Abstract
Lignin-based activated carbon fibers (LCFK) were prepared by electrospinning method and evaluated in adsorption of volatile organic compounds (VOCs). Batch adsorption experiments for various component were carried out in a fixed-bed reactor. The molecular polarity of VOCs plays a pivotal role in the monocomponent dynamic adsorption. As a result, the adsorption capacity of toluene was larger than that of methanol or acetone. In the various multicomponent atmospheres (without water), the components interact with each other and competitive adsorption phenomenon occurs, resulting in the adsorption capacity of each component decreased significantly. Also, the samples before and after adsorption were characterized via Fourier transform infrared (FTIR), X-ray photoelectron spectroscopy (XPS), and Boehm titration. The results reveal that methanol and acetone, controlled by physical adsorption, prefer to be adsorbed on polar groups on the surface of LCFK through the dipole–dipole interactions (i.e., van der Waals’ forces). Differently, the adsorption of toluene onto LCFK was controlled by physical and chemical processes, and the lactone groups have a positive contribution to the adsorption of toluene. It was also observed that water vapor can enhance the negative effect on the adsorption of VOCs, especially for toluene. The results from this study will be valuable for explaining the mechanisms of competitive adsorption among each component in the various multicomponent atmospheres and understanding the contribution of chemical functional groups on the surface of LCFK in the adsorption process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past decade, the rapid development of the chemical industry and the increase of human activities have caused serious atmospheric pollution problems. Volatile organic compounds (VOCs), as one of the main air pollutants, are high toxicity and long existence, which can not only directly endanger human health but also contribute significantly to the formation of the photochemical smog (Tiao et al. 1975; Colbeck and Mackenzie 1994; Celebioglu et al. 2016; Kamal et al. 2016). The Goteborg protocol has proposed that VOC emission by 2020 should be reduced by half as compared with that in 2000 (Zhang et al. 2017a, b). Therefore, it is of great significance and urgency to effectively control the VOC emission.
Recently, many scholars around the world have made great efforts to develop efficient VOC treatment technologies, such as absorption, adsorption, condensation, biological degradation, incineration, and catalysis oxidation. Among them, the adsorption is an economical and promising control strategy (Serna-Guerrero and Sayari 2007; Wang et al. 2014; Zhang et al. 2017a, b), and a selective and efficient adsorbent is an important factor for achieving high VOC removal efficiency. Carbonaceous materials, such as activated carbon (AC) and activated carbon fibers (ACFs), are the most common adsorbents (Suzuki 1994; Chu et al. 2015; Huang and Liang 2017). AC has been widely used for the adsorption and recovery of most VOCs due to its rapid adsorption kinetics and low cost, including alcohols, aldehydes, ketones, aromatic hydrocarbons, alkanes, ethers, and esters. (Cardoso et al. 2008; Oh et al. 2010; Wang et al. 2012). However, the adsorption capacity of VOCs on AC is limited by some factors such as its physicochemical properties, high desorption temperature, moisture, irreversible adsorption of adsorbates, and the mass transfer rate to the adsorbate surface (Chu et al. 2015; Zhang et al. 2017a, b). To overcome these restrictions, ACFs have been identified as a good candidate by virtue of their unique micropore structure, faster adsorption kinetics, and higher mass transfer rate during adsorption or desorption process (Oh et al. 2008; Lee et al. 2010; Bai et al. 2013). Alternatively, modification by acid or alkali to enhance the adsorption properties of AC is usually carried out (Yin et al. 2007; Shafeeyan et al. 2010; Elwakeel et al. 2014). Most ACFs have web-like structure and diameter is smaller than powdered ACs (Tang et al. 2007), and such unique structure characteristic makes it possible for ACFs to reactivate the fabric when it has been saturated. Additionally, the adsorption ability of ACFs is less adversely affected by moisture compared with AC (Anfruns et al. 2013; Lee et al. 2014; Chu et al. 2015). The raw materials used in preparation of ACFs are viscose, polyacrylonitrile fibers and pitch fibers (Li et al. 2015), which are poor renewable and high cost. It is noted that lignin as a precursor of carbon fibers has sparked interest in its utilization (Nar et al. 2016). More importantly, lignin which derived from waste wood and pulp of paper industry is a renewable and low-cost resource (Wang et al. 2015). As far as we know, many techniques have been developed for the preparation of lignin-based carbon fibers. Little attention, however, has been focused on the electrospinning method. Lai et al. (2014) prepared lignin-based activated carbon fibers with nanoscale diameters by this method. Furthermore, lignin-based ACFs have a larger specific surface area, high adsorption capacity, and sufficient mechanical stability. Our previous research confirmed that the electrospun nanofiber was well exhibited in enriching gaseous organic compounds (Song et al. 2017). Given this, the lignin-based activated carbon fibers prepared by electrospinning have potential application prospects in the adsorption of VOCs.
