Abstract
The lime, biochar, and fly ash are widely used to ameliorate acidic soils. The purpose of our pot experiment was to understand whether these soil amendments affected the availability of phosphorus in soils using diffusive gradients in thin films (DGT) technique. Three soil amendments, biochar (B), fly ash (F), and lime (L), were applied alone or combined to Ultisol and Alfisol at the rate of 40, 40, and 6 g/kg, respectively. The DGT and chemical extraction techniques were used to quantify the soil available (phosphorus) P dynamic in the rice growth period. The addition of amendments can effectively increase the phosphorus content extracted by 0.01 M KCl solution. The biochar amendment showed the best effect on increasing the contents of DGT-P and Olsen-P in the soil. Combined with the DGT-induced fluxes in sediments (DIFS) model, it was found that soil amendments mainly increased the adsorption rate (k) and desorption rate (k−1), and reduced soil reflect time (Tc) of phosphorus released to the pore water. The P contents accumulated in rice grains correlated more closely with the effective P concentration measured by DGT technique, compared with those with soil total P, pore water P, and Olsen-P. These results suggested that the DGT technique had significant advantage over conventional chemical extraction techniques when assessing the bioavailability of phosphorus to rice growth. Application of soil amendments increased the contents of Al-P and Ca-P of both Ultisol and Alfisol, and decreased the content of Fe-P in soils. The addition of amendments could change the affinity of Fe and phosphorus decreasing the phosphorus fixation in soils. The increase of available phosphorus in soil is mainly due to the change of pH value and the direct diffusion of phosphorus in the amendments (biochar and fly ash). In conclusion, soil amendment can affect the availability and adsorption ability of phosphorus in soil, and biochar has the best effect on the availability of soil phosphorus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strongly acidic soils are widely covered in tropical and subtropical regions of China. The soil acidification will lead to phosphorus (P) deficiency in soils, which is often considered as an important limiting factor to plant growth in strongly acidic soils (Blake et al. 2000; Koutika et al. 2016; Solomon et al. 2002). In practice, biochar, fly ash, and lime are common amendments to ameliorate acidic soils and increase the bioavailability of phosphorus in soils.
Lime can effectively reduce soil acidity and it is often used as a soil amendment to ameliorate agricultural production of strongly acidic soils (Curtin and Syers 2001; Holland et al. 2018). Many field experiments demonstrated the ameliorating effects of lime for highly acidic soils. Its ameliorating mechanism was mainly attributed to reduce soil Al toxicity, to improve soil structure, and to increase the availability of P to plants (Curtin and Syers 2001). Recent studies found that biochar as a soil amendment can effectively ameliorate the physical and chemical degeneration of highly weathered tropical and subtropical soils. More importantly, biochar increases the availability of phosphate in soils (Glaser et al. 2002; Sohi et al. 2010; Soinne et al. 2014). Biochar itself, depending on feedstock and pyrolysis temperature, also contains large amounts of phosphate (Enders et al. 2012; Hong and Lu 2018). As a result, biochar application can directly not only supply a source of plant available P into the soil, but also increase phosphorus bioavailability (Atkinson et al. 2011; Sohi et al. 2010; Parvage et al. 2013; Soinne et al. 2014). Fly ash, a by-product of coal-burning power plants, is also a good amendment to ameliorate acidic soils (Jala and Goyal 2006; Yunusa et al. 2012). This is because fly ash has very high pH and strong acid-neutralizing capacity.
