Abstract
Atmospheric contamination by heavy metal(loid)s is a widespread global issue. Recent studies have shown foliar pathway of heavy metal(loid) uptake by plants, thus menacing plant productivity and threatening health risks. In contrast to root uptake of heavy metal(loid)s, there is scarce data available on heavy metal(loid) foliar uptake, accumulation in different plant parts, changes in growth and other biophysiochemical processes/reactions, detoxification mechanisms and associated health risks due to the consumption of contaminated vegetables. This study evaluated the effect of foliar application of two potentially toxic metal(loid)s (arsenic (As) and lead (Pb)) on their uptake by Spinacia oleracea, plant growth, pigment contents, physiological changes, and activation of antioxidative enzymes. Results revealed that S. oleracea seedlings can accumulate both the metal(loid)s in their leaves via foliar pathway. Arsenic was transferred from the leaves towards the roots, while Pb was mainly sequestered in S. oleracea leaves. Both the metal(loid)s significantly decreased plant growth and pigment contents, As being more toxic than Pb. Foliar application of As and Pb did not cause lipid peroxidation and overproduction of reactive oxygen species (ROS). However, both the metal(loid)s enhanced the activities of antioxidative enzymes. We also calculated possible health risks (both non-carcinogenic and carcinogenic) due to As and Pb accumulation in the edible parts for both the adults and children. It was observed that As can induce non-carcinogenic effects (HQ > 1) in children only, while both As and Pb can cause carcinogenic hazards in both adults and children under their all applied foliar levels. Therefore, it is proposed that As and Pb contents in the atmosphere must be monitored continuously for their possible foliar uptake and accumulation in edible plant parts to avoid cancer risks. Moreover, multivariate analysis traced weak-strong correlations between metal(loid) treatments and plant response variables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent investigations have revealed that heavy metal(loid)s can also enter the plants by foliar pathway in addition to root uptake (Song et al. 2018; Uzu et al. 2010; Xiong et al. 2017). However, despite considerable progress in recent years, there exists comparatively rare data regarding foliar uptake of metal(loid)s compared to the root-plant pathway. In fact, foliar uptake and accumulation of metal(loid)s have been the focus of recent studies (Schreck et al. 2012; Shahid et al. 2017b; Xiong et al. 2014b). Some recent reports revealed the uptake and accumulation of considerable amounts of metal(loid)s via foliar pathway (Mombo et al. 2016; Schreck et al. 2014; Uzu et al. 2014). It is considered that metal(loid)s can penetrate through the cuticle layer of foliar organs and adopt symplastic or apoplastic pathway to move between cells, from where they can undergo phloem loading (Schreck et al. 2012; Shahid et al. 2017b). After being released into the phloem, metal(loid)s uptaken by foliar pathway can be distributed throughout the plant (Natasha et al. 2018a; Xiong et al. 2016). Still, the interaction between foliar metal(loid) uptake, accumulation in different plant tissues, and associated phytotoxic and health risks remained poorly understood.
During the past few years, the use of more effective and thinner filters during metal(loid) mining, production and recycling processes have resulted in the emission of a high amount of fine and ultrafine metallic particles into the atmosphere (El Hayek et al. 2017; Xiong et al. 2014a). Consequently, high amounts of fine and ultrafine airborne particles are observed in urban, peri-urban, and industrial areas (Gajbhiye et al. 2016; Huang et al. 2018; Liu et al. 2018; Xiong et al. 2018). These metal(loid)-enriched particulates are highly reactive compared to coarse emissions, consequently causing environmental and sanitary concerns. In many cases, heavy metal(loid)s get attached with these airborne particles during their release/origin from industrial and traffic sources (Huang et al. 2018; Liu et al. 2018; Schreck et al. 2013; Wang et al. 2018). These metal(loid)-enriched particulates can deposit on soil, water, and plants via wet and dry atmospheric deposition: wet deposition through precipitation and dry deposition through the wind, hurricanes, tornadoes, or maybe gravity (Gunawardena et al. 2013).
High levels of these heavy metal(loid)-enriched particles in the atmosphere can induce health hazards via inhalation by people living in nearby areas (Jadoon et al. 2018; Kim et al. 2017; Uzu et al. 2011; Uzu et al. 2009). Additionally, the health impacts may also occur due to ingesting heavy metal(loid)-polluted vegetables cultivated in the areas containing high concentrations of metal(loid)s in the atmosphere. For example, kitchen gardening in urban and peri-urban areas can be highly risky in areas of high atmospheric heavy metal(loid) pollution (Dumat et al. 2016; Xiong et al. 2017).