The VOC adsorption capacity of carbonaceous adsorbents depends on their morphology structure and surface chemical functional groups (Li et al. 2011). The surface functional groups of carbon materials are closely related to the prepared raw materials and activation or modification methods (i.e., heating and chemical treatments) (Song et al. 2013; Chu et al. 2015). The oxygen-containing groups on the surface of the carbon materials are identified as one of the most important species for adsorption, which facilitates better adsorption of the hydrophilic VOCs onto carbon surface (Liu et al. 2008). In addition, Ródenas et al. suggested that the polarity of VOC molecules is also one of the factors influencing the VOCs adsorption capacity of carbon materials (Lillo-Ródenas et al. 2005). Hydrothermal treatment and alkali modification will effectively eliminate the surface oxygen-containing groups, which will help the hydrophobic VOCs to be adsorbed on the surface of carbon materials without oxygen groups (Lillo-Ródenas et al. 2011; Baur et al. 2015). Besides, some studies suggested that there is a certain concentration of water vapor in the gas stream, which can compete with VOCs and thus affect the adsorption process (Kaplan et al. 2006; Zhou et al. 2015). However, the mechanism of interaction between chemical functional groups and water vapor has not yet been clearly explained. Further, much work so far has focus on investigating the experimental conditions of monocomponent or multicomponent VOCs adsorption, as previously reported (Gil et al. 2014; Son et al. 2016). Herein, it is worthwhile to consider the interaction mechanism of multicomponent gaseous VOCs in the adsorption process and the quantitative research on the contribution of surface chemical functional groups to the dynamic adsorption.
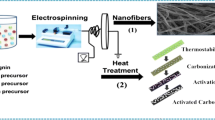
Based on the above ideas and discussions, in this study, lignin-based activated carbon fibers (LCFK) were prepared by electrospinning, pre-oxidation, carbonization, and one-step KOH activation method. Three common VOCs in industrial production, toluene, methanol, and acetone, with different polarities, were chosen as adsorbates. The main purpose of this article is to investigate the contribution of oxygen-containing functional groups on the surface of LCFK in the adsorption process and to illustrate interaction mechanism of multicomponent gaseous VOCs.
Materials and methods
Raw materials
Lignin alkali (low sulfur), poly (vinyl alcohol) (PVA), and acetic acid were obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) and used to prepare electrospinning solutions without further purification. Three kinds of VOCs, acetone, methanol, and toluene were purchased from Lingfeng Chemical Reagent Co. Ltd. (Shanghai, China), and their main properties are shown in Table 1 (Li et al. 2011; Kamal et al. 2016; Zhang et al. 2017a, b). The chemical regents are all analytical grade.
Preparation of lignin-based activated carbon fibers
Lignin alkali was used as a precursor of carbon fibers. The carbon fibers were prepared to process as reported in our previous study (Song et al. 2017; Wei et al. 2017). In a typical procedure, lignin, 10% PVA solution, and acetic acid were mixed according to the ratio of 1:1:3 (w/v/v), and the mixture solution was kept under agitation at 80 °C for 30 min. Then, cool down, the solution was used as the spinning solution to synthesize the lignin-based fiber (LF). Subsequently, the lignin-based fiber was preoxidized at 200 °C for 3 h and then annealed in high purity N2 atmosphere at 800 °C for 1 h. The resulted sample was cooled to room temperature to form the carbon fiber, which was denoted as LCF.
LCFK was obtained through KOH one-step activation method. Firstly, the LF after pre-oxidation was thoroughly mixed with KOH (the LF/KOH mass ratio was 1/3) and then annealed as above. The resulted sample was washed with HCl solution and deionized water until neutral filtrate and then dried at 105 °C for 24 h. The obtained product was ground into powder and referred to as LCFK.
Characterization
The X-ray photoelectron spectroscopy (XPS) (Thermo ESCALab 250 xl, USA) was utilized to determine the chemical states of the sample surface. The surface functional groups of carbon materials before and after adsorption were analyzed using Fourier transform infrared spectroscopy (FTIR) (vector 22 + TGA Bruker Optics, Germany) with the wavenumber range of 400 to 4000 cm−1. Furthermore, the Boehm titration was applied to illustrate the surface chemistry properties of the carbon materials.
