parent rock composition (Censi et al. 2006; Huang et al. 2007). The second component explained 25.37% of the total variance with positive loadings on Pb and Zn, but negative loadings on Hg. Because of its high level presence in water, this component could be identified as an “anthropogenic factor”. Gasoline combustion, rubber tire wear, auto plants, electroplating industries, and domestic wastewater were probably the major sources of these metals (Neal et al. 2006), which was also reflected by their strong correlations and their high values in intense anthropogenic years (Fig. 2; Table 2). The third component, with a variance of 17.05%, included Pb, Cu, and As. Cu and Pb were greatly impacted by anthropogenic activities such as mining and agricultural processes (Li et al. 2009). As was divided almost equally between the three components, displaying an equal correlation with three groups and seemed to have both natural and anthropogenic sources. Therefore, this component could be attributed to mixed sources of anthropogenic activities, especially agriculture and mining in the basin and geogenic origins.
Many authors have utilized PCA to investigate heavy metals in the fluvial ecosystem, with varying results (e.g., Krishna et al. 2009; Pekey et al. 2004). For example, results have shown that Cd and Hg were primarily from agrochemicals, phosphate fertilizers and pesticides, while As, Cu, and Pb were from urban and industrial activities such as energy production, mining, metal smelting and refining, manufacturing processes, automobile exhausts, and waste incineration (Huang et al. 2007; Neal et al. 2006; Li et al. 2009). These discrepancies were attributed to different river environments and different water variables.
Spatial variation of heavy metals
Spatial variation of heavy metals in the Manwan Reservoir
The spatial variation of the heavy metal concentrations along the Manwan Reservoir is shown in Fig. 6. The mean values of metal concentrations can be arranged in order as Fe>Mn>Cr>Zn>Pb>Cu>Ni. The Cd, Hg, and As concentration values were below the detection limit at all sites. The maximum values for Fe and Mn in the reservoir were 249.39 and 50.29 μg/L, representing the highest levels, while the maximum values for Cu and Ni in the reservoir were 2.04 and 0.37 μg/L, with the lowest levels.
Average concentration of heavy metals in surface water from different sites of the Manwan Reservoir in 2010 (μg/L); a indicates there is no significant difference among the reservoir tail, center, and head; b indicates a significant difference at the 0.05 level; c indicates a significant difference at the 0.01 level
The levels of Fe, Mn, Ni, Pb, and Zn in the Manwan Reservoir showed clear spatial variation from the reservoir tail to the head. The peak values for Fe, Mn, Ni, Pb, and Zn were very similar, with high concentrations observed at the reservoir center and the lowest concentrations observed at the reservoir tail (Fig. 6). There was a distinct peak directly after the confluence of the Mangshuai, Sanjiacun and Jingfang Rivers at the reservoir center. Concentrations downstream from the confluence to the reservoir head decreased but were distinctively higher than those at the reservoir tail. The Fe, Mn, and Pb levels were significantly different (P < 0.01) at the reservoir tail and center. The results of the heavy metal concentrations in the reservoir tail showed that construction of the Xiaowan Dam did not significantly affect the water quality of the reservoir. However, the concentrations of heavy metals increased in the reservoir center, which could be related to the metals contribution of tributaries at reservoir center. Pollution of the aquatic environment directly or indirectly by human has resulted in an impairment of water quality with respect to industrial, agricultural, and recreational activities (Salati and Moore 2010). Some scholars have reported that the concentration of Ni was about 0.3 μg/L in rivers (Yang et al. 2002) and related the increment of Ni to sewage effluents, whereas the level was about 1.46 μg/L in agricultural zones (Markich and Brown 1998; Cenci and Martin 2004). The lowest and mean concentrations of Ni in the Manwan Reservoir and its tributaries were 0.1 and 0.4 μg/L, respectively, indicating that sewage effluents might be the pollution source for the Manwan Reservoir. In addition, there were no significant differences in Ni among the reservoir tail, center, and head. The highest concentration of Pb at the reservoir center was about 1.42 times higher than that at the reservoir tail. The trend of Zn was similar to that of Pb, with high concentrations observed at the reservoir center. Zn and Pb were consistent with nonpoint and point sources in the different cross-sections and its tributaries, but there were no significant differences among the three sections of the Manwan Reservoir.
