Abstract
This paper provides an overview of the principle and latest development of the diffusive gradients in thin films (DGT) technology and its applications in environmental studies with a focus on bioavailability assessment of phosphorus and metals in sediments and soils. Compared with conventional methods, DGT, as a passive sampling method, has significant advantages: in situ measurement, time averaged concentrations and high spatial resolution. The in situ measurement avoids artificial influences including contamination of samples and sample treatment which may change the forms of chemicals. The time averaged concentration reflects representative measurement over a period of time. The high-resolution information captures the biogeochemical heterogeneity of elements of interest distributed in microenvironments, such as in the rhizosphere and the vicinity of the sediment-water interface. Moreover, DGT is a dynamic technique which simultaneously considers the diffusion of solutes and their kinetic resupply from the solid phases. All the advantages of DGT significantly promote the collection of “true” information of the bioavailable or labile forms of chemicals in the environment. DGT provides potential for applications in agriculture, environmental monitoring and the mining industry. However, the applications are still at the early testing stage. Further studies are needed to properly interpret the DGT-measured results under complex environmental conditions, and standard procedures and guideline values based on DGT are required to pave the way for its routine applications in environmental monitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The diffusive gradients in thin films (DGT) technology provides a novel approach for the in situ measurement of the labile forms of chemical elements, such as phosphorus (P), sulphur (S), arsenic (As) and metals in waters, sediments and soils (Davison and Zhang 1994, 2012). It was invented in Lancaster by Bill Davison and Hao Zhang in 1993 (DGT Research Ltd. 2014; Davison and Zhang 1994). The simple device uses a hydrogel binding layer impregnated with Chelex resin or other binding agents to accumulate ions. The binding layer is overlain by a diffusive layer of hydrogel and a filter. Ions have to diffuse through the filter and diffusive layer to reach the binding layer. It is the establishment of a constant concentration gradient in the diffusive layer that forms the basis for measuring chemical element concentrations in solution quantitatively. Deployment time can vary from 1 h to 3 months provided the binding resin is not saturated. The measurement is independent of ionic strength if the range is within 10 mM–1 M. For Chelex-100 as the binding resin, the measurement is independent of pH in the range of 5–8.3. The effect of temperature can be predicted from the known temperature dependence of the diffusion coefficient (Zhang and Davison 1995).
DGT can be used for many different purposes (DGT Research Ltd. 2014), including the following:
-
In situ measurements
-
Monitoring (time averaged concentrations)
-
Speciation (labile inorganic and/or organic species)
-
Bioavailability (effective concentration)
-
Fluxes in sediments and soils
-
Kinetic and thermodynamic constants
-
High spatial resolution measurements (sub-mm)
-
2D concentration images
This paper provides an overview of DGT theory and its recent development and applications in bioavailability assessment with comments on its future development. The main purpose of this review is to promote the use of DGT; thus, the scope is restricted to the general concepts and applications without technical details which are available elsewhere (Davison and Zhang 1994, 2012; DGT Research Ltd. 2014). For the applications, this review focuses on the uses of DGT in bioavailability assessment of phosphorus and metals in sediments and soils, while an extensive review was performed on a similar topic in water (INAP 2012).
Theory of DGT
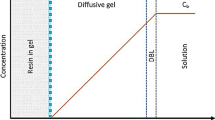
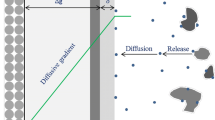
The fundamental theory behind DGT is Fick’s first law of diffusion. For deployment, the unit is immersed in water, inserted into sediments or placed in close contact with wet soils. The labile forms of chemical elements diffuse through the filter and diffusive gel and are then adsorbed on the binding gel. The process is illustrated in Fig. 1, which is explained as follows.
The principles of DGT applied in water, sediments and soils are similar (Davison and Zhang 2012). When the unit is deployed, there is a diffusive boundary layer (DBL) between the diffusive layers (including both diffusive gel and filter membrane) and the solution. Within a few minutes, a steady state linear concentration gradient is established between the solution and the binding gel (Harper et al. 1998). The flux J of an ion through the diffusive gel is given by Fick’s first law of diffusion (Eq. 1):
where D is the diffusion coefficient and dC/dx is the concentration gradient. Assuming the diffusion coefficients in the diffusive layers and DBL are the same, the concentration gradient can be calculated as (Eq. 2):
where C is the concentration of an ion in solution, C′ is the concentration at the boundary between the binding gel and the diffusive gel, ∆g is the combined thickness of the diffusive gel layer and filter layer and δ is the boundary layer thickness. If the free ions are in rapid equilibrium with the binding agent, with a large binding constant, C′ is effectively zero, provided the binding agent is not saturated. In well-stirred solutions, the boundary layer thickness δ is negligibly small. Therefore, Eq. 1 can be simplified as Eq. 3:
During the period of deployment, when the binding gel is not saturated, the flux can also be calculated based on the amount of accumulated mass (M) of an ion, exposure area (A) of the DGT unit and deployment time (t):
Combining Eqs. 3 and 4, the concentration C of an ion in the solution can be calculated as Eq. 5.
In this equation, the accumulated mass M can be measured directly in the binding-gel layer by drying it and using a beam technique such as laser ablation ICP-MS or, in the case of radionuclides, by direct counting. However, it is often obtained via elution of the ion from the binding gel into solution for routine measurements to minimize the cost. The accumulated mass can be calculated using the following Eq. 6.
where C e is the concentration of the ion in the elution solution, V e is the volume of the elution solution, V g is the volume of the gel and ƒ e is the elution efficiency. Diffusion coefficient D is related to the property of the diffusive gel and temperature. A list of diffusion coefficients for the most commonly used polyacrylamide gels cross-linked with an agarose derivative (APA) has been measured (Zhang and Davison 1999), and their tabulated values for different temperatures and elements are available from the dgtresearch.com website (DGT Research Ltd. 2014).