When deposited on plants, metal(loid)-enriched particulates can be taken up by plants via foliar organs (Shahid et al. 2017b; Xiong et al. 2017). Exposure of plants to high concentrations of metal(loid)s is generally accompanied by phytotoxic effects (Hariram et al. 2018). Foliar metal(loid) uptake has also been reported to disrupt the plant metabolic activities and adversely affect the normal physiological functioning (Klink et al. 2018; Rizvi and Khan 2018; Xiong et al. 2016). Literature indicates that overproduction of reactive oxygen species (ROS) in plants via Haber-Weiss reactions is one of the earliest and most common phenomena upon exposure to heavy metal(loid)s (Abbas et al. 2018; Shahid et al. 2017c; Shahid et al. 2019). The oxidative stress associated with overproduction of ROS in plant leaves can result in decreased photosynthetic activity, DNA and RNA damage, oxidation of proteins, and peroxidation of lipids (Abbas et al. 2018; Shahid et al. 2019). Consequently, these phytotoxic effects or physiological responses are among the most studied mechanisms/biomarkers of metal(loid) toxicity in the plant. Unlike root metal(loid) transfer, less data is available on the physiological changes induced by foliar metal(loid) uptake (Bakhat et al. 2017; Shahid et al. 2017b).
In recent years, the concept of food security and risk assessment is highly topical due to increased use and emission of metal(loid) minerals in industrial activities (Khalid et al. 2017; Mombo et al. 2016; Phi et al. 2018; Raessler 2018). However, data about health risk assessment due to the foliar uptake of heavy metal(loid)s is almost missing in the literature. Consumption of heavy metal(loid) contaminated crops/vegetables after foliar uptake can provoke numerous clinical conditions (Li et al. 2018; Sawut et al. 2018). Therefore, it can be of great use to predict possible health risks associated with foliar uptake of heavy metal(loid)s by vegetables/crops.
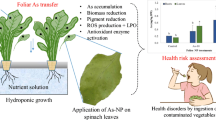
Keeping in view the above-mentioned novel aspects and knowledge gaps, the current study was performed to reveal Pb and As (the carcinogenic elements) foliar uptake mechanisms and transport inside S. oleracea, a vegetable having high nutritional and consumption value. Moreover, the possible effects of these metal(loid)s on plant biophysiochemical processes, mainly the tolerance mechanism of vegetables under foliar metal(loid) stress, are also assessed. Finally, we also estimated health risks associated with the consumption of contaminated vegetable.
Methodology
Experimental conditions and treatments
Seeds of S. oleracea were allowed to germinate in acid-washed sand for 1 week. The acid-washed sand was used to avoid the metal(loid) contamination at germination stage. After 1 week, S. oleracea seedlings (with 3–4 cm long roots) were transferred to a nutrient solution for 7 days (5 mM Ca(NO3)2, 5 mM KNO3, 1.5 mM MgSO4, 2 mM KH2PO4, 0.235 μM CuSO4, 9.11 μM MnSO4, 0.1 μM Na2MoO4, 1.53 μM ZnSO4, 24.05 μM H3BO3, and 268.6 μM Fe-EDTA). Both the metal(loid)s were exposed via foliar application to S. oleracea at two levels: 50 mg/L and 100 mg/L in solution form. A non-contaminated plants group served as control. Arsenic solutions were prepared from sodium arsenite (NaAsO2) salt and Pb solutions were prepared from lead acetate (Pb(C2H3O2)2) salt.
The adaxial surface of the three higher leaves was wetted with specific metal(loid) concentrations (Fig. 1). About 3–5 ml of prepared solution per plant was applied on leaves during each treatment. An applicator brush was used to apply treatments as used previously by Xiong et al. (2014a). Separate brushes were used for each metal(loid) application to avoid the cross-contamination. The spraying of the metal(loid) solutions was avoided to prevent the contamination of the surrounding environment by metal(loid) droplets/aerosols. In order to control growth medium contamination during treatment application, each tub was covered with a polyethene sheath. All the treatments were applied once a week for four consecutive weeks.
After exposure to employed treatments, plants were harvested and stored under appropriate conditions for further analysis. The experiment followed a completely randomized design with six replications for each treatment. Three replications were used for plant physicochemical analysis, while the remaining three replications were used for metal(loid) content analysis.
Metal(loid) content analysis
The dried S. oleracea samples were ground to powder form. Plant samples were wet digested with a di-acid mixture of nitric acid and perchloric acid (2:1). Lead contents in plant samples were determined using atomic absorption spectroscopy, while As contents were determined with hydride generation atomic absorption spectroscopy (HG-AAS) (AAS; Thermo AA®, Solar-Series).
Determination of pigment contents
S. oleracea root and leaf samples were frozen in liquid nitrogen. About 1 g of frozen leaves were ground in an ice-cold mortar. In order to extract pigment contents, hydro-acetone (80% v/v) was used followed by centrifugation at 3000g for 10 min (Lichtenthaler 1987). A spectrophotometer (AA, Solar -Series) was used to record the absorbance of mixture at 470, 646.8, and 663.2 nm.
Analysis of hydrogen peroxide (H2O2)
In order to determine H2O2 contents, about 1 g frozen S. oleracea root/shoot samples were ground in an ice-cold mortar. Hydro-acetone (80% v/v) was used as an extractant. The extract of S. oleracea samples was centrifuged for 10 min at 3000g. A spectrophotometer was used to measure the absorbance (at 390 nm) of the mixture comprised of 1 mL of the plant extract, 1 mL of potassium iodide, and 1 mL of potassium phosphate buffer.