Adsorption performance measurement
The determination of the VOC (gaseous) concentrations was analyzed by a gas chromatograph (SP-6890) with a flame ionization detector (FID) in this study. The VOC concentration was made stable in the range 3000~3050 mg/m3; the total flow rate of the column vapor inlet was controlled at the rate of 150 mL/min; and the adsorption temperature was 25 °C. The heater band was used to prevent the condensation of VOC vapor in the pipeline, and 0.1 g of absorbent was used for each adsorption test. The adsorption capacity and adsorption efficiency were calculated according to the following formula:
In Eq. (1), qe (mg/g) represents the adsorption capacity of VOCs for adsorbent; Q (L/min) is the gas flow rate; Cin (mg/m3) is the inlet concentration of VOCs, Cout (mg/m3) indicates outlet concentration of VOCs; and m (g) is the dosage of the adsorbent. In Eq. (2), η (%) is the VOC removal rate of the adsorbent and t (min) is the adsorption time.
Results and discussion
XPS analysis
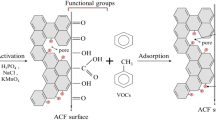
To reveal the chemical states of the as-prepared adsorbent, the obtained samples were measured by XPS spectrum. The results are given in Fig. 1 and Table 2. The main characteristic peaks of LCFK are C1s (284.6 eV), N1s (401 eV), and O1s (532 eV) from Fig. 1a. In Fig. 1b, the C1s peak was segmented into five peaks based on the binding energies of 284.6 ± 0.2 eV, 285 ± 0.2 eV, 286.5 ± 0.2 eV, 288.5 ± 0.3 eV, and 290.4 ± 0.4 eV corresponding to the graphite carbon, C—O (carbon in phenolic and ether), C=O (carbon in carbonyl groups), COOH (carbon in carboxyl groups), and π-π* (shakeup satellite peak), respectively (Liu et al. 2012). Similarly, based on the binding energy, the characteristic peak of O1s-XPS (Fig. 1c) at 531.4 ± 0.3 eV, 532.5 ± 0.3 eV, 533.5 ± 0.2 eV, 534.8 ± 0.2 eV, and 536.4 ± 0.1 eV, which are assigned to the O=C (carbonyl oxygen), C—O (oxygen in phenol hydroxyl groups) together with O=C—O (oxygen in lactone groups), C—O—C (oxygen in ether), COOH (oxygen in carboxyl groups), and oxygen in adsorbed water, respectively (Li et al. 2010). Combined with the results of Table 2, the surface of LCFK treated by KOH contains a large number of oxygen-containing functional groups, which can provide abundant active sites for LCFK to adsorb VOCs.
Monocomponent dynamic adsorption
Adsorption breakthrough curve together with the saturated adsorption capacity and removal rate of three VOCs over the LCFK was obtained, and the results were shown in Fig. 2. According to Fig. 2a, it can be seen that the breakthrough point of acetone occurs earliest as well as the complete breakthrough time was also the shortest at the same conditions. Also, the breakthrough point of toluene was the latest. However, the adsorption of methanol by LCFK was the most rapid, that is, the time from breakthrough to adsorption saturation was the shortest. With the integral operation of three breakthrough curves, the saturated adsorption capacity and removal rate of LCFK were obtained (Fig. 2b). It clearly shows that the adsorption capacity and removal rate of toluene were largest among the three VOCs with the value of 169.41 mg/g and 72.5%, respectively. This is because the adsorption performance of VOCs on LCFK is also influenced by the molecular polarity of VOCs. The surface of LCFK is predominantly hydrophobic, attached with slightly polar due to the present of oxygen-containing groups. The nonpolar prominent feature makes LCFK preferentially adsorb nonpolar toluene in quantity. In contrary, both methanol and acetone are polar molecules, of which the polarity of methanol is higher than that of acetone (Zhang et al. 2005). Herein, methanol is more readily absorbed on the polar groups on the surface of LCFK, and its adsorption capacity is greater than that of acetone. Further, according to the above XPS analysis results, a certain amount of polar carbonyl and lactone groups was observed on the surface of the LCFK. The interaction between carbonyl (or lactone) groups on the LCFK and the benzene ring of toluene can also improve the adsorption capacity because of the electron donor-acceptor mechanism (Singh et al. 2008). As a consequence, the adsorption capacity of toluene was significantly greater than that of the others on the same conditions, and the adsorption of toluene onto LCFK may be controlled by physical and chemical processes.