It needs to be noted that there are some assumptions for Eq. 5, such as the omissions of the concentration on binding layer C′ and DBL thickness δ. The first omission is not regarded as problematic. For the influence of omitting DBL thickness δ in Eq. 5, it was suggested that the error caused would be much less than the typical experimental errors (e.g. 10 %) (Davison and Zhang 2012). The assumptions hold for most conditions, but there are exceptions and challenges, especially in systems with complex heterogeneous ligands (Davison and Zhang 2012).
Development of DGT
Since its invention 20 years ago, DGT technology has been developing rapidly. Significant developments have been made in two aspects of the technology: new binding gels for various analytes and 2D high-resolution measurements.
The analytes that can be measured by DGT are determined by the binding agent in use (Table 1). It is known that the Chelex resin can take up trace metals, as it contains paired iminodiacetate ions which act as chelating groups in binding polyvalent metal ions. The binding agent for the first DGT was Chelex resin (Davison and Zhang 1994; Zhang and Davison 1995). After that, the ferrihydrite gel with strong affinity for phosphorus was used to measure labile phosphorus (Zhang et al. 1998) and silver iodide was included in the gel to take up sulphide (Teasdale et al. 1999). The radioactive caesium was adsorbed by a gel containing ammonium molybdophosphate (Murdock et al. 2001). Recently, the Zr oxide gel was developed to measure phosphorus and inorganic arsenic with high capacities (Ding et al. 2010; Sun et al. 2013, 2014). It needs to be mentioned that the DGT method has recently been developed to measure organic compounds (Chen et al. 2012) which is beyond the scope of this review.
Besides measurements of individual chemical elements, techniques for simultaneous measurements of multiple elements have been developed. Two separate gels of silver iodide and Chelex-100 were used together to measure sulphide and metals in sediments (Motelica-Heino et al. 2003). A mixed binding layer (MBL) containing a mixture of ferrihydrite and Chelex-100 was developed to measure phosphorus and cations (Mason et al. 2005). A titanium dioxide gel-assembled DGT has been used to simultaneously measure arsenic, phosphorus and metals (Bennett et al. 2010; Panther et al. 2010, 2013). The hydrous zirconium oxide (Zr oxide) has been combined with silver iodide to measure both phosphorus and sulphide (Ding et al. 2012) and combined with Chelex to measure phosphorus and iron (Xu et al. 2013). The mixed Amberlite and ferrihydrite gel has been recently developed to measure potassium and phosphorus (Zhang et al. 2013). Recently, it has been found that the capacities of the Zr oxide DGT for As in freshwater and seawater were 5∼19 times and 3∼13 times more than those reported for the commonly used ferrihydrite and Metsorb DGTs, respectively (Sun et al. 2014).
Another significant development in DGT is the 2D high-resolution measurement, which provides new evidences for the micro-scale biogeochemical heterogeneity of sediments (Stockdale et al. 2009). The scales have generally reached sub-millimetre level using various technologies, including proton-induced X-ray emissions (Davison et al. 1997), computer-imaging densitometry (Teasdale et al. 1999; Devries and Wang 2003; Jezequel et al. 2007; Robertson et al. 2008; Ding et al. 2013), laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) (Warnken et al. 2004; Stockdale et al. 2008; Santner et al. 2012; Stahl et al. 2012) and 2D slicing (Ding et al. 2011, 2012) (Table 2). Besides measurements for a single analyte in 2D high resolution, simultaneous measurements of multiple analytes have received attention, which enable the investigation of the relationships among multiple elements during their transport processes in pore waters and from the solid phases, e.g. Zn, Mn, Fe and As (Davison et al. 1997), iron and sulphur (Jezequel et al. 2007; Robertson et al. 2008), phosphorus, vanadium and arsenic (Stockdale et al. 2008, 2010), phosphorus and sulphide (Ding et al. 2012) and iron and phosphorus (Xu et al. 2013).
Development of new binding gels enables DGT to analyse diverse analytes in the environment, especially those of environmental significance, and to collect high-resolution information on a 2D level. The simultaneous measurements of multiple analytes are helpful to capture their coherent dynamics between pore waters and solid phases, and the 2D high-resolution measurements provide novel information of their micro-scale heterogeneity. Further development is needed to increase the range of analytes measured both individually and simultaneously, and easy and cheap methods are needed to make the 2D high-resolution measurements more accessible. One of the main issues should be the comparability of the results from different binding gels. Standardized DGT methods are required to ensure that the DGT results measured using different binding gels and under different conditions can be meaningfully compared. The capacity of a binding gel layer for DGT response is a key limiting factor for a wide application of DGT. Taking the complex background in natural environments into consideration, it is vitally important to develop and apply high-capacity DGT techniques to ensure a reliable and robust measurement especially under challenging environments.
Applications of DGT in bioavailability assessment
Applications of DGT in soil P assessment
Phosphorus (P) is an essential element for plant growth and is also added to animal feed (Bomans et al. 2005). In soils, phosphorus exists in different forms: associated with soil particles, adsorbed on Fe-Al oxides or Ca-carbonates, incorporated in organic matter and, to a much lesser extent, in soluble form dissolved in the soil solution (Bomans et al. 2005). Since only a small portion of P is available for plant uptake, it is often amended via fertilizer application, and P becomes one of the most expensive nutrients in agriculture (van Raij et al. 2002). Economic benefit can be achieved via accurate assessment of P status in soils to maximize fertilizer efficiency (Mason et al. 2010).