The highest value of Cr was observed at the reservoir head, where the reservoir dock and fishing ground were found, the domestic sewage and bait were discharged directly into the reservoir, and the ships were operated and gas emitted. Consequently, there were significant differences (P < 0.05) between the reservoir head and tail. However, high concentrations of Cr in the whole reservoir were probably due to two causes: a naturally high concentration of Cr in soils or bearing minerals such as cinnabar soil, pyroxenes, and silicates (He et al. 2004), and anthropogenic activities such as fish-farming, deforestation and land reclamation, or pesticides used in the agricultural fields around the reservoir (He et al. 2004; Fu et al. 2008). The highest Cu value was observed at the reservoir tail, which is situated downstream of a cement plant and is the area where the industrial wastewater was discharged. Nevertheless, there were no significant differences in the levels at the reservoir tail, center, and head. The high Fe and Mn concentrations in the Manwan Reservoir were not unexpected because the composition of the fluvial ecosystem is predominantly controlled by lithogenic influences from the source areas (Facetti et al. 1998). This is also because iron and manganese oxides are among the major mineral components of suspended matter, exerting a particularly important role in the sorption reactions of trace metals (Renoldi et al. 1997; Külahcı and Şen 2008; Song et al. 2010). Therefore, the presence of Fe and Mn in the Manwan Reservoir was probably involved in the transport and water self-purification processes of metals.
Variation of heavy metals in the main stream and tributaries of the Manwan Reservoir
According to previous researchers (Jian et al. 2005, 2006; Li et al. 2008b; Song et al. 2010), tributaries carry a lot of heavy metals and import pollutants into the main stream and it is noteworthy that the heavy metal contamination levels of tributaries are often very high. This is further supported by different contamination levels of heavy metals between the tributaries and main stream of the Manwan Reservoir shown in Figs. 7 and 8. The statistical comparison of the main stream and tributaries using one-way ANOVA showed no statistically significant differences (P > 0.05) in Cr, Ni, and Pb at the reservoir center (Fig. 7) or in Cr, Fe, Ni, and Zn at the reservoir head (Fig. 8), indicating that the main stream and tributaries contributed equally to the metals concentrations. The average concentration of Cu showed a statistically significant difference between the main stream and tributaries (P < 0.01). The high level of Cu in the main stream could be related to the construction of Xiaowan hydropower station, which is located upstream of the Manwan Reservoir, the exploitation of the Heihui River (a main tributary of Lancang River which is known as the “industrial corridor”), or the development of counties along the main stream, such as Yun, Fengqing, and Nanjiang. Fe and Zn had high values in the main stream and Mn had high values in the tributaries, exhibiting statistically significant differences (P < 0.05) at the reservoir center, indicating that the main stream contributed more to Fe and Zn, whereas the tributaries contributed more to Mn. In the head of the Manwan Reservoir (Fig. 8), the average concentration of Pb (P < 0.05) in the main stream was higher than that in the tributaries, while the average concentration of Mn (P < 0.05) in the main stream was lower than that in the tributaries. Average concentrations of Cr between the main stream and tributaries were not significantly different at the reservoir center or head, indicating that Cr concentrations in the reservoir were probably related to a high concentration in soils or parent matters, which are natural factors (He et al. 2004). In the main stream and tributaries of the reservoir center and head, significant differences in Mn could be caused by soil erosion and other anthropogenic inputs (Külahcı and Şen 2008; Song et al. 2010). Impacts of the tributaries on the concentration of heavy metals in the Manwan Reservoir can be seen obviously through above analyses.