Analysis of lipid peroxidation
The assay of lipid peroxidation was carried out according to Hodges et al. (1999). For this purpose, about 1 g of S. oleracea samples were ground in an ice-cold mortar. The samples were incubated at 95 °C with butyl hydroxytoluene and trichloroacetic acid in the presence and absence of thiobarbituric acid. The samples were centrifuged at 3000g for 10 min, followed by recording the absorbance of supernatant at 532 nm.
Antioxidative enzyme activity
About 250 mg of S. oleracea shoot samples were ground with an ice-cooled pestle and mortar under liquid nitrogen in 0.1 M phosphate buffer (pH − 7.0). The mixture was centrifuged twice at 4500g at 4 °C for 30 min.
Superoxide dismutase (SOD) analysis
The SOD activity was evaluated following the protocol of Dhindsa et al. (1981). A 3 ml reaction assay was composed of 200 μl enzyme extract, 13 mM methionine, 0.1 mM EDTA, 75 μM NBT, 50 mM phosphate buffer (pH − 7.8), and 60 μM riboflavin. The SOD activity expresses its amount essential to induce 50% NBT reduction.
Catalase (CAT) assay
For CAT activity, a 3 ml mixture assay of S. oleracea samples was prepared using 200 μl enzyme extract in 15 mM H2O2 and 50 mM phosphate buffer (pH − 7.0) (Aebi 1984). A spectrophotometer was used to record the absorbance of mixture at 240 nm for 45 s (25 °C). The CAT activity expresses micromolar of H2O2 degraded per minute per milligram protein.
Peroxidase (POD) assay
The mixture for POD analysis was comprised of 200 μl enzyme extract, 50 mM phosphate buffer (pH − 6), 12 mM guaiacol, and 15 mM H2O2 (Hemeda and Klein 1990). A spectrophotometer was used to record the absorbance of mixture at 470 nm for 90 s (25 °C). The POD activity expresses micromolar of guaiacol oxidized per minute per milligram protein.
Health risk assessment
Estimated daily intake (EDI) of Pb and as
The EDI values of Pb and As after foliar application were calculated as reported by Xiong et al. (2014b) using the following Eq. (1).
For detail of different parameters used to calculate EDI, please see the Supplementary Table 1.
Hazard quotient (HQ) of Pb and As
Hazard quotients of Pb and As were determined following Xiong et al. (2014b) using the following Eq. (2).
For detail of different parameters used to calculate HQ, please see the Supplementary Table 1.
Cancer risk assessment
The possible cancer risk (CR) by consuming As or Pb contaminated S. oleracea was calculated as following (Xiong et al. 2014b).
For detail of different parameters used to calculate CR, please see the Supplementary Table 1.
Translocation factor (TF)
Translocation factor was calculated using the following formula:
Statistical analysis
Analysis of variance (ANOVA) was carried out using Statistix (Ver 8.1), while Pearson correlation and principal component analysis (PCA) were perfumed using XLSTAT (ver. 19.4).
Results
Metal(loid) accumulation in S. oleracea
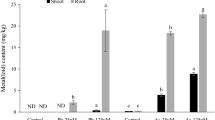
The accumulation of As in leaves and roots of S. oleracea, after foliar application of As solutions, is shown in Table 1. Plants absorbed a significant amount of As when exposed to foliar As-solution treatments. The maximum concentration of As (1.2 and 4.67 mg/kg, respectively in shoot and roots) was found in S. oleracea treated with the low-applied dose of As (As-50 ppm) with visible leaf tissue damage. At higher As level (As-100 ppm), S. oleracea accumulated a lesser amount of As (1.13 and 2.7 mg/kg, respectively, in shoot and roots) compared to As-50, which can be due to high As toxicity at As-100 applied level as well as low biomass.
In case of Pb, the increase in the exogenous foliar concentration of Pb did not cause a parallel increase in metal accumulation in leaves (1.73 and 1.36 mg/kg, respectively, for Pb-50 and Pb-100). Lead was absorbed by the foliar plant organs but did not evoke tissue disruption. No concentration of Pb was found in roots of S. oleracea suggesting that Pb tends to remain in aerial/aboveground plant tissues under foliar Pb-solution application.
When the two metal(loid)s were compared, Pb foliar uptake was slightly higher compared to As. However, the transfer of As from leaves to roots was higher than Pb.
Effect of metal(loid) accumulation on the dry weight
Results indicated that As and Pb accumulation in the plants exerted toxic effects and significantly decreased the dry weight of S. oleracea compared to control. Foliar application of As significantly decreased S. oleracea biomass than control. A linear trend was experienced between As applied concentrations and the decrease in dry weight of both the root and shoot. Although a slightly more toxic effect was observed at As-100, the effect was non-significant between As-50 and As-100. A significant reduction in dry weight of S. oleracea was also observed at the high level of Pb in plant leaves and roots compared to control (Table 1).