It is recognized that the total concentrations of P in soils fail to provide sufficient information for the assessment of its bioavailability. However, the established soil testing methods for assessment of available phosphorus (P), such as Colwell (Colwell 1963) and Olsen (Olsen et al. 1954), have been shown to fail to reliably predict plant P requirements over a range of soil types (e.g. calcareous, acidic with high iron or aluminium) (Holdford et al. 1985; McBeath et al. 2005; Mason et al. 2010). Recently, it has been demonstrated that the DGT method predicted plant responsiveness to applied P more accurately than Colwell P (Burkitt et al. 2002; Moody 2007; Mason et al. 2010, 2013). In an experiment comparing the various soil test methods for wheat response to applied P, it has been found that, compared with DGT, the Colwell, Olsen and resin methods are more likely to measure more non-labile forms of P in soils (Mason et al. 2013).
Since the development of a binding gel for P analyses (Zhang et al. 1998), it has been applied for soil P assessment in numerous studies, with successful demonstrations of plant yield response to P fertilizers (Tandy et al. 2011; Six et al. 2012) and more specifically for wheat (McBeath et al. 2007; Mason et al. 2010), tomato (Menzies et al. 2005) and maize (Six et al. 2013) responses to P fertilizers. Mason et al. (2008) demonstrated that for analysis of P in soils, the Fe oxide binding gel is highly specific to P and less subjected to anionic interferences compared to the resin method. However, conflicting results for the performance of DGT for the assessment of plant availability for P have been reported for rice (Six et al. 2013) and pasture (Burkitt et al. 2011). However, Burkitt et al. (2011) acknowledged that their conclusion was not firm due to a number of issues identified in their study (e.g. low P fertilizer effectiveness, possible solubilization of less labile P forms by pasture root exudates and potential increase in the amount of P adsorbed during soil storage).
The DGT technology has provided an effective way of assessing P availability for plants; thus, it provides a more precise recommendation for P fertilizer application in nutrient management (Zhang et al. 2013). Further work needs to consider a wide range of plant types, soil types and climate conditions. This would allow the establishment of guideline values for P fertilizer application based on the DGT measurements.
Applications of DGT in sediment P assessment
Eutrophication has been one of the major threats to water quality worldwide, with the basic cause in most cases likely to be excess P inputs (McGarrigle et al. 2010). However, even though external sources are being regulated, eutrophication of rivers and lakes has become a major and persistent problem, e.g. in Ireland (Taylor et al. 2012). With regulated inputs, the contribution of P from the internal sources of sediments becomes important. An accurate assessment of P status in sediments should consider the diffusive process of pore water P coupled with its kinetic resupply from the solid phase. Traditional chemical extraction methods can hardly satisfy such a requirement, as they are developed based on operationally defined response to chemical reagents rather than on a true reflection of P lability (Condron and Newman 2011). Due to its in situ feature, DGT technology is responding to the kinetic solid-solution interaction of P rather than a pseudo-equilibrium between extractant and soil/sediment (Davison and Zhang 2012).
The purpose of using DGT in the measurement of sediment P includes three aspects, measurement of dissolved reactive phosphorus (DRP) concentration, observation of heterogeneous distribution of DRP on a small scale and assessment of sediment reactivity in resupplying DRP. Zhang et al. (1998) first applied DGT to measure the concentration of DRP in sediments at a vertical spatial resolution of 1 mm. A steep increase of DRP was observed with increasing sediment depth, reflecting a risk of sediment P to be released to overlying water. The concentration gradients of DRP were frequently obtained with DGT especially in the vicinity of the sediment-water interface (SWI) in later studies (Monbet et al. 2008; Pichette et al. 2009; Ding et al. 2010; Santner et al. 2010; Xu et al. 2012), implying that DGT is potentially a new tool in the assessment of internal P load. The feasibility needs a further study, including establishment of a quantitative relationship between the DGT-measured concentration and the flux of DRP from sediment.
A distinct heterogeneous distribution of DRP in sediments on a small scale was observed from DGT measurement at sub-millimetre and 2D levels (Ding et al. 2011, 2012, 2013). This observation discovered small, discrete spots (termed microniche with diameters of 1 to several mm) with elevated P concentrations in a sediment profile. The formation of the sulphide microniche previously reported has been attributed to strong decomposition of enriched organic matter in localized zones (Stockdale et al. 2009). The bioturbation of the tubificid worm significantly increased the chemical heterogeneity of DRP, whose effects were much more pronounced than observed at a routine spatial resolution (∼1 cm) (Ding et al. 2011). Simultaneous measurements of DRP with dissolved sulphide further observed their simultaneous release in a microniche with a diameter of ∼3 mm and in locally aggregated zones with a length over 1 cm. This new phenomenon was attributed to the simultaneous reductions of Fe(III) and sulphate and the associated release of Fe-bound P in sediments (Ding et al. 2012). However, the depletion of DRP at the sulphidic microniche was also observed in a sediment profile from a productive lake (Stockdale et al. 2008). The inverse relationships between DRP and dissolved sulphide imply complex biogeochemical processes controlling their cycling.