Average concentration of water heavy metals from the stream order of the Manwan Reservoir center in 2010 (μg/L); a indicates there is no significant difference among the reservoir tail, center, and head; b indicates a significant difference at the 0.05 level; c indicates a significant difference at the 0.01 level
Average concentration of water heavy metals from the stream order of Manwan Reservoir head in 2010 (μg/L); a indicates there is no significant difference among the reservoir tail, center, and head; b indicates a significant difference at the 0.05 level; c indicates a significant difference at the 0.01 level
Enrichment factor analysis
In this study, a normalized enrichment factor for metal concentrations above uncontaminated background levels (Abrahim and Parker 2008) was calculated for the Manwan Reservoir. However, there is a lake of background concentrations for the tested metals in the study area, so the water heavy metal values at the reservoir tail were taken as the reference values to calculate the enrichment factors for the reservoir center and head based on the geographic location. The EF values were interpreted as the levels of heavy metal pollution suggested by Birch (2003) and the assessment criteria were generally based on the EF values. According to Birch suggestion, we considered that EF < 1 as no enrichment, 1 < EF < 3 as minor enrichment, and 3 < EF < 5 as moderate enrichment.
The EF values are shown in Fig. 9. The enrichment of different elements at the reservoir center was ordered as Mn>Zn>Pb>Ni>Cr>Cu and the enrichment of different elements at the reservoir head was ordered as Mn>Pb>Cr>Zn>Cu>Ni. It is clear that there were different enrichment series between the reservoir center and head, but there was a similarity of the enrichment series in that the largest EF value at both sites was for Mn with an EF value of 2 (minor enrichment) and 2.46 (minor enrichment) at the reservoir center and head, respectively. This indicated that Mn was the most enriched element in the water tested. Therefore, the transport processes of Mn and tributary discharges were probably the cause of high EF values. However, other heavy metals with EF values lower than 1 at both the reservoir center and head fall into the non-enriched group of elements in the study area. This indicated that their sources might be upstream of the reservoir without any additional inputs or that they were insignificantly affected by tributary discharges. The difference in spatial enrichment also reflected the difference in water self-purification processes for heavy metals at different sections of the Manwan Reservoir.
Conclusions
From this study, several major conclusions can be drawn.
The annual average heavy metal values were far lower than the limit value of the Environmental Qualitative Standards of Surface Water in China (GB3838-2002 2002), except for Hg which was higher than the limit value in some years. Therefore, the heavy metals were not the major problem of heavy metal pollution in the Manwan Reservoir from 1989 to 2005. The concentration of heavy metals showed considerable variation in different seasons. Higher values for metals generally occurred in the high flow periods before and after dam construction. Hg, Pb, Cu, and Zn exhibited greater seasonal variation as compared with other elements. There was evidence of the accumulation of heavy metals in the reservoir after dam construction, and the increasing concentration of heavy metals led to decreased water quality. The PCA results show that Zn, Cu, and Cd (PC1) were related to the “natural factor”, Pb, Cu, and Hg (PC2) were considered “anthropogenic factors”, and Pb, Cu, and As were regarded as mixed sources of anthropogenic factors, especially agriculture and mining in the basin and geogenic origins.
The spatial variation of heavy metals in the Manwan Reservoir shows that the highest metal concentrations in water were at the reservoir center, whereas the lowest metal concentrations were detected at the cross-sections close to the dam site (i.e., Cu, Ni, and Zn). This pattern indicated the existence of settling processes, which transferred the heavy metals towards the sediment when they were carried across the Manwan Reservoir center. Therefore, the spatial variation of metals in the Manwan Reservoir may be due to the decreased flow velocity, which is closely related to the capability of water self-purification. The EF results indicated no other enriched element in the sampling water at the reservoir center and head as compared with the reservoir tail with the exception of Mn, which showed minor enrichment at the reservoir center and head, reflecting the presence of water self-purification processes in the Manwan Reservoir. It is necessary for future researchers to study heavy metal concentrations in sediments in order to characterize the water self-purification processes of the entire Manwan Reservoir and to restore the reservoir water and sediment qualities as well as its ecosystem functioning after dam construction.
References
Abrahim, G. M. S., & Parker, R. J. (2008). Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environmental Monitoring and Assessment, 136(1–3), 227–238.
Alkarkhi, F. M. A., Ismail, N., & Easa, A. M. (2008). Assessment of arsenic and heavy metal contents in cockles (Anadara granosa) using multivariate statistical techniques. Journal of Hazardous Materials, 150(3), 783–789.
Allinson, G., Nishikawa, M., De Silva, S. S., Laurenson, L. J. B., & De Silva, K. (2002). Observations on metal concentrations in tilapia (Oreochromis mossambicus) in reservoirs of South Sri Lanka. Ecotoxicology and Environmental Safety, 51(3), 197–202.