To further evaluate the influence of chemical functional groups on the adsorption performance of LCFK, the FTIR spectra of LCFK before and after adsorption were determined (Fig. 3). The obvious peaks around 3430 cm−1 on both of the test adsorbents demonstrated the existence of the O-H stretching vibration of water. The band at 2353 cm−1 corresponds to the —N=C=O. In particular, the peaks at 1630 cm−1 are due to the C=O vibration from —COO— and —CONH2. The band at 1405 cm−1 could attribute to the COO− stretching vibration. Another peak at 1068 cm−1 corresponds to the stretching vibrations of C-O band in phenol or ether. The results are in good consistence with the observation from XPS analysis. As shown in Fig. 3, the peak intensities of C=O, C—O, and COO− decreased and the band of —N=C=O disappeared after adsorption, which could imply the interactions of the surface oxygen-containing functional groups with VOCs (Guo et al. 2014; Song et al. 2018).
Multicomponent competitive adsorption and mechanism analysis
To examine the influence of different component atmospheres and water vapor on the adsorption of VOCs onto LCFK, the adsorption experiments of water vapor on multicomponent VOCs were conducted, and the results are shown in Fig. 4. It represented the breakthrough curves and adsorption capacity under various adsorption atmospheres composed of toluene, methanol, and acetone. As shown in Fig. 4a, compared with the monocomponent dynamic adsorption, the adsorption breakthrough time of the three VOCs under the two-component condition was shortened, and the change of methanol was the most obvious. This is manifested in the fact that the breakthrough point of the dynamic adsorption of the monocomponent methanol occurs at 20 to 25 min, while advanced to about 8.5 min in the two-component atmospheres. Meanwhile, the adsorption capacity of each component on the LCFK was significantly reduced when various gases coexisted (Fig. 4b), compared with that in the monocomponent dynamic adsorption. The existence of methanol or acetone has a remarkable impact on the toluene adsorption with the adsorption capacity decreased by 40.09% and 46.83%, respectively. Similarly, when toluene or acetone coexist with methanol, methanol adsorption capacity decreases by 49.85% and 60.31%, respectively. Moreover, when toluene or methanol coexist with acetone, acetone adsorption capacity decreases by 40.35% and 28.09%, respectively. Furthermore, when methanol, acetone, and toluene coexist, the adsorption capacities of toluene, methanol, and acetone decrease by 63.49%, 75.44%, and 58.85%, respectively. What is noticeable is that the effect of water vapor on toluene is more significant compared to methanol and acetone, which is mainly manifested in the results that toluene adsorption capacity decreases dramatically by 48.79%; the methanol adsorption capacity decreases by 12.42%; and the acetone adsorption capacity decreases by 24.88% when 40% of water vapor was introduced into the VOC atmosphere (compared with the adsorption capacity of each component when three VOCs coexist without water vapor).
The breakthrough curves (a) and adsorption capacity (b) under various adsorption atmospheres composed of toluene, methanol, and acetone. Conditions: 3000 mg/m3 toluene (if only), 3000 mg/m3 methanol (if only), 3000 mg/m3 acetone (if only), 40% relative humidity (RH) (if only), gas flow rate 150 mL/min, adsorption temperature 298 K, amount of adsorbent 0.1 g, and a balance of N2 in the atmosphere
It is clear that the above results observed that the adsorption capacity of each component was greatly adversely affected in the multicomponent adsorption atmospheres. However, there is no significant difference between the total adsorption capacity and the adsorption capacity of any each component, which means that LCFK also has a better removal effect for low-concentration VOCs in multicomponent adsorption. Additionally, as shown in Fig. 4a, the adsorption breakthrough curves of methanol in the multicomponent adsorption atmospheres were quite different from that of monocomponent adsorption; the phenomenon of outlet concentration rising first and then decreasing was observed in the adsorption breakthrough curves. When toluene and methanol coexist, the maximum outlet concentration of methanol is 1.05 times of the inlet concentration, and when it is coexisted with acetone, the maximum outlet concentration is 1.09 times of the inlet concentration. This is mainly because methanol, compared with toluene and acetone, has smaller molecular weight and lower boiling point, and when it coexists with toluene or acetone, competitive adsorption occurs. Methanol molecules adsorbed on LCFK were replaced by toluene or acetone with higher molecular weight, leading to a rise in the outlet concentration of methanol above the inlet concentration. Further, when the three VOCs coexist, the competitive adsorption of methanol adsorbed on the LCFK surface by substitution of toluene and acetone was more significant. The maximum outlet concentration of methanol was 1.19 times of the inlet concentration. It was interesting to note that the methanol was not displaced completely by the toluene and/or acetone in the multicomponent adsorption atmospheres.