As DGT is a dynamic technique which can demonstrate the resupply of sediment solids, the measurement of DRP with DGT is especially useful in the characterization of sediment P reactivity. An easy way is to compare the DGT-measured DRP concentration to the real concentration of DRP in pore water. Their ratio (R) close to 1 indicates highly reactive sediment, whereas R < < 1 indicates inert sediments. The R values collected in several studies were mostly less than 0.5 except for a profile from Lake Wellington, which reflected a limited resupply of DRP from solid reactive P pools (Monbet et al. 2008; Ding et al. 2010; Xu et al. 2012). The surface sediments generally had lower values of R compared to deeper sediments, which is further demonstrated by higher sediment response time (Tc) and lower partitioning coefficient (K d) (Monbet et al. 2008). The R values in the sediment of an algal-dominated region of Lake Taihu were lower than those in a macrophyte-dominated region (Ding et al. 2010). Capping of polluted sediments with Fe- and Al-rich soil also decreased R values because the capped sediments had larger K d values and greater adsorption capacity (Q max) (Xu et al. 2012).
Recently, a promising technique was developed for the high-resolution imaging of labile phosphorus (P) in sediments in combination with DGT (Ding et al. 2013). This technique was based on the surface coloration of the Zr oxide binding gel using the conventional molybdenum blue method following the DGT uptake of P to this gel. The accumulated mass of the P in the gel was then measured using computer-imaging densitometry (CID). The use of this technique has observed small, discrete spots with elevated P concentrations in a sediment profile. The high-resolution 2D measurement of P in sediment profiles thus provides novel information for the characterization of P in sediments, which deserves more attention.
The current development of DGT for sediment P assessment is still mainly at the laboratory level with limited field applications. Field applications under various benthic environments are needed, and the link between DGT-measured P results and eutrophication mechanisms and trophic levels should be properly established in the future.
Applications of DGT in the bioavailability assessment of metals in soils
It is well known that the total concentrations of metals in soils bear little information of their availability to plants (Nolan et al. 2003; Degryse et al. 2009). Traditionally, extraction methodologies, including neutral salts (such as CaCl2 (Novozamsky et al. 1993) and NaNO3 (Sanka and Dolezal 1992)), diluted or mild acids (0.1 M HCl (Baker and Amacher 1982)) and organic extractants including ethylene-diamine-tetra-acetic acid (EDTA) (Ure et al. 1993) and diethylenetriaminepentaacetic acid (DTPA) (Lindsay and Norvell 1978), are applied to assess the bioavailability of metals in soils, but they are operationally defined and have been found with only limited/varying success (McLaughlin et al. 2000; Menzies et al. 2007).
It has been recognized that, in conventional methods of testing soil solution, metal speciation may change during sampling and extraction and the kinetics of metal resupply from solid phase to solution are not considered (Hooda and Zhang 2008). Meanwhile, the bioavailability of metals in a given soil is dependent on both their concentrations in the soil solution and their rate of transport through the soil (Hooda and Zhang 2008). DGT measures directly the mean flux of labile species in soils to the device during the deployment. Therefore, it provides a novel and promising approach for the measurement of bioavailable metal concentrations in soils (Zhang et al. 2001). The effective concentration, C E, measured by DGT has been shown to give a better correlation to plant uptake than any other measurement (Zhang et al. 2001; Song et al. 2004; Nolan et al. 2005; Koster et al. 2005). The main reason is that DGT mimics the main mechanism of plant uptake by lowering the concentration locally and inducing diffusive supply and release from the solid phase (Lehto et al. 2006). It automatically accounts for all soil properties, including pH and organic matter content.
DGT assessment of bioavailability is most accurate under conditions where the diffusive transport of an element from soil to the plant roots is rate-limiting for its uptake (Degryse et al. 2009). If plant uptake is slow, competitive cations may affect the plant uptake, while they have no effect on DGT flux, whereas labile complexes do not contribute to the plant uptake but they are measured by DGT. When the plant has little affinity for the element or when the supply is large and the plant uptake is saturated, the DGT flux and plant uptake may not correlate well (Degryse et al. 2009). In mining tailings when the pH is low, competition with other cations that are present at very high concentrations may hinder the accumulation of metals by the chelating resins (Conesa et al. 2010). Another important factor affecting DGT deployment in soils is moisture. Soil should be moist enough (field capacity or above) in order to maintain contact with the membrane (Hooda et al. 1999). Based on an experiment dealing with Cd, the DGT flux reached the highest level when the soil moisture content was at the maximum water holding capacity (MWHC) level (Hooda et al. 1999). Therefore, when assessing bioavailability using the DGT results, soil moisture should be considered and preferably consistent soil moisture is considered for comparison and assessment purposes.
The applications of DGT in the bioavailability assessment of metals in soils have received wide attention in recent years (Table 3), with both positive (Emily et al. 2012; Liu et al. 2012; Tatiana Garrido and Jorge Mendoza 2013; Senila et al. 2012) and negative (Muhammad et al. 2012; Williams et al. 2012) results. Researches have also provided cautionary comments or conditions on the use DGT for metal bioavailability assessment; for example, DGT prediction of plant availability was best in anaerobic compared to aerobic soils (Mundus et al. 2012). The DGT technique was fairly predictive of bioavailability in the greenhouse conditions but not in the field (Agbenin and Welp 2012). The DGT approach was demonstrated to be a dynamic in situ measuring technique that can be used as a surrogate of bio-indicators if the cationic correction is taken into account (Ferreira et al. 2013).
Besides plant bioavailability assessment, a recent study assessed metal bioavailability to earthworms using DGT, showing that DGT can be used to mimic the earthworm uptake of heavy metals in contaminated soils (Bade et al. 2012). The DGT results were found highly correlated with physiologically based extraction test (PBET) results for As, Pb, Cu and Zn in contaminated sites (Bade et al. 2013). It has also demonstrated that DGT results reasonably predict mono-methylmercury uptake by clams from the aqueous phase and provide the basis for application of the DGT device as a surrogate for a sentinel organism for monitoring bioavailable mono-methylmercury (Clarisse et al. 2012).