The available P in soils refers to P present in the soil solution, which is reversibly adsorbed on the solid phase or present in labile precipitates. Soil scientists had developed several chemical extraction tests to quantify the plant available P in soil. However, these chemical extraction tests are often debated because they do not mimic the entire process that controls the availability of phosphorus to plants (Nawara et al. 2017). In fact, they could extract large amounts of P that are not available for plant uptake. As an alternative to traditional chemical extraction, the diffusive gradient in thin film technique (DGT) has been introduced to quantify plant available P in soils (Ding et al. 2010; Mason et al. 2010; Menzies et al. 2005; Six et al. 2013; Zhang et al. 1998a, b; Vogel et al. 2017). Previous studies had demonstrated that the DGT technique provided a novel approach for the in situ measurement of the labile forms of chemical elements in soils and sediments (Zhang et al. 2014). The working mechanism of DGT for assessing availability of P in soils is similar to the uptake of phosphorus by plant roots. For example, Menzies et al. (2005) used the DGT technique to predict the availability of phosphorus in a pot experiment. They found that the DGT predicted maize uptake for P significantly more accurately than traditional chemical extraction. Vogel et al. (2017) recommended that the DGT technique was a reliable method to assess the performance of P fertilizers for maize growth. The field experiments on Australian soils indicated that DGT technique was superior to other soil P tests for predicting plant growth on the tropical soils (Mason et al. 2010; Speirs et al. 2013). In addition, the DGT method has a much better correlation to plant available P in soils than chemical extraction tests (Zhang et al. 1998; Menzies et al. 2005; Tandy et al. 2011; Six et al. 2013). These studies concluded that DGT technique could be used to estimate the available phosphorus content and to predict effectively the availability of phosphorus to plant.
Although the ameliorating effects of three amendments on soil properties and crop growth have been extensively studied, little work dealt with the available phosphorus dynamic induced by soil amendments. The DGT technique has been used to predict the availability of P in soils and sediments (Ding et al. 2011, 2018), but there have been very few investigations into the interactions of soil amendment with phosphorus dynamic. In this study, we combined the DGT technique and chemical extraction to assess the availability of phosphorus to plant uptake after soil amendments were applied. The purpose of the study was to evaluate the effect of soil amendments on bioavailability of phosphorus in acidic soils and to understand the mechanism of soil amendment in improving soil phosphorus dynamic using DGT technique.
Materials and methods
Soils and amendments
The two soils (Ultisol and Alfisol) were collected from the surface soils (0–20 cm) of subtropical region. The Ultisol was taken from a low-hilly location developed on Quaternary red earth in Xuancheng, Anhui Province (N 30° 56.6′, E 118° 45.2′). The Alfisol was developed on alluvial material in Wenzhou, Zhejiang Province (N 28° 23.2′, E 121° 04.3′). The soils were air dried at room temperature and then ground to pass through a 2-mm sieve. The biochar used in this study was produced from rice straw by slow pyrolysis at 400 °C for 2 h in a muffle furnace under an oxygen-limited condition. The biochar was ground to pass through a 1-mm sieve to obtain similar particle size. The fly ash used in this work was obtained from coal-burning power plant, Eastern China. The lime was a chemical agent. The basic properties of soils and amendments are given in Table 1.
Pot experiment
About 5 kg mixture of soil and amendment of each soil were weighed and placed into plastic pots. The application rates of biochar (B), fly ash (F), and lime (L) were 40, 40, and 6 g kg−1, respectively. The eight treatments were performed: control (no amendment), 40 g biochar kg−1 (B), 40 g fly ash kg−1 (F), 6 g lime kg−1 (L), 40 g biochar kg−1 + 40 g fly ash kg−1 (B + F), 40 g biochar kg−1 + 6 g lime kg−1 (B + F), 40 g fly ash kg−1 + 6 g lime kg−1 (FL), and 40 g biochar kg−1 + 40 g fly ash kg−1 + 6 g lime kg−1 (BFL). Three replicates were used per treatment. The experiment was carried out in a glasshouse. Each treatment was fertilized with 150 mg N, 100 mg P, and 100 mg K per kilogram soil. After mixing thoroughly the soil and amendment, the mixtures of soil and amendment were incubated at a saturated water content. After 60 days of incubation, rice (Oryza sativa) seedlings of uniform size were planted in each pot. The soil moisture was adjusted to be suitable for rice growth. The soil samples were collected at the tillering stage, jointing stage, and mature stage of rice, respectively. After the mature stage, the grain, aboveground straw, and roots of rice were harvested separately. Plant materials were washed first with tap water and then with deionized water. They were then oven dried at 70 °C for 48 h and weighed to determine their dry-weight biomass. Dried rice grains were ground to a fine powder using a stainless steel grinder for P content analyses.