The toxicity of As and Pb varied in different plant tissues. In leaves, As was more toxic compared to Pb, despite the fact that more Pb was accumulated in leaves than As. This can be due to higher toxicity of As to S. oleracea compared to Pb. The root biomass of S. oleracea was also lower for As treatments compared to Pb treatments. This can be due to high As accumulation in S. oleracea roots compared to Pb.
Effect of metal(loid) accumulation on pigment content
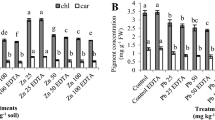
Both the levels of As exogenous solution applications showed a significant decrease in pigment contents over the control (Table 2). Among all the As treatments, As-100 solution (100 ppm) showed the highest decrease in pigment content indicating the disruption of chlorophyll biosynthesis. The linear trend in pigment content reduction was observed in the plants treated with As solutions.
The application of Pb had no significant effect on S. oleracea pigment contents compared to control (Table 2). A slight decrease in pigment content was observed at the lower level (Pb-50) while the higher level of Pb (Pb-100) did not show any difference over the control.
Effect of metal(loid) accumulation on H2O2 production and lipid peroxidation
Arsenic-induced lipid peroxidation and generation of H2O2 are presented in Table 3. In leaves of S. oleracea, no significant difference in H2O2 content was observed. However, there was a linear decrease in H2O2 production for both applied levels of As. The same trend with lower H2O2 production compared to control was observed in roots under As-100 treatments.
The TBARS content increased at higher As level (As-100) but the increase was not statistically significant. Roots of all As-treated plants did not show any significant difference in TBARS over the control. In case of Pb, no change was observed in H2O2 contents at any applied level in roots and leaves (Table 3). Similar to H2O2 production, plants did not show any alteration in TBARS content under Pb treatments in roots and leaves.
Antioxidant enzyme activities
The activities of antioxidative enzymes in S. oleracea leaves increased differently with respect to As treatment level (Table 4). Arsenic solutions at both levels non-significantly increased the enzymatic activities. Increase in SOD, CAT, and POD was, respectively, 123%, 166%, and 349% for As-50 and 15%, 111%, and 213% for As-100 solution application.
Solution application of Pb also exhibited the same trend on the activities of antioxidative enzymes as observed for As solution application (Table 4). Increase in SOD, CAT, and POD was, respectively 12%, 87%, and 135% for Pb-50 and 2%, 197%, and 47% for Pb-100.
Health risk assessment
In this study, the carcinogenic and non-carcinogenic risk assessment parameters were calculated in adults and children to predict any possible health hazards due to foliar uptake of heavy metal(loid)s (Table 5). In case of As, the EDI values were 0.000188 and 0.000178 for adults and 0.000367 and 0.000346 for children, respectively, for As-50 and As-100. In the case of Pb, these values were higher compared to As: 0.00027 and 0.00021 for adults and 0. 000529 and 0.000414 for adults, respectively, at Pb-50 and Pb-100. The HQ values remained below 1 (limit hazard value) for all the treatments of Pb and As for adults, but the HQ was higher than 1 for As exposure in children. The HQ values were higher for As than Pb in case of adults and several times higher for children.
The cancer risk factor (CR) showed values higher than threshold limit (10−4) in both adults and children for both the metal(loid)s, however, risk being more pronounced for As than Pb. This shows that there can be a risk of cancer via foliar transfer of As and Pb to edible plant parts. The transfer factors (TF) of both the metal(loid)s remained below 1. The TF was higher for As showing its higher transfer towards roots compared to Pb.
Discussions
Heavy metal(loid) accumulation and its effect on the dry weight of plants
Toxicity to living organisms induced by heavy metal(loid) stress is a complicated phenomenon. Heavy metal(loid)s have been reported to induce numerous toxic effects to plants such as oxidative stress, osmotic disturbance, specific ion toxicity, and nutrient deficiency (Abbas et al. 2018). In this way, heavy metal(loid)s can seriously affect various biochemical and physiological mechanisms linked to plant growth, survival, and development (Kováčik et al. 2018). Plants have been reported to uptake toxic metal(loid)s such as As, Pb, and Cu by roots from polluted soils as well as by leaves (foliar organs) from the atmosphere, and can accumulate these heavy metal(loid)s in various parts of plants (Natasha et al. 2018a; Xiong et al. 2016).
Under current experimental conditions, As content in shoot increases up to 1.20 and 1.13 mg/kg DW in leaves, respectively, at As-50 and As-100 ppm solution applications. Previously, As uptake by plants after foliar application of As solution has been reported by Bondada et al. (2004). They demonstrated that a high concentration of As was absorbed in Chinese brake after foliar application of As. It was reported that the fronds are capable of accumulating high levels of As in addition to roots, indicating that the fronds are also key structures to uptake metal(loid)s. Recently, Natasha et al. (2018a) also reported As uptake in spinach after foliar application of As nanoparticles. In this study, solution application of Pb also enhanced the accumulation of Pb inside the plant leaves without translocating towards the root. This shows that after foliar Pb uptake by S. oleracea leaves, its transfer towards roots does not take place freely. Several previous studies reported foliar uptake of Pb by different types of plants (Uzu et al. 2010; Xiong et al. 2017).