In addition to the above analysis, when toluene and methanol or acetone coexist, the benzene ring of toluene acts as electron acceptor to combine with the polar lactone groups as electron donor to form a stable electron-donating receptor complex (Singh et al. 2008). In the case of LCFK, the surface of LCFK is primarily nonpolar, toluene, as a nonpolar molecule, can be preferentially and more adsorbed on LCFK to maintain a high adsorption capacity. Herein, it can be inferred that the molecular polarity of adsorbate and the oxygen-containing functional groups on the surface of LCFK play a pivotal role during the competitive adsorption process. Further, it is shown in Fig. 4b the introduced water vapor can severely inhibit the adsorption of toluene on the LCFK. It is attributed to the water molecule preferentially adsorbed on the oxygen-containing functional groups on the LCFK and then more water molecule is adsorbed through hydrogen bonding. As a result, capillary condensation occurs in micropores of LCFK and forms the water cluster, which occupies the sites in the pores competitively. In contrast, when water vapor is introduced, methanol is only slightly inhibited in a multicomponent atmosphere. It can be ascribed to the smaller molecular kinetic diameters, simple structure, and higher diffusion rate of methanol in the multicomponent atmospheres (Yamamoto et al. 2010). Additionally, according to the XPS together with FTIR analysis of LCFK (Fig. 1 and Fig. 3), which found that LCFK contains a large number of hydroxyl functional groups. The presence of water vapor has little effect on the adsorption of methanol with hydroxyl group. Consequently, it can be concluded that the molecular polarity of VOCs decides their adsorption performance onto LCFK, and toluene and acetone should be removed in an atmosphere with low humidity.
It has been pointed out that the VOC adsorption performance onto carbon materials is not only closely related to the properties of adsorbate molecules (i.e., polarity, boiling point, and molecular structure) but also the characteristics of adsorbents (specific surface area, pore structure as well as surface chemical functional groups) are also vital factors (Liu et al. 2008). In the present study, LCFK prepared by KOH activation has a large specific surface area with a value of 1147.16 m2/g, which is conducive to the adsorption of small molecule adsorbates. Additionally, the micropore volume accounts for 66.93% of the total volume, which gives remarkable adsorption sites or active sites for VOCs. Further, a large number of oxygen-containing groups are formed on the surface of LCFK, which contributed to the adsorption process of VOCs. Although the aforementioned characteristics of adsorbents would directly determine the adsorption performance of VOCs onto LCFK, the relationship between the oxygen-containing functional groups and the polarity of the adsorbent was only analyzed qualitatively. More evidence was needed to reasonably and adequately illustrate the contribution of functional groups in the adsorption process.
From what has been discussed, it can be inferred that the multicomponent adsorption is a complicated processes controlled by many factors. Boehm’s titration method was utilized to determine the content of oxygen-containing groups from quantitative view on LCFK before and after adsorption in order to better illustrate the contribution of surface chemical functional groups in the adsorption process. As shown in Fig. 5, the number of carboxyl, phenolic hydroxyl, and lactone groups of LCFK significantly decreases in the presence of various adsorbates after adsorption. It was interesting noted that, according to the electron donor-acceptor mechanism, the lactone groups as electron donor to combine with the aromatic ring of toluene as electron acceptor to form a stable electron donor-acceptor complex, which can also enhance the adsorption capacity. Herein, it also indicated that competitive adsorption occurs between toluene and methanol and/or acetone in the multicomponent adsorption process. Moreover, the adsorption potential energy of toluene is greater than that of methanol and acetone when the lactone groups are combined with toluene. Subsequently, methanol and/or acetone can only occupy residual active sites. Further, as reported in the literature, the adsorption of phenols on a carbon layer involves the π-π dispersive interaction (Liu et al. 2010). In the case of LCFK, the π-π dispersive interaction occurs between the π-electrons in the benzene ring of toluene and those in the carbon layer of LCFK. Although the dispersive interaction is slight, it can also play a positive role in enhancing the adsorption of VOCs onto LCFK.
To sum up, these correlation analyses also demonstrated that methanol and acetone are adsorbed on LCFK mainly via dipole–dipole interactions, which is a physical adsorption process. Meanwhile, the toluene adsorption process includes two aspects. On the one hand, the toluene molecule was adsorbed onto LCFK through a strong affinity between the adsorbate and the adsorbent; on the other hand, a stable electron donor-acceptor complex was formed owing to the aromatic ring of toluene as electron acceptor to combine with carbonyl or lactone as electron donor. Consequently, the adsorption of toluene onto LCFK was controlled by physical and chemical processes. It was worth noting that the stronger adsorption of toluene or acetone can displace the weaker adsorption of methanol during the multicomponent adsorption processes. Futhermore, water vapor can enhance the negative effect on the adsorption of VOCs, especially for toluene. The competitive adsorption process and related mechanisms are shown in Fig. 6.