Soil and plant analyses
The basic properties of studied soils were determined using routine procedures (Zhang and Gong 2012). Soil pH was determined by a pH meter (PB-21, Sartorius) in 1:2.5 soil to water suspension. Soil organic carbon (SOC) was measured by oxidation method with potassium dichromate and cation exchange capacity (CEC) by the ammonium acetate method (pH = 7). Soil exchangeable acidity was extracted with 1 M KCl solution and determined by titration with 0.02 M NaOH. The pH of the biochar was measured by adding biochar to deionized water in a ratio of 1:20 (w/v). The way of other properties of biochars were the same as soil. Soil total P (TP) was determined by digesting the soil samples with NaOH. The P concentrations in the plant tissue were determined by digesting the plant sample with H2SO4 solution. The orthophosphate in the digest solutions was determined using the colorimetric molybdate–ascorbic acid procedure (Murphy and Riley 1962).
Soil available P tests
The available P in soils was extracted by 0.01 M KCl and 0.5 M NaHCO3 (Olsen-P) solutions, respectively. The Olsen-P was extracted by adding 20 ml of 0.5 M NaHCO3 (pH = 8.5) to 1.0 g of soil, shaking for 30 min, and filtering through a filter paper. The concentration of Olsen-P was also determined using the colorimetric molybdate–ascorbic acid procedure (Murphy and Riley 1962).
Soil inorganic P form analysis
The chemical forms of inorganic P in the soils were analyzed using the modified scheme developed by Hedley et al. (1982). The soil samples were sequentially extracted by each of the following extractants:1.0 M NH4Cl for loosely bound phosphate, 30 ml of 0.5 M NH4F (pH 8.2) for aluminum phosphate (Al-P), 0.1 M NaOH and 0.1 M Na2CO3 for iron phosphate (Fe-P), 0.3 M Na3C6H5O7·2H2O for occluded phosphate (Oc-P), and 0.5 M H2SO4 for calcium phosphate (Ca-P). The concentrations of P in the extracts were immediately determined using colorimetric molybdate–ascorbic acid procedure (Murphy and Riley 1962).
Diffusive gradient in thin films (DGT) measurements
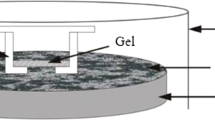
In our study, the Zr-oxide DGT device was used to measure the labile P in soils. The measured DGT-P includes soluble reactive P (SRP) in the pore water and weakly bound P that is dynamically released from the soil solids (Ding et al. 2018; Harper et al. 2000). The development of Zr-oxide DGT and its application has been reported previously (Ding et al. 2010, 2011). The DGT units in our study were purchased from EasySensor Ltd. (www.easysensor.net). The DGT units consist of a Zr-oxide series fixed film, agarose diffusion gel (0.80 mm), and polyvinylidene fluoride (PVDF) membrane (0.45 μm pore size and 0.10 mm thickness). The detailed description of the DGT device and calculation of DGT parameters can be found elsewhere (Zhang et al. 1995, 1998; Ding et al. 2018).
Deployment process of DGT-P: 30 g soil samples were adjusted to the 70% of water-holding capacity. The soils were stirred evenly and covered with plastic film to prevent water evaporation. After the soils were balanced 48 h at the constant temperature (25 °C), a small amount of soils were put into the DGT hole using a clean plastic spoon. The DGT device was vibrated gently on the table in order to make the soil in full contact with the membrane surface of the DGT unit. Then, the DGT device was transferred to the plastic wrap with a small amount of deionized water. After 24 h in the constant temperature, soil particles were removed from the DGT device by deionized water. The DGT device was stored under 4 °C condition. To measure the phosphorus concentrations in the pore water, a portion of the soil paste prepared for the DGT measurements was added to 50 ml polyethylene tubes and centrifuged at 4000 r/min−1 for 10 min. The collected supernatant was filtered using a 0.45-μm pore size filter paper. After filtration, the supernatant was stored under 4 °C condition for measuring P concentrations in the pore water. The fixed films in the DGT devices were eluted with 1.8 ml of 1 M NaOH solution overnight at room temperature. The resin gels were eluted with 1 ml of 1 M HNO3 for 24 h. The DGT-P and DET-P contents were analyzed using the molybdate-ascorbic acid procedure (Murphy and Riley 1962).