For the applications in bioavailability assessment of metals in soils, DGT has provided a promising approach. Further work is required to have more plant species tested and to have more metals analysed under various field conditions including soil and climate conditions.
Conclusion
Based on the concepts of diffusion and absorption, DGT is now an established technique quantitatively measuring concentrations of a wide range of analytes in waters, sediments and soils. It provides an effective way for the assessment of P in soils for nutrient management, P in sediment for eutrophication assessment and metals in soils for bioavailability assessment.
DGT works well under the assumption of the establishment of a constant diffusive gradient during deployment, and the results rely on the feature of the binding gels. Further development is needed to have more analytes measured both individually and simultaneously, and easy and cheap methods are needed to have the 2D high-resolution measurements performed. The current applications of DGT are still mainly for research purposes. Standardized methodologies, especially under complicated field conditions, are needed to render the results derived from DGT more meaningful, so that guideline values from DGT for environmental monitoring and management could be established in the future.
References
Agbenin, J. O., & Welp, G. (2012). Bioavailability of copper, cadmium, zinc, and lead in tropical savanna soils assessed by diffusive gradient in thin films (DGT) and ion exchange resin membranes. Environmental Monitoring and Assessment, 184(4), 2275–2284.
Bade, R., Oh, S., & Shin, W. S. (2012). Diffusive gradients in thin films (DGT) for the prediction of bioavailability of heavy metals in contaminated soils to earthworm (Eisenia foetida) and oral bioavailable concentrations. Science of the Total Environment, 146, 127–136.
Bade, R., Oh, S., Shin, W. S., & Hwang, I. (2013). Human health risk assessment of soils contaminated with metal(loid)s by using DGT uptake: a case study of a former Korean metal refinery site. Human and Ecological Risk Assessment: An International Journal, 19(3), 767–777.
Baker, D. E., & Amacher, M. C. (1982). Nickel, copper, zinc, and cadmium. In A. L. Page, R. H. Miller, & D. R. Keeney (Eds.), Methods of soil analysis: part 2. Chemical and microbiological methods (pp. 323–336). Madison, WI: American Society of Agronomy/Soil Science Society of America.
Bennett, W. W., Teasdale, P. R., Panther, J. G., Welsh, D. T., & Jolley, D. F. (2010). New diffusive gradients in a thin film technique for measuring inorganic arsenic and selenium (IV) using a titanium dioxide based adsorbent. Analytical Chemistry, 82(17), 7401–7407.
Bomans, E., Fransen, K., Gobin, A., Mertens, J., Michiels, P., Vandendriessche, H., & Vogels, N. (2005). Addressing phosphorus related problems: final report to the European Commission. Leuven-Heverlee: Soil Service of Belgium.
Burkitt, L. L., Moody, P. W., Gourley, C. J., & Hannah, M. C. (2002). A simple phosphorus buffering index for Australian soils. Australian Journal of Soil Research, 40(3), 497–513.
Burkitt, L. L., Mason, S. D., & Dougherty, W. J. (2011). A preliminary assessment of the ability of the DGT soil phosphorus test to predict pasture response in Australian pasture soils. Australia. Dairy Australia Ltd, UT13919 Final (Abstract only). http://ecite.utas.edu.au/73967 (last access date: 20/06/2014)
Chen, C. E., Zhang, H., & Jones, K. C. (2012). A novel passive water sampler for in situ sampling of antibiotics. Journal of Environmental Monitoring, 14(6), 1523–1530.
Clarisse, O., Lotufo, G. R., Hintelmann, H., & Best, E. H. (2012). Biomonitoring and assessment of monomethylmercury exposure in aqueous systems using the DGT technique. Science of the Total Environment, 416, 449–454.
Colwell, J. D. (1963). The estimation of the phosphorus fertiliser requirements of wheat in southern New South Wales by soil analysis. Australian Journal of Experimental Agriculture and Animal Husbandry, 3, 190–198.
Condron, L. M., & Newman, S. (2011). Revisiting the fundamentals of phosphorus fractionation of sediments and soils. Journal of Soils and Sediments, 11(5), 830–840.
Conesa, H. M., Schulin, R. R., & Nowack, B. (2010). Suitability of using diffusive gradients in thin films (DGT) to study metal bioavailability in mine tailings: possibilities and constraints. Environmental Science and Pollution Research, 17(3), 657–664.
Davison, W., & Zhang, H. (1994). In situ speciation measurements of trace components in natural waters using thin-film gels. Nature, 367, 546–548.
Davison, W., & Zhang, H. (2012). Progress in understanding the use of diffusive gradients in thin films (DGT)—back to basics. Environmental Chemistry, 9, 1–13.
Davison, W., Fones, G. R., & Grime, G. W. (1997). Dissolved metals in surface sediment and a microbial mat at 100 μm resolution. Nature, 387(6636), 885–888.
Degryse, F., Smolders, E., Zhang, H., & Davison, W. (2009). Predicting availability of mineral elements to plants with the DGT technique: a review of experimental data and interpretation by modelling. Environmental Chemistry, 6, 198–218.
Devries, C. R., & Wang, F. Y. (2003). In situ two-dimensional high-resolution profiling of sulfide in sediment interstitial waters. Environmental Science and Technology, 37(4), 792–797.
DGT Research Ltd. (2014). DGT—for measurements in waters, soils and sediments. http://www.dgtresearch.com/dgtresearch/dgtresearch.pdf (last access date: 20/6/2014)
Ding, S. M., Di, X., Sun, Q., Yin, H. B., & Zhang, C. S. (2010). Measurement of dissolved reactive phosphorus using the diffusive gradients in thin films technique with a high-capacity binding phase. Environmental Science and Technology, 44, 8169–8174.