When two metal(loid)s were compared, Pb was more accumulated in leaves, while As was more translocated towards roots. This reveals that heavy metal(loid) accumulation in plant leaves and their transfer towards roots greatly vary with metal(loid) type.
Metal(loid) accumulation in plants causes a significant decrease in plant dry biomass in the present study. Metal(loid) toxicity and the decrease in dry weight due to the root uptake system have widely been reported in the literature (Abbas et al. 2018; Chowdhury et al. 2018; Pourrut et al. 2011). But the toxicity of metal(loid)s after foliar absorption of heavy metal(loid)s has not been well-established yet. The foliar application of metal(loid)s caused a significant decrease in the dry matter of plants. There was a linear trend between metal(loid) accumulation and dry weight reduction, for both the metal(loid)s.
Effect of metal(loid) accumulation on pigment contents
In the present study, the application of As solutions reduced the plant pigment contents at both applied levels. At As-50, the reduction observed was 49%, 56%, 52%, and 33%, and at As-100, about 57%, 61%, 59%, and 37%, respectively, in Chl-a, Chl-b, Chl-a+b, and carotenoid contents. In contrast, the application of Pb treatments did not induce any alteration in chlorophyll content at Pb-100 while Pb-50 showed 19%, 32%, 14%, and 11% reduction in Chl-a, Chl-b, Chl-a+b, and carotenoid contents, respectively. This showed that toxicity of metal(loid)s to S. oleracea varied with their type. Recently, Xiong et al. (2017) reported the decrease in chlorophyll content of cabbage and lettuce when exposed to CuO NPs. The plants showed a reduction of photosynthesis even at 10 mg NPs/plant.
Effect of metal(loid) accumulation on H2O2 induction and lipid peroxidation
Numerous recent landmarks in scientific research have revealed that in plants, heavy metal(loid)-induced oxidative stress has been associated with the damage of several macromolecules such as DNA, RNA, proteins, and lipids (Shahid et al. 2012; Shahid et al. 2017c). Arsenic and Pb can directly cause enhanced production of ROS and other free radicals and indirectly by limiting the activities of antioxidative enzymes (Abbas et al. 2018; Pourrut et al. 2011). Under current experimental conditions, H2O2 production and lipid peroxidation did not vary significantly after As and Pb foliar application. This can be due to the low level of metal(loid) uptake and accumulation in S. oleracea tissues, which were unable to cause overproduction of ROS.
Similarly, numerous previous studies have shown that antioxidant enzymes can scavenge and interconvert ROS from one into another form (Shahid et al. 2014). Consequently, the low level of H2O2 under current experimental conditions can be due to the transformation of ROS from one to another form (Shahid et al. 2018). It is also possible that plant may not experience high H2O2 (ROS) production under foliar metal(loid) uptake in contrast to root uptake. However, there is no literature data available to support these assumptions. Similarly, the exposure of As NPs to S. oleracea did not provoke the production of ROS in leaves and roots (Natasha et al. 2018a).
Effect of metal(loid) accumulation on antioxidant enzyme activities
In order to cope with ROS-induced oxidative stress, plants have well-established tolerance mechanism of antioxidative enzyme systems that can tolerate the potential toxicity of ROS (Abbas et al. 2018). One of the protective mechanisms adopted by plants is the induction of the antioxidant defense system, which involves the sequential and simultaneous action of several enzymes (Natasha et al. 2018b, 2019). In the present study, the maximum increase in CAT and POD activity was 165% and 349% observed at the lower dose of As (As-50) solutions while the high dose of As (As-100) showed lesser alteration in enzymatic activities. The activities of SOD, CAT, and POD increased, respectively, by 12%, 87%, and 197% at Pb-50 and 2%, 135%, and 47% at Pb-100 treatment. This revealed that plant defense mechanisms become activated after foliar uptake of metal(loid)s. However, the current study is unable to explain the mechanism and intensity of activation of these defense mechanisms.
Health risk assessment
Previously, environmental contamination by heavy metal(loid)s was mainly evaluated for their possible toxic effects on plant growth (Shahid et al. 2011). Nowadays, health hazards associated with accumulation of heavy metal(loid)s in the edible plant parts have become highly topical under the umbrella of food security (Antoniadis et al. 2019; Mombo et al. 2016). In this study, different risk assessment parameters (EDI, HQ, and CR) showed that there was a very low chance of non-carcinogenic risks in adults due to low accumulation of both the metal(loid)s in plant tissues. However, HQ was > 1 for As in children, showing possible non-carcinogenic health risks.