Theoretical calculation
The time-averaged concentration of phosphate (PDGT) at the DGT interface was calculated using Eq. 1 according to Zhang and Davison (1995).
where M is the accumulated P mass in the DGT-binding layer (ng), Δg is the thickness of the diffusion layer (cm), D is the diffusion coefficient of P in the diffusive gel (cm2 s−1), and A is the surface area of the DGT-sampling window, and t is the deployment time.
The ratio R, a measure of resupply from the solid phase, is the comparison of PDGT with the independently measured P concentration in the soil pore water (PDET) (both determined at the 70% water-holding capacity of soil).
The distribution coefficient between the solid and dissolved phases, Kd, for P was calculated as the ratio of labile (easily exchangeable) solid phase pool concentration (POlsen) to SRP concentration in the pore water (PDET) (Eq. 3). In this study, we assumed Olsen-P as labile solid phase pool of P.
The DGT theory introduces a concept of PE that represents the effectively available P from both soil solution and the solid-phase labile pool. PE was calculated using Eq. 4 (Zhang et al. 2004)
where Rdiff is the hypothetical ratio of the PDGT when no resupply from the solid phase occurred. Rdiff was calculated using the DIFS dynamic numerical model (DGT-induced fluxes in sediments and soils) (Harper et al. 2000).
The desorption (k−1) and adsorption (k1) rate constants were determined using Eqs. 5 and 6, respectively (Ding et al. 2018; Harper et al. 1998; Zhang et al. 1998).
Data analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis of data. A one-way analysis of variance (ANOVA) was carried out to determine significant differences between treatments using LSD’s test with a significant level of p < 0.05.
Results and discussion
Total P content in soils and plants
The effect of soil amendments on total P content in soils and rice grains was shown in Fig. 1. Application of the three amendments had no significant effect on total P content of soils except BF-treated Ultisol. This was mainly due to the fact that biochar contains abundant phosphorus (Table 1), and the increased phosphorus in soils comes from direct input of biochar. Application of soil amendments significantly (p < 0.05) increased the total P content of rice grains grown in Alfisol except lime-amended soil (Fig. 1). For Ultisol, only BFL treatment significantly increased the total P content of rice grain, whereas soil amendments alone and combined had no significant effects on the total P content of rice.
Available P content in soils measured by chemical tests
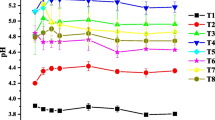
The phosphorus extracted with 0.01 M KCl solution for 24 h was defined as the short term available phosphorus for plant uptake (Nawara et al. 2017). As could be seen from Fig. 2, the addition of amendments could effectively increase the short-term plant availability of phosphorus in the soils. The combined application of soil amendments significantly (p < 0.05) increased the contents of short-term plant available phosphorus in Ultisol and Alfisol, compared with the treatments of control and amendment alone. The increased availability of phosphorus was because application of amendment-reduced soil acidity, which lead to the release of more free water-soluble phosphate ions.
Effects of soil amendments (biochar, fly ash, and lime) alone and combination on the KCl-extractable P concentrations in soils. Error bars represent standard error of the means (n = 3). Different letters indicate significant differences between treatments at p < 0.05 level. B biochar; F fly ash; L lime
The Olsen-P was generally considered to be an unstable pool of phosphorus in the soil or the total amount of phosphorus that plants could uptake (Nawara et al. 2017). The effect of soil amendments alone and combined on Olsen-P was shown in Fig. 3. The content of Olsen-P in soils decreased as rice grows. In Ultisol, Olsen-P content was reduced from 60.93 mg kg−1 at the tillering stage to 45.25 mg kg−1 at the mature stage. In Alfisol, Olsen-P content was reduced from 30.60 mg kg−1 at the tillering stage to 9.67 mg kg−1 at the mature stage. The decreasing rate in Alfisol was much larger than that in Ultisol. In Ultisol, treatments containing biochar or fly ash increased the content of available phosphorus in soil at the tillering stage compared with control treatment. At the jointing stage, only B + F treatment significantly increase the content of available phosphorus in soil. In the mature period, amendments alone and combined had no significant effects on available phosphorus content in soil. In Alfisol, B and B + F + L treatments significantly increased the content of available phosphorus in soil at the tillering stage, while only B treatment significantly increased Olsen-P content in the jointing and mature stage. The F and L treatments significantly decreased the Olsen-P content in soil at the tillering stage of rice. In the whole rice growth stage, F and L treatments reduced soil available P content.