Barba-Brioso, C., Fernández-Caliani, J. C., Miras, A., Cornejo, J., & Galán, E. (2010). Multi-source water pollution in a highly anthropized wetland system associated with the estuary of Huelva (SW Spain). Marine Pollution Bulletin, 60(8), 1259–1269.
Bergamaschi, L., Rizzio, E., Valcuvia, M. G., Verza, G., Profumo, A., & Gallorini, M. (2002). Determination of trace elements and evaluation of their enrichment factors in Himalayan lichens. Ecological Engineering, 120(1), 137–144.
Birch, G. (2003). A scheme for assessing human impacts on coastal aquatic environments using sediments. In C. D. Woodcoffe, & R. A. Furness (Eds.), Coastal GIS 2003. Wollongong University Papers in Center for Maritime Policy, 14, Australia. (http://www.ozcoasts.org.au/indicators/).
Bockelmann, B. N., Fenrich, E. K., Lin, B., & Falconer, R. A. (2004). Development of an ecohydraulics model for stream and river restoration. Ecological Engineering, 22(4–5), 227–235.
Bombino, G., Tamburino, V., & Zimbone, S. M. (2006). Assessment of the effects of check-dams on riparian vegetation in the Mediterranean environment: a methodological approach and example application. Ecological Engineering, 27(2), 134–144.
Brainwood, M. A., Burgin, S., & Maheshwari, B. (2004). Temporal variations in water quality of farm dams: impacts of land use and water sources. Agricultural Water Management, 70(2), 151–175.
Bu, H. M., Tan, X., Li, S. Y., & Zhang, Q. F. (2010). Temporal and spatial variations of water quality in the Jinshui River of the South Qinling Mts., China. Ecotoxicology and Environmental Safety, 73(5), 907–913.
Buschmann, J., Berg, M., Stengel, C., Winkel, L., Sampson, M. L., Trang, P. T. K., et al. (2008). Contamination of drinking water resources in the Mekong delta floodplains: arsenic and other trace metals pose serious health risks to population. Environment International, 34(6), 756–764.
Cenci, R. M., & Martin, J. M. (2004). Concentration and fate of trace metals in Mekong River Delta. Science of the Total Environment, 332, 167–182.
Censi, P., Spoto, S. E., Saiano, F., Sprovieri, M., Mazzola, S., Nardone, G., et al. (2006). Heavy metals in coastal water systems. A case study from the northwestern Gulf of Thailand. Chemosphere, 64(7), 1167–1176.
Chen J., Wang, F., Xia, X., & Zhang, L. (2002). Major element chemistry of the Changjiang (Yangtze River). Chemical Geology, 187, 231–255.
Chen, L. H., Zeng, Z. G., & He, D. M. (2003). Coordinating the relationships between interest parties in development of the international river—A case study of Lancang-Mekong. World Regional Studies, 12(1), 71–78. (In Chinese).
Chen X., Yan, Y., Fu, R., Dou, X., & Zhang, E. (2008). Sediment transport from the Yangtze River, China, into the sea over the Post-Three Gorge Dam Period: A discussion. Quaternary International, 186, 55–64.
Coker, E. H. (2003). Conversion of a flood control system to a sustainable system: the energy requirements for pipeline transport of silt. Environmental Science & Technology, 34(17), 3730–3736.
Deely, J. M., & Fergusson, J. E. (1994). Heavy metal and organic matter concentrations and distributions in dated sediments of a small estuary adjacent to a small urban area. Science of the Total Environment, 153(1–2), 97–111.
Facetti, J., Dekov, V. M., & Van Grieken, R. (1998). Heavy metals in sediments from the Paraguay River: a preliminary study. Science of the Total Environment, 209(1), 79–86.
Feng, Y., He, D. M., & Gan, S. (2005). Analysis on the changes of water resource system of the Lancang River and its impacts to Great Mekong sub-regional cooperation. World Regional Studies, 14(4), 49–56 (in Chinese).