Ding, S. M., Jia, F., Di, X., Sun, Q., Zhang, L., Fan, C. X., & Zhang, C. S. (2011). High-resolution, two-dimensional measurement of dissolved reactive phosphorus in sediments using the diffusive gradients in thin films technique in combination with a routine procedure. Environmental Science and Technology, 45, 9680–9686.
Ding, S. M., Sun, Q., Di, X., Jie, F., He, X., & Zhang, C. S. (2012). High-resolution simultaneous measurements of dissolved reactive phosphorus and dissolved sulfide: the first observation of their simultaneous release in sediments. Environmental Science and Technology, 46, 8297–8304.
Ding, S. M., Wang, Y., Xu, D., Zhu, C. G., & Zhang, C. S. (2013). Gel-based coloration technique for the submillimeter-scale imaging of labile phosphorus in sediments and soils with diffusive gradients in thin films. Environmental Science and Technology, 47, 7821–7829.
Emily, E., Chapman, V., Dave, G., & Murimboh, J. D. (2012). Bioavailability as a factor in risk assessment of metal-contaminated soil. Water, Air, & Soil Pollution, 223(6), 2907–2922.
Ferreira, D., Ciffroy, P., Tusseau-Vuillemin, M.-H., Bourgeault, A., & Garnier, J.-M. (2013). DGT as surrogate of biomonitors for predicting the bioavailability of copper in freshwaters: an ex situ validation study. Chemosphere, 91(3), 241–247.
Harper, M. P., Davison, W., Zhang, H., & Tych, W. (1998). Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes. Geochimica et Cosmochimica Acta, 62, 2757–2770.
Holdford, I. C., Morgan, J. M., Bradley, J., & Cullis, B. R. (1985). Yield responsiveness and response curvature as essential criteria for the evaluation and calibration of soil phosphorus tests for wheat. Australian Journal of Soil Research, 23, 167–180.
Hooda, P. S., & Zhang, H. (2008). Chapter 9 DGT measurements to predict metal bioavailability in soils. In R. Naidu (Ed.), Developments in soil science (Chemical bioavailability in terrestrial environments, Vol. 32, pp. 169–185). Amsterdam: Elsevier B.V.
Hooda, P. S., Zhang, H., Davison, W., & Edwards, A. C. (1999). Measuring bioavailable trace metals by diffusive gradients in thin films (DGT): soil moisture effects on its performance in soils. European Journal of Soil Science, 50, 285–294.
International Network for Acid Prevention (INAP) (2012). Diffusive gradients in thin-films (DGT): a technique for determining bioavailable metal concentrations. INAP.
Jezequel, D., Brayner, R., Metzger, E., Viollier, E., Prevot, F., & Fievet, F. (2007). Two-dimensional determination of dissolved iron and sulfur species in marine sediment pore-waters by thin-film based imaging. Thau lagoon (France). Estuarine, Coastal and Shelf Science, 72(3), 420–431.
Koster, M., Reijnders, L., van Oost, N. R., & Peijnenburg, W. J. (2005). Comparison of the method of diffusive gels in thin films with conventional extraction techniques for evaluating zinc accumulation. Environmental Pollution, 133, 103–116.
Lehto, N., Davison, W., Zhang, H., & Tych, W. (2006). Theoretical comparison of how soil processes affect uptake of metals by diffusive gradients. Journal of Environmental Quality, 35, 1903–1913.
Lindsay, W. L., & Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Science Society of America Journal, 42, 421–428.
Liu, J. L., Feng, X. B., Qiu, G. L., Anderson, C. W., & Yao, H. (2012). Prediction of methyl mercury uptake by rice plants (Oryza sativa L.) using the diffusive gradient in thin films technique. Environmental Science and Technology, 46(20), 11013–11020.
Mason, S. D., Hamon, R. E., Nolan, A. L., Zhang, H., & Davison, W. (2005). Performance of a mixed binding layer for measuring anions performance of a mixed binding layer for measuring anions thin films technique. Analytical Chemistry, 77, 6339–6346.
Mason, S. D., Hamon, R. E., Zhang, H., & Anderson, J. (2008). Investigating chemical constraints to the measurement of phosphorus in soils using diffusive gradients in thin films (DGT) and resin methods. Talanta, 74(4), 779–787.
Mason, S., McNeill, A., McLaughlin, M. J., & Zhang, H. (2010). Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant and Soil, 337, 243–258.
Mason, S. D., McLaughlin, M. J., Johnston, C., & McNeill, A. (2013). Soil test measures of available P (Colwell, resin and DGT) compared with plant P uptake using isotope dilution. Plant and Soil. doi:10.1007/s11104-013-1833-7.
McBeath, T. M., Armstrong, R. D., Lombi, E., McLaughlin, M. J., & Holloway, R. E. (2005). Responsiveness of wheat to liquid and granular phosphorus fertilisers in southern Australia. Soil Research, 43(2), 203–212.
McBeath, T. M., McLaughlin, M. J., Armstrong, R. D., Bell, M., Bolland, M. D., Conyers, M. K., Holloway, R. E., & Mason, S. D. (2007). Predicting the response of wheat (Triticum aestivum L.) to liquid and granular phosphorus fertilisers in Australian soils. Soil Research, 45(6), 448–458.
McGarrigle, M., Lucey, J., & Cinnéide, M. Ó. (2010). Water quality in Ireland (2007–2009). Wexford: Environmental Protection Agency.
McLaughlin, M. J., Zarcinas, B. A., Stevens, D. P., & Cook, N. (2000). Soil testing for heavy metals. Communications in Soil Science and Plant Analysis, 31, 1661–1700.