In the case of CR, there is a chance of cancer risk in both adults and children under As and Pb treatments. Arsenic was several times more lethal than Pb. This can be due to higher toxicity and carcinogenic risk of As compared to Pb. Arsenic and its compounds have been classified as highly toxic (carcinogenic) at the global scale (ATSDR 2007). The International Agency for Research on Cancer (IARC) has classified it as Group-1 human carcinogen. Arsenic is ranked top among the top 20 priority toxic elements (ATSDR 2007). Therefore, it is proposed that monitoring of metal(loid)s, especially As, must be carried out for its atmospheric concentration, possible foliar uptake, and accumulation in edible plant parts to avoid cancer risks.
Principal component analysis
Nowadays, the use of multivariate analysis has gained considerable importance to trace out possible trends and relationships among data set (Shahid et al. 2018). In fact, the multivariate analysis (such as principal component analysis) takes into account the variance and correlation of various response variables in relation to input factors (Natasha et al. 2018a; Shahid et al. 2018). The PCA groups the input and response variables into various groups based on similarity/difference in their variance and correlation.
In this study, PCA divided response variables of S. oleracea into different groups for As treatments (Fig. 2a). The pigment and growth parameters were grouped together showing their similar trend of decrease (toxicity) in response to As stress. On the other hand, As contents were grouped with antioxidative enzymes showing an increase in their activities with As foliar treatments. The other physiological responses (ROS and lipid peroxidation) were scattered in the PCA graph due to their varied responses with respect to As treatments. However, in roots, ROS and lipid peroxidation have been grouped together, which may be due to their similar effects under As stress.
In case of Pb, the pigment contents and growth parameters (dry weights) were grouped together (Fig. 2b). All other response variables of S. oleracea were scattered in the PCA graph. This shows that under Pb stress, different physiological response variables (ROS, lipid peroxidation and antioxidative enzymes) did not show similar trends. This also proposed that the responses of plants and trends in different response variables vary greatly for different metal(loid)s.
When different treatments of As (control, As-50, As-100) and Pb (control, Pb-50, Pb-100) were compared using their overall effects on different biochemical responses of S. oleracea, PCA placed all the treatments separately for both the metal(loid)s (Fig. 3a, b). This shows that all the treatments of both the metal(loid)s induced overall different effects in the biophysiochemical traits of S. oleracea.
The grouping of different plant response variables and applied treatments was also confirmed by the Pearson correlation matrix (Supplementary Table 2A, 2B). The variables grouped together showed a moderate to a strong linear correlation matrix. This showed that the overall effect of different treatments of As and Pb on plant responses differed from each other as well as from control treatment. This also proposed that under certain conditions, simple statistical analysis may not show any significant difference among different treatments, but the multivariate analysis separates them based on their overall effect to different response variables. Therefore, multivariate analysis may be preferred over simple statistical analysis under conditions where great variation in data and complicity in treatments do not show clear linear trends.
Conclusions
The findings of this study proposed that S. oleracea can accumulate As and Pb via foliar pathway. However, As has high shoot-root transfer index compared to Pb. After foliar uptake, both the metal(loid)s can significantly reduce plant growth and pigment contents and slightly affect ROS production and lipid peroxidation. Similarly, plant defense mechanism also gets activated under foliar uptake of As and Pb. Moreover, foliar-applied As accumulation in edible plant parts can induce non-carcinogenic effects (HQ > 1) in children, while both the metal(loid)s can cause carcinogenic hazards in both adults and children under their all applied foliar levels. Therefore, atmospheric contamination by As and Pb may be monitored and controlled to avoid their health risks via foliar accumulation in edible plant parts. Multivariate analysis revealed that different metal(loid) treatments, their applied levels, and the plant physiological/defense responses showed varied effect.