Feng, Y., He, D. M., Gan, S., & Gu, Y. (2006). Linkages of transboundary water allocation and its eco-thresholds with international laws. Chinese Science Bulletin, 51(Suppl), 25–32.
Friese, K., Schmidt, G., De Lena, J. C., Nalini, H. A., & Zachmann, D. W. (2010). Anthropogenic influence on the degradation of an urban lake—the Pampulha reservoir in Belo Horizonte, Minas Gerais, Brazil. Limnologica, 40(2), 114–125.
Fu, K. D., He, D. M., & Lu, X. X. (2008). Sedimentation in the Manwan reservoir in the Upper Mekong and its downstream impacts. Quaternary International, 186(1), 91–99.
GB3838-2002. (2002). National standard of the People’s Republic of China: Environmental quality standards for surface water. Beijing, China.
Han, Y. M., Du, P. X., Cao, J. J., & Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Science of the Total Environment, 355(1–3), 176–186.
Hassan, S., Schmieder, K., & Böcker, R. (2010). Spatial patterns of submerged macrophytes and heavy metals in the hypertrophic, contaminated, shallow reservoir Lake Qattieneh/Syria. Limnologica, 40(1), 54–60.
He, D. M., & Tang, Q. (2000). Chinese international rivers (pp. 165–175). Beijing: Science Press (in Chinese).
He, D. M., Zhao, W. J., & Chen, L. H. (2004). Ecological change in the Manwan reservoir area and its causes. Journal of Yunnan University, 26(3), 220–226 (in Chinese).
Hu, W. W., Wang, G. X., Deng, W., & Li, S. N. (2008). The influence of dams on ecohydrological conditions in the Huaihe River basin, China. Ecological Engineering, 33(3–4), 233–241.
Huang, S., Liao, Q. L., Hua, M., Wu, X. M., Bi, K. S., Yan, C. Y., et al. (2007). Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere, 67(11), 2148–2155.
Idris, A. M. (2008). Combining multivariate analysis and geochemical approaches for assessing heavy metal level in sediments from Sudanese harbors along the Red Sea coast. Microchemical Journal, 90(2), 159–163.
Jain, C. K., Singhal, D. C., & Sharma, M. K. (2005). Metal pollution assessment of sediment and water in the river Hindon, India. Environmental Monitoring and Assessment, 105(1–3), 193–207.
Jian, M. F., You, H., & Ni, C. Y. (2005). Characteristics of heavy metals contaminated sediments in Raohe River of Poyang Lake. Journal of Jiangxi Normal University (Natural Sciences Edition), 29(4), 363–366 (in Chinese).
Jian, M. F., You, H., & Ni, C. Y. (2006). Characteristics of heavy metals contaminant status and migration in Raohe River of Lake Poyang. Journal of Lake Sciences, 18(2), 127–133 (in Chinese).
Kidd, P. S., Dominguez-Rodriguez, M. J., Diez, J., & Monterroso, C. (2007). Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere, 66(8), 1458–1467.
Kite, G. (2001). Modelling the Mekong: hydrological simulation for environmental impact studies. Journal of Hydrology, 253(1–4), 1–13.
Kowalkowski, T., Zbytniewski, R., Szpejna, J., & Buszewski, B. (2006). Application of chemometrics in river water classification. Water Research, 40(4), 744–752.
Krishna, A. K., Satyanarayanan, M., & Govil, P. K. (2009). Assessment of heavy metal pollution in water using multivariate statistical techniques in an industrial area: a case study from Patancheru, Medak District, Andhra Pradesh, India. Journal of Hazardous Materials, 167(1–3), 366–373.
Külahcı, F., & Şen, Z. (2008). Multivariate statistical analyses of artificial radionuclides and heavy metals contaminations in deep mud of Keban Dam Lake, Turkey. Applied Radiation and Isotopes, 66(2), 236–246.
Kummu, M., & Varis, O. (2007). Sediment-related impacts due to upstream reservoir trapping, the Lower Mekong River. Geomorphology, 85(3–4), 275–293.
Li, S. Y., & Zhang, Q. F. (2010). Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. Journal of Hazardous Materials, 176(1–3), 579–588.