Conclusions
In this work, lignin-based activated carbon fibers (LCFK) were prepared by electrospinning method for volatile organic compound (VOCs) removal. The adsorption performance of different components of adsorbates onto LCFK was investigated. The large specific surface area and microporous structure of LCFK contribute to adsorption, whereas the effect of chemical functional groups is concerned with the polarity of VOCs. For monocomponent dynamic adsorption, the molecular polarity of VOCs plays a vital role in the adsorption process. The adsorption capacity for toluene (169.41 mg/g) was larger than that of methanol (133.06 mg/g) or acetone (105.48 mg/g). For multicomponent adsorption, methanol and acetone prefer to be adsorbed on polar groups on the surface of LCFK through the dipole–dipole interactions, and their adsorption process was observed as physical adsorption. Differently, the adsorption of toluene onto LCFK was controlled by physical and chemical processes, and the lactone groups are helpful for the adsorption of toluene. The introduction of water vapor significantly enhances the competition among each component in the multicomponent atmospheres, resulting in the adsorption capacity decreased, especially for toluene. The reason is that water molecule has a greater polarity and can be preferentially adsorbed on the surface oxygen-containing functional groups and occupies the active sites. Subsequently, water cluster is formed in the LCFK pore via hydrogen bonding, which hinders the adsorption of VOCs. This study comprehensively illustrated the contribution of oxygen-containing functional groups in the adsorption process, which will provide a theoretical basis for the simultaneous removal of multicomponent VOCs onto LCFK.
References
Anfruns A, Montesmorán MA, Gonzalezolmos R, Martin MJ (2013) H2O2-based oxidation processes for the regeneration of activated carbons saturated with volatile organic compounds of different polarity. Chemosphere 91(1):48–54. https://doi.org/10.1016/j.chemosphere.2012.11.068
Bai Y, Huang ZH, Kang F (2013) Synthesis of reduced graphene oxide / phenolicresin-based carbon composite ultrafine fibers and their adsorption performance for volatile organic compounds and water. J Mater Chem A 1(33):9536–9543. https://doi.org/10.1039/C3TA10545H
Baur GB, Yuranov I, Renken A, Kiwi-Minsker L (2015) Activated carbon fibers for efficient VOC removal from diluted streams: the role of surface functionalities. Adsorption 21(4):479–488. https://doi.org/10.1007/s10450-015-9667-7
Cardoso B, Mestre AS, Carvalho AP, Pires J (2008) Activated carbon derived from cork powder waste by KOH activation: preparation, characterization, and VOCs adsorption. Ind Eng Chem Res 47(16):5841–5846. https://doi.org/10.1021/ie800338s
Celebioglu A, Sen HS, Durgun E, Uyar T (2016) Molecular entrapment of volatile organic compounds (VOCs) by electrospun cyclodextrin nanofibers. Chemosphere 144:736–744. https://doi.org/10.1016/j.chemosphere.2015.09.029
Chu LL, Deng SW, Zhao RS, Zhang Z, Li C, Kang XJ (2015) Adsorption / desorption performance of volatile organic compounds on electrospun nanofibers. RSC Adv 5(124):102625–102632. https://doi.org/10.1039/C5RA22597C
Colbeck I, Mackenzie AR (1994) Air pollution by photochemical oxidants: air quality monographs. Volume 1. Elsevier Science B.V, Amsterdam, p 388
Elwakeel KZ, El-Sayed GO, Abo El-Nassr SM (2014) Removal of ferrous and manganous from water by activated carbon obtained from sugarcane bagasse. Desalin Water Treat 55(2):471–483. https://doi.org/10.1080/19443994.2014.919606
Gil RR, Ruiz B, Lozano MS, Martín MJ, Fuente E (2014) VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem Eng J 245(6):80–88. https://doi.org/10.1016/j.cej.2014.02.012
Guo YY, Li YR, Wang J, Zhu TY, Ye M (2014) Effects of activated carbon properties on chlorobenzene adsorption and adsorption product analysis. Chem Eng J 236(1):506–512. https://doi.org/10.1016/j.cej.2013.10.017