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi N, Khan M, Amjad M, Hussain M, Natasha (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Antoniadis V, Shaheen SM, Levizou E, Shahid M, Niazi NK, Vithanage M, Ok YS, Bolan N, Rinklebe J (2019) A critical prospective analysis of the potential toxicity of trace element regulation limits in soils worldwide: are they protective concerning health risk assessment?-a review. Environ Int 127:819–847
ATSDR (2007) Toxicological profile for arsenic. Agency for Toxic Substances and Disease Registry
Bakhat HF, Zia Z, Fahad S, Abbas S, Hammad HM, Shahzad AN, Abbas F, Alharby H, Shahid M (2017) Arsenic uptake, accumulation and toxicity in rice plants: possible remedies for its detoxification: a review. Environ Sci Pollut Res 24:9142–9158
Bondada BR, Tu S, Ma LQ (2004) Absorption of foliar-applied arsenic by the arsenic hyperaccumulating fern (Pteris vittata L.). Sci Total Environ 332:61–70
Chowdhury NR, Das R, Joardar M, Ghosh S, Bhowmick S, Roychowdhury T (2018) Arsenic accumulation in paddy plants at different phases of pre-monsoon cultivation. Chemosphere 210:987–997
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dumat C, Xiong T, Shahid M (2016) Agriculture urbaine durable: Opportunité pour la transition écologique. OmniScriptum, Germany, Germany, pp 1–80
El Hayek E, El Samrani A, Lartiges B, Kazpard V, Aigouy T (2017) Lead bioaccumulation in Opuntia ficus-indica following foliar or root exposure to lead-bearing apatite. Environ Pollut 220:779–787
Gajbhiye T, Pandey SK, Kim K-H, Szulejko JE, Prasad S (2016) Airborne foliar transfer of PM bound heavy metals in Cassia siamea: a less common route of heavy metal accumulation. Sci Total Environ 573:123–130
Gunawardena J, Egodawatta P, Ayoko GA, Goonetilleke A (2013) Atmospheric deposition as a source of heavy metals in urban stormwater. Atmos Environ 68:235–242
Hariram M, Sahu R, Elumalai SP (2018) Impact assessment of atmospheric dust on foliage pigments and pollution resistances of plants grown nearby coal based thermal power plants. Arch Environ Contam Toxicol 74:56–70
Hemeda HM, Klein B (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Huang H, Jiang Y, Xu X, Cao X (2018) In vitro bioaccessibility and health risk assessment of heavy metals in atmospheric particulate matters from three different functional areas of Shanghai, China. Sci Total Environ 610:546–554
Jadoon WA, Khpalwak W, Chidya RCG, Abdel-Dayem SMMA, Takeda K, Makhdoom MA, Sakugawa H (2018) Evaluation of levels, sources and health hazards of road-dust associated toxic metals in Jalalabad and Kabul cities, Afghanistan. Arch Environ Contam Toxicol 74:32–45
Khalid S, Shahid M, Dumat C, Niazi NK, Bibi I, Gul Bakhat HFS, Abbas G, Murtaza B, Javeed HMR (2017) Influence of groundwater and wastewater irrigation on lead accumulation in soil and vegetables: implications for health risk assessment and phytoremediation. Int J Phytoremediation:0–0
Kim HS, Kim K-R, Kim W-I, Owens G, Kim K-H (2017) Influence of road proximity on the concentrations of heavy metals in Korean urban agricultural soils and crops. Arch Environ Contam Toxicol 72:260–268
Klink A, Polechońska L, Dambiec M, Białas K (2018) A comparative study on macro-and microelement bioaccumulation properties of leaves and bark of Quercus petraea and Pinus sylvestris. Arch Environ Contam Toxicol 74:71–79
Kováčik J, Dresler S, Peterková V, Babula P (2018) Metal-induced oxidative stress in terrestrial macrolichens. Chemosphere 203:402–409
Li X, Li Z, Lin C-J, Bi X, Liu J, Feng X, Zhang H, Chen J, Wu T (2018) Health risks of heavy metal exposure through vegetable consumption near a large-scale Pb/Zn smelter in Central China. Ecotoxicol Environ Saf 161:99–110
Lichtenthaler HK (1987): [34] chlorophylls and carotenoids: pigments of photosynthetic biomembranes, Plant Cell Membranes Academic Press, pp. 350–382
Liu Y, Xing J, Wang S, Fu X, Zheng H (2018) Source-specific speciation profiles of PM 2.5 for heavy metals and their anthropogenic emissions in China. Environ Pollut 239:544–553
Mombo S, Foucault Y, Deola F, Gaillard I, Goix S, Shahid M, Schreck E, Pierart A, Dumat C (2016) Management of human health risk in the context of kitchen gardens polluted by lead and cadmium near a lead recycling company. J Soils Sediments 16:1214–1224
Natasha SM, Dumat C, Khalid S, Rabbani F, Farooq ABU, Amjad M, Abbas G, Niazi NK (2018a) Foliar uptake of arsenic nanoparticles by spinach: an assessment of physiological and human health risk implications. Environ Sci Pollut Res Int 26:20121–20131
Natasha SM, Niazi NK, Khalid S, Murtaza B, Bibi I, Rashid MI (2018b) A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Pollut 234:915–934
Natasha, Shahid M, Khalid S, Dumat C, Pierart A, Niazi NK (2019) Biogeochemistry of antimony in soil-plant system: ecotoxicology and human health. Applied Geochemistry
Phi TH, Chinh PM, Cuong DD, Ly LTM, Van Thinh N, Thai PK (2018) Elemental concentrations in roadside dust along two National Highways in Northern Vietnam and the health-risk implication. Arch Environ Contam Toxicol 74:46–55
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol 213:113–136