Li, S. Y., Gu, S., Liu, W. Z., Han, H. Y., & Zhang, Q. F. (2008). Water quality in relation to the land use and land cover in the Upper Han River basin, China. Catena, 75(2), 216–222.
Li, M., Wu, J. C., Zhang, X. L., & Zhou, X. J. (2008b). Assessment on heavy metal pollution at five estuaries of Poyang Lake. Journal of Nanchang University (Natural Sciences), 32(5), 483–485 (in Chinese).
Li, J. L., He, M., Han, W., & Gu, Y. F. (2009). Analysis and assessment on heavy metal sources in the coastal soils developed from alluvial deposits using multivariate statistical methods. Journal of Hazardous Materials, 164(2–3), 976–981.
Ligon, F. K., Dietrich, W. E., & Trush, W. J. (1995). Downstream ecological effects of dams. Bioscience, 45(3), 183–192.
Loska, K., & Wiechuła, D. (2003). Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere, 51(8), 723–733.
Maingi, J. K., & Marsh, S. E. (2002). Quantifying hydrologic impacts following dam construction along the Tana River, Kenya. Journal of Arid Environments, 50(1), 53–79.
Markich, S. J., & Brown, P. L. (1998). Relative importance of natural and anthropogenic influences on the fresh surface water chemistry of the Hawkesbury-Nepean River, south-eastern Australia. Science of the Total Environment, 217(3), 201–230.
Mishra, V. K., Kim, K. H., Kang, C. H., & Choi, K. C. (2004). Wintertime sources and distribution of airborne lead in Korea. Atmospheric Environment, 38(17), 2653–2664.
Müller, B., Berg, M., Yao, Z. P., Zhang, X. F., Wang, D., & Pfluger, A. (2008). How polluted is the Yangtze river. Water quality downstream from the Three Gorges Dam. Science of the Total Environment, 402, 232–247.
Neal, C., Neal, M., Hill, L., & Wickham, H. (2006). The water quality of the River Thames Basin of south/south-eastern England. Science of the Total Environment, 360(1–3), 254–271.
Okadera, T., Watanabe, M., & Xu, K. Q. (2006). Analysis of water demand and water pollutant discharge using a regional input–output table: an application to the City of Chongqing, upstream of the Three Gorges Dam in China. Ecological Economics, 58(2), 221–237.
Oyarzun, R., Oyarzún, J., Lillo, J., Maturana, H., & Higueras, P. (2007). Mineral deposits and Cu–Zn–As dispersion–contamination in stream sediments from the semiarid Coquimbo Region, Chile. Environmental Geology, 53(2), 283–294.
Paul, J. F., Comeleo, R. L., & Copeland, J. (2002). Landscape metrics and estuarine sediment contamination in the mid-Atlantic and Southern New England regions. Journal of Environmental Quality, 31(3), 836–845.
Pekey, H., Karaka, D., & Bakoglu, M. (2004). Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analyses. Marine Pollution Bulletin, 49, 809–818.
Pizarro, J., Vergara, P. M., Rodríguez, J. A., & Valenzuel, A. M. (2010). Heavy metals in northern Chilean rivers: spatial variation and temporal trends. Journal of Hazardous Materials, 181(1–3), 747–754.
Poff, N. L., & Hart, D. D. (2002). How dams vary and why it matters for the emerging science of dam removal. Bioscience, 52(8), 659–668.
Ravichandran, M., Baskaran, M., Santschi, P. H., & Bianchi, T. (1995). History of trace metal pollution in Sabine–Neches Estuary, Beaumont, Texas. Environmental Science & Technology, 29(6), 1495–1503.
Renoldi, M., Camusso, M., & Tartari, G. (1997). The highly polluted Lambro river (N. Italy): dissolved and solid transport of Cu, Cr and Fe. Water, Air, & Soil Pollution, 95(1–4), 99–118.
Salati, S., & Moore, F. (2010). Assessment of heavy metal concentration in the Khoshk River water and sediment, Shiraz, Southwest Iran. Environmental Monitoring and Assessment, 164(1–4), 677–689.
Samara, C., & Vousta, D. (2005). Size distribution of airborne particulate matter and associated heavy metals in the roadside environment. Chemosphere, 59(8), 1197–1206.