Menzies, N. W., Kusumo, B., & Moody, P. W. (2005). Assessment of P availability in heavily fertilized soils using the diffusive gradients in thin films (DGT) technique. Plant and Soil, 269, 1–9.
Menzies, N. W., Donn, M. J., & Kopittk, P. M. (2007). Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environmental Pollution, 145, 121–130.
Monbet, P., McKelvie, I. D., & Worsfold, P. J. (2008). Combined gel probes for the in situ determination of dissolved reactive phosphorus in porewaters and characterization of sediment reactivity. Environmental Science and Technology, 42(14), 5112–5117.
Moody, P. W. (2007). Interpretation of a single-point P buffering index for adjusting critical levels of the Colwell soil P test. Australian Journal of Soil Research, 45, 55–62.
Motelica-Heino, M., Chris Naylor, C., Zhang, H., & Davison, W. (2003). Simultaneous release of metals and sulfide in lacustrine sediment. Environmental Science and Technology, 37(19), 4374–4381.
Muhammad, I., Puschenreiter, M., & Wenzel, W. W. (2012). Cadmium and Zn availability as affected by pH manipulation and its assessment by soil extraction, DGT and indicator plants. Science of the Total Environment, 416, 490–500.
Mundus, S., Lombi, E., Holm, P. E., Zhang, H., & Husted, S. (2012). Assessing the plant availability of manganese in soils using diffusive gradients in thin films (DGT). Geoderma, 183–184, 92–99.
Murdock, C., Kelly, M., Chang, L. Y., Davison, W., & Zhang, H. (2001). DGT as an in situ tool for measuring radiocesium in natural water. Environmental Science and Technology, 35, 4530–4535.
Nolan, A. L., Lombi, E., & McLaughlin, M. J. (2003). Metal bioaccumulation and toxicity in soils—why bother with speciation? Australian Journal of Chemistry, 56(3), 77–91.
Nolan, A. L., Zhang, H., & McLaughlin, M. J. (2005). Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. Journal of Environmental Quality, 34, 496–507.
Novozamsky, I., Lexmond, T. T., & Houba, V. J. (1993). A single extraction procedure of soil for evaluation of uptake of some heavy metals by plants. International Journal of Environmental Analytical Chemistry, 51, 47–58.
Olsen, S. R., Cole, C. V., Watanabe, F. S., & Dean, L. A. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture, CircularNo. 939.
Panther, J. G., Teasdale, P. R., Bennett, W. W., Welsh, D. T., & Zhao, H. J. (2010). Titanium dioxide-based DGT technique for in situ measurement of dissolved reactive phosphorus in fresh and marine waters. Environmental Science and Technology, 44(24), 9419–9424.
Panther, J. G., Stewart, R. R., Teasdale, P. R., Bennett, W. W., Welsh, D. T., & Zhao, H. J. (2013). Titanium dioxide-based DGT for measuring dissolved As(V), V(V), Sb(V), Mo(VI) and W(VI) in water. Talanta, 105, 80–86.
Pichette, C., Zhang, H., & Sauve, S. (2009). Using diffusive gradients in thin-films for in situ monitoring of dissolved phosphate emissions from freshwater aquaculture. Aquaculture, 286, 198–202.
Puschenreiter, M., Wittstock, F., Friesl-Hanl, W., & Wenzel, W. W. (2013). Predictability of the Zn and Cd phytoextraction efficiency of a Salix smithiana clone by DGT and conventional bioavailability assays. Plant and Soil. doi:10.1007/s11104-013-1597-0.
Robertson, D., Teasdale, P. R., & Welsh, D. A. (2008). A novel gel-based technique for the high resolution, two-dimensional determination of iron (II) and sulfide in sediment. Limnology and Oceanography: Methods, 6, 502–512.
Sanka, M., & Dolezal, M. (1992). Prediction of plant contamination by cadmium and zinc based on soil extraction method and contents in seedlings. International Journal of Environmental Analytical Chemistry, 46, 87–96.
Santner, J., Prohaska, T., Luo, J., & Zhang, H. (2010). Ferrihydrite containing gel for chemical imaging of labile phosphate species in sediments and soils using diffusive gradients in thin films. Analytical Chemistry, 82(18), 7668–7674.
Santner, J., Zhang, H., Leitner, D., Schnepf, A., Prohaska, T., Puschenreiter, M., & Wenzel, W. (2012). High-resolution chemical imaging of labile phosphorus in the rhizosphere of Brassica napus L. cultivars. Environmental and Experimental Botany, 77, 219–226.
Senila, M., Levei, E. A., & Senila, L. R. (2012). Assessment of metals bioavailability to vegetables under field conditions using DGT, single extractions and multivariate statistics. Chemistry Central Journal, 6, 119.
Six, L., Pypers, P., Degryse, F., Smolders, E., & Merckx, R. (2012). The performance of DGT versus conventional soil phosphorus tests in tropical soils—an isotope dilution study. Plant and Soil, 359(1–2), 267–279.
Six, L., Smolders, E., & Merckx, R. (2013). The performance of DGT versus conventional soil phosphorus tests in tropical soils—maize and rice responses to P application. Plant and Soil, 366, 49–66. doi:10.1007/s11104-012-1375-4.
Song, J., Zhao, F. J., Luo, Y. M., McGrath, S. P., & Zhang, H. (2004). Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environmental Pollution, 128, 307–315.
Stahl, H., Warnken, K. W., Sochaczewski, L., Glud, R. N., Davison, W., & Zhang, H. (2012). A combined sensor for simultaneous high resolution 2-D imaging of oxygen and trace metals fluxes. Limnology and Oceanography: Methods, 10, 389–401.