Huang S, Liang C (2017) A conceptual study on the formulation of a permeable reactive pavement with activated carbon additives for controlling the fate of non-point source environmental organic contaminants. Chemosphere 193:438–446. https://doi.org/10.1016/j.chemosphere.2017.11.028
Kamal MS, Razzak SA, Hossain MM (2016) Catalytic oxidation of volatile organic compounds (VOCs)—a review. Atmos Environ 140:117–134. https://doi.org/10.1016/j.atmosenv.2016.05.031
Kaplan D, Nir I, Shmueli L (2006) Effects of high relative humidity on the dynamic adsorption of dimethyl methylphosphonate (DMMP) on activated carbon. Carbon 44(15):3247–3254. https://doi.org/10.1016/j.carbon.2006.06.036
Lai CL, Zhou ZP, Zhang LF, Wang XX, Zhou QX, Zhao Y, Wang YC, Wu XF, Zhu ZT, Fong H (2014) Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J Power Sources 247(3):134–141. https://doi.org/10.1016/j.jpowsour.2013.08.082
Lee KJ, Shiratori N, Lee GH, Miyawaki J, Mochida I, Yoon SH, Jang J (2010) Activated carbon nanofiber produced from electrospun polyacrylonitrile nanofiber as a highly efficient formaldehyde adsorbent. Carbon 48(15):4248–4255. https://doi.org/10.1016/j.carbon.2010.07.034
Lee T, Ooi CH, Othman R, Yeoh FY (2014) Activated carbon fiber - the hybrid of carbon fiber and activated carbon. Rev Adv Mater Sci 36(2):118–136. https://doi.org/10.1016/j.microrel.2013.12.011
Li N, Wang Z, Zhao K, Shi Z, Gu Z, Xu S (2010) Synthesis of single-wall carbon nanohorns by arc-discharge in air and their formation mechanism. Carbon 48(5):1580–1585. https://doi.org/10.1016/j.carbon.2009.12.055
Li L, Liu S, Liu J (2011) Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J Hazard Mater 192(2):683–690. https://doi.org/10.1016/j.jhazmat.2011.05.069
Li PP, Xiong JM, Ge ML, Sun JC, Zhang W, Song YY (2015) Preparation of pitch-based general purpose carbon fibers from catalytic slurry oil. Fuel ProcessTechnol 140:231–235. https://doi.org/10.1016/j.fuproc.2015.09.011
Lillo-Ródenas MA, Cazorla-Amorós D, Linares-Solano A (2005) Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 43(8):1758–1767. https://doi.org/10.1016/j.carbon.2005.02.023
Lillo-Ródenas MA, Cazorla-Amorós D, Linares-Solano A (2011) Benzene and toluene adsorption at low concentration on activated carbon fibers. Adsorption 17(3):473–481. https://doi.org/10.1007/s10450-010-9301-7
Liu Y, Li Z, Shen W (2008) Surface chemical functional groups modification of porous carbon. Recent Pat Chem Eng 1(1):27–40. https://doi.org/10.2174/1874478810801010027
Liu QS, Zheng T, Wang P, Jiang JP, Li N (2010) Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem Eng J 157(2):348–356. https://doi.org/10.1016/j.cej.2009.11.013
Liu ZH, Qiu JR, Hao L, Tan ZQ, Yan ZQ, Zhang ML, Zeng HC, Yang H (2012) Effect of SO2 and NO on removal of VOCs from simulated flue gas by activated carbon fibers at low temperature. J Fuel Chem Technol 40(1):93–99. https://doi.org/10.1016/S1872-5813(12)60008-5
Nar M, Rizvi HR, Dixon RA, Chen F, Kovalcik A, D'Souza N (2016) Superior plant based carbon fibers from electrospun poly-(caffeyl alcohol) lignin. Carbon 103:372–383. https://doi.org/10.1016/j.carbon.2016.02.053
Oh GY, Ju YW, Jung HR, Lee WJ (2008) Preparation of the novel manganese-embedded PAN-based activated carbon nanofibers by electrospinning and their toluene adsorption. J Anal Appl Pyrol 81(2):211–217. https://doi.org/10.1016/j.jaap.2007.11.006
Oh KJ, Park DW, Kim SS, Park SW (2010) Breakthrough data analysis of adsorption of volatile organic compounds on granular activated carbon. Korean J Chem Eng 27(2):632–638. https://doi.org/10.2478/s11814-010-0079-9
Serna-Guerrero R, Sayari A (2007) Applications of pore-expanded mesoporous silica. 7. Adsorption of volatile organic compounds. Environ Sci Technol 41(13):4761–4766. https://doi.org/10.1021/es0627996
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrol 89(2):143–151. https://doi.org/10.1016/j.jaap.2010.07.006
Singh KP, Malik A, Sinha S, Ojha P (2008) Liquid-phase adsorption of phenols using activated carbons derived from agricultural waste material. J Hazard Mater 150(3):626–641. https://doi.org/10.1016/j.jhazmat.2007.05.017