Shine, J. P., Ika, R. V., & Ford, T. E. (1995). Multivariate statistical examination of spatial and temporal patterns of heavy metal contamination in New Bedford Harbor marine sediments. Environmental Science & Technology, 29(7), 1781–1788.
Singh, K. P., Malik, A., Mohan, D., & Sinha, S. (2004). Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Research, 38(18), 3980–3992.
Song, Y. X., Ji, J. F., Mao, C. P., Yang, Z. F., Yuan, X. Y., Aokyo, G. A., et al. (2010). Heavy metal contamination in suspended solids of Changjiang River—environmental implications. Geoderma. doi:10.1016/j.geoderma.2010.07.020.
Tiffan, K. F., Garland, R. D., & Rondorf, D. W. (2002). Quantifying flow-dependent changes in subyearling fall Chinook salmon rearing habitat using two-dimensional spatially explicit modeling. North American Journal of Fisheries Management, 22(3), 713–726.
van Vliet, P. C. J., van der Zee, S. E. A. T. M., & Ma, W. C. (2005). Heavy metal concentrations in soil and earthworms in a flood plain grassland. Environmental Pollution, 138(3), 505–516.
Wang, Z. Z., & Zhang, X. M. (2000). Eco-environmental and ecological resources in Manwan Hydropower Station Reservoir, Yunnan (pp. 1–13). Kunming: Yunnan Science and Technology (in Chinese).
World Commission on Dams. (2000). Dams and development: a new framework for decision-making. London: Earthscan.
Yang, S. Y., Li, C. X., Jung, H. S., & Lee, H. J. (2002). Discrimination of geochemical compositions between the Changjiang and the Huanghe sediments and its application for the identification of sediment source in the Jiangsu coastal plain, China. Marine Geology, 186(3–4), 229–241.
Yao, W. K., Yang, Z. F., Liu, Z., Cui, B. S., Dong, S. K., & Liu, J. (2005). Spatio-temporal characteristics of Lancangjiang River water quality along middle reaches. Journal of Soil Water Conservation in China, 19(6), 148–152 (in Chinese).
Yao, W. K., Cui, B. S., Dong, S. K., & Liu, J. (2006). Spatio-temporal characteristics of Lancangjiang River water temperatures along the representative reaches disturbed by hydroelectric power projects. Acta Scientiae Circumstantiae, 26(6), 1031–1037 (in Chinese).
Zhang, Y. X., Liu, J. Q., & Wang, L. Q. (2005). Changes in water quality in the downstream of Lancang River after the construction of Manwan hydropower station. Resources and Environment in the Yangtze Basin, 14(4), 501–506 (in Chinese).
Zhang, M. K., Wang, L. P., & He, Z. L. (2007). Spatial and temporal variation of nitrogen exported by runoff from sandy agricultural soils. Journal of Environmental Sciences, 19(9), 1086–1092.
Zhang, W. G., Feng, H., Chang, J. N., Qu, J. G., Xie, H. X., & Yu, L. Z. (2009). Heavy metal contamination in surface sediments of Yangtze River intertidal zone: an assessment from different indexes. Environmental Pollution, 157(5), 1533–1543.
Zhao, C. H., Zhu, Z. H., & Zhou, D. Z. (2000). World rivers and dams (pp. 382–384). Beijing: China Water Power Press (in Chinese).
Zhou, Y. W., Zhao, B., Peng, Y. S., & Chen, G. Z. (2010). Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments. Marine Pollution Bulletin, 60(8), 1319–1324.
Acknowledgments
This research was funded by the National Natural Sciences Foundation of China (no. 50939001, No. 40871237) and the Fundamental Research Funds for the Central Universities. We wish to thank Xiaoyan Li, Ying Mei, and Jing Dai for their assistance with field sampling; we also express our sincere appreciations to the Asian International Rivers Center and Yunnan University as they provided the historical data of water heavy metals of the Manwan Reservoir.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Q., Liu, S., Deng, L. et al. Spatio-temporal variation of heavy metals in fresh water after dam construction: a case study of the Manwan Reservoir, Lancang River. Environ Monit Assess 184, 4253–4266 (2012). https://doi.org/10.1007/s10661-011-2260-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2260-y