Stockdale, A., Davison, W., & Zhang, H. (2008). High-resolution two dimensional quantitative analysis of phosphorus, vanadium and arsenic, and qualitative analysis of sulfide, in a freshwater sediment. Environmental Chemistry, 5(2), 143–149.
Stockdale, A., Davison, W., & Zhang, H. (2009). Micro-scale biogeochemical heterogeneity in sediments: a review of available technology and observed evidence. Earth-Science Reviews, 92(1–2), 81–97.
Stockdale, A., Davison, W., & Zhang, H. (2010). 2D simultaneous measurement of the oxyanions of P, V, As, Mo, Sb, W and U. Journal of Environmental Monitoring, 12(4), 981–984.
Sun, Q., Chen, Y. F., Xu, D., Wang, Y., & Ding, S. M. (2013). Detailed performance test of the hydrous zirconium oxide-based DGT technique for measurement of dissolved reactive phosphate. Journal of Environmental Sciences. doi:10.1016/S1001-0742(12)60140-5.
Sun, Q., Chen, J., Zhang, H., Ding, S. M., Li, Z., Williams, P. N., Cheng, H., Zhu, Y. X., Wu, L. H., & Zhang, C. S. (2014). Improved diffusive gradients in thin films (DGT) measurement of total dissolved inorganic arsenic in waters and soils using a hydrous zirconium oxide binding layer. Analytical Chemistry, 86(6), 3060–3067.
Tandy, S., Mundus, S., Yngvesson, J., de Bang, T. C., Lombi, E., Schjoerring, J. J., & Husted, S. (2011). The use of DGT for prediction of plant available copper, zinc and phosphorus in agricultural soils. Plant and Soil, 346(1–2), 167–180.
Tatiana Garrido, R., & Jorge Mendoza, C. (2013). Application of diffusive gradient in thin film to estimate available copper in soil solution. Soil and Sediment Contamination: An International Journal, 22(6), 654–666. doi:10.1080/15320383.2013.756447.
Taylor, D., McElarney, Y., Greene, S., Barry, C., Foy, B., & Jordan, P. (2012). An assessment of aquatic ecosystem responses to measures aimed at improving water quality in the Irish ecoregion, (2007-W-MS-3-S1), STRIVE synthesis report. Wexford: Environmental Protection Agency.
Teasdale, P. R., Sean Hayward, S., & Davison, W. (1999). In situ, high-resolution measurement of dissolved sulfide using diffusive gradients in thin films with computer-imaging densitometry. Analytical Chemistry, 71, 2186–2191.
Ure, A. M., Quevauviller, P., Muntau, H., & Griepink, B. (1993). Speciation of heavy metals in soils and sediments: an account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. International Journal of Environmental Analytical Chemistry, 51(1), 135–151.
van Raij, B., Cantarella, H., & Quaggio, J. A. (2002). Rationale of the economy of soil testing. Communications in Soil Science and Plant Analysis, 33, 2521–2536.
Warnken, K. W., Zhang, H., & Davison, W. (2004). Analysis of polyacrylamide gels for trace metals using diffusive gradients in thin films and laser ablation inductively coupled plasma mass spectrometry. Analytical Chemistry, 76(20), 6077–6084.
Williams, P. N., Zhang, H., Davison, W., Zhao, S. Z., Lu, Y., Dong, F., Zhang, L., & Pan, Q. (2012). Evaluation of in situ DGT measurements for predicting the concentration of Cd in Chinese field-cultivated rice: impact of soil Cd:Zn ratios. Environmental Science and Technology, 46(15), 8009–8016.
Xu, D., Ding, S., Sun, Q., Zhang, J., Wu, W., & Jia, F. (2012). Evaluation of in situ capping with clean soils to control phosphate release from sediments. Science of the Total Environment, 438, 334–341.
Xu, D., Chen, Y. F., Ding, S. M., Sun, Q., Wang, Y., & Zhang, C. S. (2013). Diffusive gradients in thin films technique equipped with a mixed binding gel for simultaneous measurements of dissolved reactive phosphorus and dissolved iron. Environmental Science and Technology, 47, 10477–10484.
Zhang, H., & Davison, W. (1995). Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Analytical Chemistry, 67, 3391–3400.
Zhang, H., & Davison, W. (1999). Diffusional characteristics of hydrogels used in DGT and DET techniques. Analytica Chimica Acta, 398, 329–340.
Zhang, H., Davison, W., Gadi, R., & Kobayashi, T. (1998). In situ measurement of dissolved phosphorus in natural waters using DGT. Analytica Chimica Acta, 370, 29–38.
Zhang, H., Zhao, F. J., Sun, B., Davison, W., & McGrath, S. P. (2001). A new method to measure effective soil solution concentration predicts copper availability to plants. Environmental Science and Technology, 35, 2602–2607.
Zhang, Y. L., Mason, S., McNeill, A., & McLaughlin, M. J. (2013). Optimization of the diffusive gradients in thin films (DGT) method for simultaneous assay of potassium and plant-available phosphorus in soils. Talanta, doi:10.1016/j.talanta.2013.03.023.
Acknowledgments
Dr. Chaosheng Zhang thanks Hong Kong Baptist University for provision of the prestigious University Fellowship, enabling him to visit the university in 2013 as part of his sabbatical leave from the National University of Ireland, Galway. This review was partly sponsored by the Chinese “111” Project (No. B08037) awarded to Sichuan University, China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, C., Ding, S., Xu, D. et al. Bioavailability assessment of phosphorus and metals in soils and sediments: a review of diffusive gradients in thin films (DGT). Environ Monit Assess 186, 7367–7378 (2014). https://doi.org/10.1007/s10661-014-3933-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3933-0