Effects of soil amendments (biochar, fly ash, and lime) alone and combination on the Olsen-P concentrations of soils in the three growth periods of rice. Error bars represent standard error of the means (n = 3). Different letters indicate significant differences between treatments at p < 0.05 level. B biochar; F fly ash; L lime
Labile P concentration measured by DGT
Figure 4 showed the changes of DGT-P content in soils. The content of DGT-P in soils decreased with the growth of rice, which was consistent with the Olsen-P changes. In Ultisol, the DGT-P in soil reduced from 0.0647 mg L−1 in the tillering stage to 0.0416 mg L−1 at the mature stage, which decreased by 35.7%. In Alfisol, the DGT-P reduced from 0.0214 mg L−1 at the tillering stage to 0.0104 mg L−1 at the mature stage, which decreased by 51.4%. The application of biochar alone and combined showed higher DGT-p values. In Ultisol, DGT-p values of B + F + L treatment had the highest values, while the biochar treatment of Alfisol had their highest DGT-p value. In Ultisol, treatments containing biochar increased soil DGT-P content at the tillering stage, but only BFL treatment reached significant level. At the jointing stage and mature stage, these three amendments had decreased effects on DGT-P content in soil, especially F + L treatment. In Alfisol, at the tillering stage, all the seven treatments increased the content of DGT-P in soil, but only the biochar treatment reached the significant level. At the jointing stage, biochar (B) and BL treatments still significantly increased DGT-P content in soil. At the mature stage, the DGT-P content in Alfisol with amendments was still higher than that in control treatment. However, only B + F + L had significant increase in DGT-P.
Effects of soil amendments (biochar, fly ash, and lime) alone and combination on the DGT-P concentrations of soils in the three growth periods of rice. Error bars represent standard error of the means (n = 3). Different letters indicate significant differences between treatments at p < 0.05 level. B biochar; F fly ash; L lime
Previous studies demonstrated that DGT-P and plant uptake had strong correlation if diffusive transport of phosphate from soil to plant roots was not limited (Degryse et al. 2009). The rice roots at the tillering stage have the high rate of absorption for soil phosphorus, therefore, the soil at the tillering stage of rice was used to perform DIFS model analysis. The PDGT and PDET, which was regarded as the phosphorus content in soil pore water at the tillering stage of rice were shown in Table 2. Results indicated that treatments containing biochar (B and BFL) increased PDGT and PDET contents of two soils. This suggested that biochar amendment could improve the pool and bioavailability of phosphorus in soils. In contrast, the application of fly ash and lime has no significant effect on the PDET content of soils.
Based on Olsen-P, PDGT, and PDET values and other parameters, the parameters that reflect soil P diffusion and kinetics of P resupply from soil solid matrix were calculated by DIFS model. The calculated parameters were given in Table 2. The P concentrations in pore water (PDET) of Ultisol and Alfisol were 0.094 and 0.037 mg L−1, respectively, while the effective P concentrations (PE) calculated as the ratio between PDGT and Rdiff were 0.33 and 0.12 mg L−1, respectively (Table 2). The R value indicates the magnitude of the phosphorus resupply from the solid phase to the soil solution. The R ratio of Ultisol and Alfisol were 0.69 and 0.57, respectively, indicating that these soils were intermediate capacity for the solid phase to resupply the mobile and kinetically labile phosphate. The application of biochar and fly ash increased consistently the R and Kd values, while lime decreased these values. Modeling with DIFS showed that biochar and fly ash decreased the Tc values, while lime increased Tc value. Biochar and fly ash increased the k1 and k−1 values, whereas lime decreased k1 and k−1. The magnitude of decrease or increase depended on soil types. The k1 and k−1 values of B + F + L treatment were 4.96 (× 10−3 s−1) and 6.52 (× 10−6 s−1) for Ultisol, and 2.15 (× 10−3 s−1) and 2.65 (× 10−6 s−1) for Alfisol, respectively. The changes in kinetics modeled by DIFS confirmed that effects of soil amendments on available P dynamics. The biochar and fly ash reduced the ability of the solids in retaining P (Kd), and increased the resupplying SRP to the pore water (R). The rate of desorption (reflected by k−1) was larger than the rate of adsorption (reflected by k1) (Table 2). However, the changes in these kinetic parameters are contrary to those induced by lime amendment that has a high-immobilization efficiency for P.