Son HK, Sivakumar S, Rood MJ, Kim BJ (2016) Electrothermal adsorption and desorption of volatile organic compounds on activated carbon fiber cloth. J Hazard Mater 301:27–34. https://doi.org/10.1016/j.jhazmat.2015.08.040
Song M, Jin BS, Xiao R, Yang L, Wu YM, Zhong ZP, Huang YJ (2013) The comparison of two activation techniques to prepare activated carbon from corn cob. Biomass Bioenergy 48(1):250–256. https://doi.org/10.1016/j.biombioe.2012.11.007
Song M, Zhang W, Chen YS, Luo JM, Crittenden JC (2017) The preparation and performance of lignin-based activated carbon fiber adsorbents for treating gaseous streams. Front Chem Sci Eng 11(3):328–337. https://doi.org/10.1007/s11705-017-1646-y
Song M, Wei YX, Cai SP, Yu L, Zhong ZP, Jin BS (2018) Study on adsorption properties and mechanism of Pb2+ with different carbon based adsorbents. Sci Total Environ 618:1416–1422. https://doi.org/10.1016/j.scitotenv.2017.09.268
Suzuki M (1994) Activated carbon fiber: fundamentals and applications. Carbon 32(4):577–586. https://doi.org/10.1016/0008-6223(94)90075-2
Tang D, Zheng Z, Lin K, Luan J, Zhang J (2007) Adsorption of p-nitrophenol from aqueous solutions onto activated carbon fiber. J Hazard Mater 143(1):49–56. https://doi.org/10.1016/j.jhazmat.2006.08.066
Tiao GC, Box GEP, Hamming WJ (1975) Analysis of Los Angeles photochemical smog data: a statistical overview. J Air Pollut Control Assoc 25(3):260–268. https://doi.org/10.1080/00022470.1975.10470082
Wang H, Jahandar LM, Fayaz M, Hashisho Z, Philips JH, Anderson JE, Nichols M (2012) Adsorption and desorption of mixtures of organic vapors on beaded activated carbon. Environ Sci Technol 46(15):8341–8350. https://doi.org/10.1021/es3013062
Wang S, Zhang L, Long C, Li A (2014) Enhanced adsorption and desorption of VOCs vapor on novel micro-mesoporous polymeric adsorbents. J Colloid Interface Sci 428:185–190. https://doi.org/10.1016/j.jcis.2014.04.055
Wang S, Li Y, Xiang H, Zhou Z, Chang T, Zhu M (2015) Low cost carbon fibers from bio-renewable lignin/poly (lactic acid) (PLA) blends. Compos Sci Technol 119:20–25. https://doi.org/10.1016/j.compscitech.2015.09.021
Wei YX, Song M, Yu L, Tang XH (2017) Preparation of ZnO-loaded lignin-based carbon fiber for the electrocatalytic oxidation of hydroquinone. Catal 7(6):180. https://doi.org/10.3390/catal7060180
Yamamoto T, Kataoka S, Ohmori T (2010) Characterization of carbon cryogel microspheres as adsorbents for VOC. J Hazard Mater 177(1):331–335. https://doi.org/10.1016/j.jhazmat.2009.12.036
Yin CY, Aroua MK, Daud WMAW (2007) Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep Purif Technol 52(3):403–415. https://doi.org/10.1016/j.seppur.2006.06.009
Zhang CT, Wang JK, Wang YL (2005) Solubility of ceftriaxone disodium in acetone, methanol, ethanol, N,N-Dimethylformamide, and formamide between 278 and 318 K. J Chem Eng Data 50(5):1757–1760. https://doi.org/10.1021/je0501989
Zhang X, Gao B, Creamer AE, Cao CC, Li YC (2017a) Adsorption of VOCs onto engineered carbon materials: a review. J Hazard Mater 338:102–123. https://doi.org/10.1016/j.jhazmat.2017.05.013
Zhang XY, Gao B, Zheng YL, Hu X, Creamer AE, Annable MD, Li YC (2017b) Biochar for volatile organic compound (VOC) removal: sorption performance and governing mechanisms. Bioresour Technol 245:606–614. https://doi.org/10.1016/j.biortech.2017.09.025
Zhou FY, Chao L, Yao YY, Sun LJ, Gong F, Li DW, Pei KM, Lu WY, Chen WX (2015) Activated carbon fibers as an effective metal-free catalyst for peracetic acid activation: implications for the removal of organic pollutants. Chem Eng J 281:953–960. https://doi.org/10.1016/j.cej.2015.07.034
Funding
The study is financially supported by the National Key Research and Development Program (2018YFB0605200).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, F., Song, M., Wei, Y. et al. The contribution of oxygen-containing functional groups to the gas-phase adsorption of volatile organic compounds with different polarities onto lignin-derived activated carbon fibers. Environ Sci Pollut Res 26, 7195–7204 (2019). https://doi.org/10.1007/s11356-019-04190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04190-6