Raessler M (2018) The arsenic contamination of drinking and groundwaters in Bangladesh: featuring biogeochemical aspects and implications on public health. Arch Environ Contam Toxicol 75:1–7
Rizvi A, Khan MS (2018) Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol Environ Saf 157:9–20
Sawut R, Kasim N, Maihemuti B, Hu L, Abliz A, Abdujappar A, Kurban M (2018) Pollution characteristics and health risk assessment of heavy metals in the vegetable bases of northwest China. Sci Total Environ 642:864–878
Schreck E, Foucault Y, Sarret G, Sobanska S, Cécillon L, Castrec-Rouelle M, Uzu G, Dumat C (2012) Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: mechanisms involved for lead. Sci Total Environ 427:253–262
Schreck E, Laplanche C, Le Guédard M, Bessoule J-J, Austruy A, Xiong T, Foucault Y, Dumat C (2013) Influence of fine process particles enriched with metals and metalloids on Lactuca sativa L. leaf fatty acid composition following air and/or soil-plant field exposure. Environ Pollut 179:242–249
Schreck E, Dappe V, Sarret G, Sobanska S, Nowak D, Nowak J, Stefaniak EA, Magnin V, Ranieri V, Dumat C (2014) Foliar or root exposures to smelter particles: consequences for lead compartmentalization and speciation in plant leaves. Sci Total Environ 476-477:667–676
Shahid M, Pinelli E, Pourrut B, Silvestre J, Dumat C (2011) Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicol Environ Saf 74:78–84
Shahid M, Pinelli E, Dumat C (2012) Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J Hazard Mater 219:1–12
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E (2014): Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants, reviews of environmental contamination and toxicology volume 232. Springer, pp. 1-44
Shahid M, Dumat C, Khalid S, Schreck E, Xiong T, Niazi NK (2017a) Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J Hazard Mater 325:36–58
Shahid M, Rafiq M, Niazi NK, Dumat C, Shamshad S, Khalid S, Bibi I (2017c) Arsenic accumulation and physiological attributes of spinach in the presence of amendments: an implication to reduce health risk. Environ Sci Pollut Res 24:16097–16106
Shahid M, Pinelli E, Dumat C (2018) Tracing trends in plant physiology and biochemistry: need of databases from genetic to kingdom level. Plant Physiol Biochem 127:630–635
Shahid M, Shamshad S, Farooq ABU, Rafiq M, Khalid S, Dumat C, Zhang Y, Hussain I, Niazi NK (2019) Comparative effect of organic amendments on physio-biochemical traits of young and old bean leaves grown under cadmium stress: a multivariate analysis. Environ Sci Pollut Res 26:11579–11590
Song J, Zhang H, Duan C, Cui X (2018) Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress. J For Res 29:1497–1505
Uzu G, Sobanska S, Aliouane Y, Pradere P, Dumat C (2009) Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environ Pollut 157:1178–1185
Uzu G, Sobanska S, Sarret G, Munoz M, Dumat C (2010) Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ Sci Technol 44:1036–1042
Uzu G, Sauvain JJ, Baeza-Squiban A, Riediker M, Hohl MS, Val S, Tack K, Denys S, Pradere P, Dumat C (2011) In vitro assessment of the pulmonary toxicity and gastric availability of lead-rich particles from a lead recycling plant. Environ Sci Technol 45:7888–7895
Uzu G, Schreck E, Xiong T, Macouin M, Lévêque T, Fayomi B, Dumat C (2014) Urban market gardening in Africa: foliar uptake of metal (loid) s and their bioaccessibility in vegetables; implications in terms of health risks. Water Air Soil Pollut 225:2185
Wang Z, Watanabe I, Ozaki H, Zhang J (2018) Enrichment and bioavailability of trace elements in soil in vicinity of railways in Japan. Arch Environ Contam Toxicol 74:16–31
Xiong T-T, Leveque T, Austruy A, Goix S, Schreck E, Dappe V, Sobanska S, Foucault Y, Dumat C (2014a) Foliar uptake and metal (loid) bioaccessibility in vegetables exposed to particulate matter. Environ Geochem Health 36:897–909
Xiong T, Leveque T, Shahid M, Foucault Y, Mombo S, Dumat C (2014b) Lead and cadmium phytoavailability and human bioaccessibility for vegetables exposed to soil or atmospheric pollution by process ultrafine particles. J Environ Qual 43:1593–1600
Xiong T, Austruy A, Pierart A, Shahid M, Schreck E, Mombo S, Dumat C (2016) Kinetic study of phytotoxicity induced by foliar lead uptake for vegetables exposed to fine particles and implications for sustainable urban agriculture. J Environ Sci 46:16–27
Xiong T, Dumat C, Dappe V, Vezin H, Schreck E, Shahid M, Pierart A, Sobanska S (2017) Copper oxide nanoparticle foliar uptake, phytotoxicity, and consequences for sustainable urban agriculture. Environ Sci Technol 51:5242–5251
Xiong T, Zhang T, Dumat C, Sobanska S, Dappe V, Shahid M, Xian Y, Li X, Li S (2018) Airborne foliar transfer of particular metals in Lactuca sativa L.: translocation, phytotoxicity, and bioaccessibility. Environ Sci Pollut Res 26:20064–20078. https://doi.org/10.1007/s11356-018-3084-x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 38.6 kb)
Rights and permissions
About this article
Cite this article
Natasha, Shahid, M. & Khalid, S. Foliar application of lead and arsenic solutions to Spinacia oleracea: biophysiochemical analysis and risk assessment. Environ Sci Pollut Res 27, 39763–39773 (2020). https://doi.org/10.1007/s11356-019-06519-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06519-7