Relationship between chemical test, DGT, and plant measurements
A correlation analysis was performed to investigate the relationships between the total P content of rice grain and soil P test values, i.e., total P in soils (TP), KCl-extractable P, Olsen-P, P in soil pore water (DEP-P), and DGT-P (Table 3). Highly significant relations were observed between the total P content in rice grains and total P in soil (r = 0.785, p < 0.01), Olsen-P (r = 0.799, p < 0.01), and DGT-P concentrations in the soils (r = 0.832, p < 0.01). The P concentrations measured by DGT were significantly linearly correlated with the P contents accumulated in rice grains, indicating that the DGT measured P concentrations was the best predictor for rice P contents among these soil P tests. The DGT-P concentrations were significantly correlated with the total P and concentrations of Olsen-P and DET-P in soils. The correlation for the P content accumulated by rice grain and soil P using the DGT technique was better than those obtained by total P in soils, chemical extraction, and pore water measurements. DGT-measured P included the contributions from soil solution, the solid phase labile pool and the kinetics of exchange between the two states (Zhang et al. 2001). The best correlation between P contents in rice and DGT-P appeared that the kinetics of supply from the solid phase to solution was an important factor affecting P uptake by rice. In contrast, the correlations between rice P and soil P concentrations measured by other methods were considerably weaker because they could not replicate the dynamic P uptake processes by rice. The results showed that DGT offers the possibility of simple prediction of P bioavailability in soil and can be used for the assessment of P bioavailability in soil.
The R value represents the effect of phosphorus diffusion in soil pores and the interaction of soil solid phase supplementation of phosphorus to pore water. The application of biochar and fly ash increased the R value, whereas lime application decreased the R value (Table 2). R-Rdiff represents the ability of soil to provide phosphorus to pore water due to the consumption of DGT. The application of biochar and fly ash could increase the value of R-Rdiff suggesting that the application of biochar and fly ash could improve soil solid phase’s ability to supplement phosphorus in plant roots. The phosphorus effective concentration (PE) represents soil solution P and easily desorbable P in soil. The Ultisol had higher PE than the Alfisol. The application of fly ash and lime could not increase PE in Ultisol, but they increased PE in Alfisol. There is a significant negative correlation between system response time (Tc) with sorption rate constant (k1) and desorption rate constant (k−1) in the soils (p < 0.05; r = 0.651 and 0.563, respectively). From Table 2 we could observe that k1 (soil adsorption rate) was much greater than the soil desorption rate (k−1), and biochar and fly ash could increase the adsorption rate and desorption rate of soils, reduce the response time (Tc), which might be the main reasons that biochar and fly ash increased soil phosphorus bioavailability. Compared with the control, modeling with DIFS showed that B, F, and B + F + L treatments remarkably decreased Tc values, while increased k1 and k−1.
Chemical forms of inorganic P in soils
The chemical forms of inorganic phosphorus in soils were shown in Fig. 5. The Fe-P, Al-P, and Ca-P accounted for 26.3%, 15.1%, and 3.8% of total phosphorus in Ultisol. In Alfisol, the Fe-P, Al-P, and Ca-P were 11.6%, 16.2%, and 11.8% of total phosphorus, respectively. The proportion of Fe-P in Ultisol was larger than that in Alfisol, while the proportion of Ca-P in Alfisol was much higher than that in Ultisol. This was because Ultisol had much higher free iron oxide content than Alfisol. The application of the three amendments alone or combined increased the content of Ca-P and reduced the content of Fe-P in soils. Except for lime treatment (L), application of amendments had an increasing trend of Al-P content in soils. Compared with the control, biochar increased the Al-P content by 8.3%, and fly ash by 7.3%, whereas lime decreased the Al-P content by 11.0% in Ultisol. The effects of combined application of amendments on chemical forms of phosphorus were more than amendments alone. The addition of amendments significantly decreased the Fe-P content in soils except for biochar treatment (B) in Ultisol, and biochar, fly ash, and biochar + fly ash treatments in Alfisol. It might be expected that the change of chemical forms of phosphate was mainly due to the pH change induced by soil amendments. Correlation analysis showed that there was a significant correlation between Fe-P and soil pH value. The increase of soil pH value leads to the formation of precipitation of free Fe3+ in soil. As a result, the soil loses many highly active P-adsorption sites (Murphy and Stevens 2010), which resulted in the decrease of Fe-P content in soils. The application of soil amendment alone or combined increased the Ca-P contents in soils compared with the control treatment (Fig. 5), while the combined application of soil amendments significantly (p < 0.05) increased the Ca-P content in soils. The treatments containing lime had higher Ca-P contents. The increase of Ca-P content in soil contributed to the input of free Ca ion from soil amendments. The lime amendment can release more Ca ion into soils than biochar and fly ash amendments, which lead to the formation of calcium phosphate precipitates. It concluded that application of soil amendments substantially changed the chemical forms of phosphorus, which greatly affected the availability of phosphorus in soils. The effects of soil amendments largely depended on the amendment type.
Conclusions
Application of the three amendments alone and combined affected the availability and chemical forms of phosphorus in soils. The combined application of the three amendments had greater effects on the P availability than individual application of biochar, fly ash, and lime. The biochar and fly ash increased the available phosphorus content of Ultisol and Alfisol, whereas lime could reduce the availability of phosphorus in soils. The biochar and fly ash are the best choice for increasing available P of acidic soils. The P bio-availabilities assessed using DGT methods could predicted P bio-availabilities induced by soil amendments. The DGT technique could use as a prediction tool for P bioavailability when compared to the traditional chemical extraction procedures. The use of DGT can be a promising tool to study bio-available P concentrations in amended soils.
References
Atkinson CJ, Fitzgerald JD, Hipps NA (2011) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Blake L, Mercik S, Koerschens M, Moskal S, Poulton PR, Goulding KWT, Weigel A, Powlson DS (2000) Phosphorus content in soil, uptake by plants and balance in three European long-term field experiments. Nutr Cycl Agroecosyst 56:263–275
Curtin D, Syers JK (2001) Lime-induced changes in indices of soil phosphate availability. Soil Sci Soc Am J 65:147–152
Degryse F, Smolders E, Zhang H, Davison W (2009) Predicting availability of mineral elements to plants with the DGT technique: a review of experimental data and interpretation by modelling. Environ Chem 6:198–218
Ding SM, Xu D, Sun Q, Yin HB, Zhang CS (2010) Measurement of dissolved reactive phosphorus using the diffusive gradients in thin films technique with a high-capacity binding phase. Environ Sci Technol 44:8169–8174
Ding SM, Jia F, Xu D, Sun Q, Zhang L, Fan C, Zhang CS (2011) High-resolution, two-dimensional measurement of dissolved reactive phosphorus in sediments using the diffusive gradients in thin films technique in combination with a routine procedure. Environ Sci Technol 45:9680–9686
Ding S, Chen M, Cui J, Wang D, Lin J, Zhang C, Tsange DCW (2018) Reactivation of phosphorus in sediments after calcium-rich mineral capping: implication for revising the laboratory testing scheme for immobilization efficiency. Chem Eng J 331:720–728
Enders A, Hanley K, Whitman T, Joseph S, Lehmann J (2012) Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour Technol 114:644–653
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal–a review. Biol Fert Soils 35:219–230
Harper MP, Davison W, Tych W (2000) DIFS–a modelling and simulation tool for DGT induced trace metal remobilisation in sediments and soils. Environ Model Softw 15:55–66
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976
Holland JE, Bennett AE, Newton AC, White PJ, McKenzie BM, George TS, Pakeman RJ, Bailey JS, Fornara DA, Hayes RC (2018) Liming impacts on soils, crops and biodiversity in the UK: a review. Sci Total Environ 610-611:316–332
Hong C, Lu SG (2018) Does biochar affect the availability and chemical fraction of phosphate in soils? Environ Sci Pollut Res 25:8725–8734
Jala S, Goyal D (2006) Fly ash as a soil ameliorant for improving crop production–a review. Bioresour Technol 97:1136–1147
Koutika LS, Mareschal L, Epron D (2016) Soil P availability under eucalypt and acacia on ferralic Arenosols, Republic of the Congo Geoderma Regional 7:153–158
Mason S, McNeill A, McLaughlin MJ, Zhang H (2010) Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant Soil 337:243–258
Menzies NW, Kusumo B, Moody PW (2005) Assessment of P availability in heavily fertilized soils using the diffusive gradient in thin films (DGT) technique. Plant Soil 269:1–9
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Ana Chim Ac 27:31–36
Murphy PNC, Stevens RJ (2010) Lime and gypsum as source measures to decrease phosphorus loss from soils to water. Water Air Soil Pollut 212:101–111
Nawara S, Van Dael T, Merckx R, Amery F, Elsen A, Odeurs W, Vandendriessche H, Mcgrath S, Roisin C, Jouany C, Pellerin S, Denoroy P, Eichler-Lobermann B, Börjesson G, Goos P, Akkermans W, Smolders E (2017) A comparison of soil tests for available phosphorus in long-term field experiments in Europe. Euro J Soil Sci 68:873–885
Parvage MM, Ulen B, Eriksson J, Strock J, Kirchmann H (2013) Phosphorus availability in soils amended with wheat residue char. Biol Fertil Soil 49:245–250
Six L, Smolders E, Merckx R (2013) The performance of DGT versus conventional soil phosphorus tests in tropical soils–maize and rice responses to P application. Plant Soil 366:49–66
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82
Soinne H, Hovi J, Tammeorg P, Turtola E (2014) Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 219–220:162–167
Solomon D, Lehmann J, Mamo T, Fritzsche F, Zech W (2002) Phosphorus forms and dynamics as influenced by land use changes in the sub-humid Ethiopian highlands. Geoderma 105:21–48
Speirs SD, Scott BJ, Moody PW, Mason SD (2013) Soil phosphorus tests II: a comparison of soil test-crop response relationships for different soil tests and wheat. Crop Past Sci 64:469–479
Tandy S, Mundus S, Yngvesson J, de Bang T, Lombi E, Schjoerring J, Husted S (2011) The use of DGT for prediction of plant available copper, zinc and phosphorus in agricultural soils. Plant Soil 346:167–180
Vogel C, Sekine R, Steckenmesser D, Lombi E, Steffens D, Adam C (2017) Phosphorus availability of sewage sludge-based fertilizers determined by the diffusive gradients in thin films (DGT) technique. J Plant Nutr Soil Sci 180:594–601
Yunusa IAM, Loganathan P, Nissanka SP, Manoharan V, Burchett MD, Skilbeck CG, Eamus D (2012) Application of coal fly ash in agriculture: a strategic perspective. Crit Rev Environ Sci Technol 42(6):559–600
Zhang H, Davison W (1995) Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal Chem 67:3391–3400
Zhang G L, Gong Z T (2012) Soil survey laboratory methods (in Chinese). Science Press, Beijing
Zhang H, Davison W, Gadi R, Kobayahsi T (1998a) In situ measurement of dissolved phosphorus in natural waters using DGT. Ana Chim Acta, 370;29–38
Zhang H, Davison W, Knight B, McGrath S (1998b) In situ measurements of solution concentrations and fluxes of trace metals in soils using DGT. Environ Sci Technol 32:704–710
Zhang C, Ding S, Xu D, Tang Y, Wong MH (2014) Bioavailability assessment of phosphorus and metals in soils and sediments: a review of diffusive gradients in thin films (DGT). Environ Monit Assess 186:7367–7378
Funding
This research was supported by the National Key Research & Development Program of China (2016YFD0200302).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Hong, C., Su, Y. & Lu, S. Phosphorus availability changes in acidic soils amended with biochar, fly ash, and lime determined by diffusive gradients in thin films (DGT) technique. Environ Sci Pollut Res 25, 30547–30556 (2018). https://doi.org/10.1007/s11356-018-3086-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3086-8