Abstract
In this work, the production of Scenedesmus obliquus in a continuous flat-plate laboratory-scale photobioreactor (PBR) under alternated day–night cycles was tested both experimentally and theoretically. Variation of light intensity according to the four seasons of the year were simulated experimentally by a tunable LED lamp, and effects on microalgal growth and productivity were measured to evaluate the conversion efficiency of light energy into biomass during the different seasons. These results were used to validate a mathematical model for algae growth that can be applied to simulate a large-scale production unit, carried out in a flat-plate PBR of similar geometry. The cellular concentration in the PBR was calculated in both steady-state and transient conditions, and the value of the maintenance kinetic term was correlated to experimental profiles. The relevance of this parameter was finally outlined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the diminishing fossil fuel supply, the research of alternative sources for fuel production is currently becoming a strong need, in particular to sustain the transportation sector. It is more and more recognized that biodiesel produced from microalgal-derived oil could substitute petroleum-derived transport fuels in the near future, in an eco-sustainable way [1, 2].
Although a number of relevant considerations are in favor of developing such a process at the industrial scale, some technical challenges still remain unsolved. In particular, when moving microalgal cultivation from laboratory to industry, careful planning is required [3]. The technology for large-scale production of microalgae already exists, but is mainly limited to open pond systems, and current commercial production is for high added value products only. So far, no industrial plants finalized to obtain low-value products such as lipids for biodiesel from microalgae are operated throughout the world [4]. In general, the approach to large-scale production of oil from microalgae must be approached in such a way to make the process environmentally and economically acceptable [3].
To become economically feasible, the cultivation of microalgae requires high biomass and lipid productivity per unit area and minimum plant investment and operating costs. In the case of PBRs, the efficient utilization of the photosynthetically active radiation (PAR) is one of the key parameters that could make this technology promising for higher throughputs [5].
In particular, in an actual PBR operated outdoor at mid-latitudes, the light conditions are widely variable, so that a number of issues must be thoroughly addressed, i.e. reactor orientation, light variation during the day, reflection of light, light gradient in the reactor [6]. Furthermore, continuous bioreactors operated at steady state are more reliable and reproducible [7], because they provide a higher controllability than batch ones, making continuous operations more advantageous at industrial scale. This operation mode is recently the object of intensive research [8–11], as well as the effect of light on growth and the modeling of light distribution in PBRs [9, 12–18]. Unfortunately, at present most of the PBR configurations proposed are by far too expensive in view of any industrial exploitation. To find out a solution able to reduce the capital and the operating costs of the process it is mandatory to test new ideas about PBRs, both experimentally and theoretically, to provide a sound design procedure. In fact, the prediction of PBRs behavior is fundamental for feasibility studies regarding large-scale production units.
In this work, the algal growth under simulated sunlight regimes of the four seasons at mid-latitudes was investigated. The aim is to evaluate the effect of seasonal irradiation on microalgae growth, biomass productivity and energy conversion to biomass, to better understand this process, and to give a contribution towards its industrialization and large-scale application with continuous PBRs. The species S. obliquus might be a promising microalga for large-scale lipid production because of its relatively high biomass growth rate which results in acceptable lipid and fatty acid productivity [19], and its satisfactory resistance to environmental changes. Based on laboratory experimental results, a mathematical model was also developed and applied to evaluate the key operating variables in S. obliquus cultivation and to simulate the PBR behavior in view of large-scale units design.
Materials and methods
Microalgae, media composition and growth analysis
The microalgae S. obliquus 276-7 (obtained from SAG-Goettingen) was maintained and cultured in sterilized BG11 medium. Medium was buffered with 10 mM HEPES pH 8 to avoid alterations due to excess CO2 supply. The medium was sterilized in an autoclave for 20 min at 121 °C to prevent any contamination. Algal growth was measured by daily changes in optical density measured at 750 nm, by a UV–Visible UV 300 spectrophotometer (Spectronic Unicam) and cells number (in a Bürker Counting Chamber). For dry weight determinations, cells were harvested with a filter (0.2 μm), which was dried at 80 °C for 4 h in a laboratory oven, and then weighed.
Equipment
Growth experiments were performed in a vertical flat-plate polycarbonate reactors (see [20] for the schematic of the experimental setup), with a working volume of 250 mL, sterilized in an autoclave. The depth of the reactor (1.2 cm) was minimized to reduce the cells self-shading and to allow a maximum utilization of light. The surface exposed to light is 208 cm2. The mixing in the culture is ensured by a CO2–air (5 % v/v) flow fed through a sieved silicone tube placed at the reactor bottom. The gas mixture is regulated using suitable valves and flow meters. The total gas flow rate is 1 L h−1 for each panel. The fresh medium is fed at a constant rate by a peristaltic pump (Watson-Marlow sci400, flow rate range 25–250 mL day−1), and a mixture of medium and cells is withdrawn from the PBR at the same rate by an overflow tube, and is collected in a sterilized tank. This system can be approximated to a CSTR, as demonstrated by tracer experiments: at time 0 a pulse of 2.5 mL of a Methylene Blue solution (10 g L−1) was fed to the reactor and a typical elution profile of the tracer obtained is shown in Fig. 1. The continuous curve here reported corresponds to a CSTR elution profile with 10 % of dead volume.
All the experiments were carried out in continuous mode, with and without recycle. When operating with recycle, part of the algal suspension from the collector tank was pumped back to the reactor inlet by the same peristaltic pump, to increase the biomass concentration in the reactor. The PBR operating conditions are summarized in Table 1.
Alternated day–night cycles were generated with a LED lamp (Photon System Instruments, SN-SL 3500-22). Photon flux density was measured upon both the reactor front panel and after the back one using a photoradiometer (HD 2101.1 from Delta OHM), which quantifies the PAR in the range 400–700 nm wavelengths. The light intensity as a function of time was simulated so that to provide the PBR with the same PAR amount of energy received under natural conditions at the selected latitude. PVGIS Solar Irradiation Data (http://re.jrc.ec.europa.eu/pvgis) is an online available database of typical day evolution of irradiation on a given surface for any earth location and time of year. This software was used as the source of irradiation data for the location of Padova, Italy. An incident angle of 35° was applied, as the default setting of the database, to exploit the maximum solar energy. Four months were selected as representatives of each season: January for winter, April for spring, July for summer and October for autumn. The direct PAR irradiation data reaching the reactor’s surface was fitted with the equation (Originlab™):
where
I dir (0) = direct PAR at the reactor front panel (z = 0), [μmol photons m−2 s−1];
t = hour at which PAR was calculated (0 ÷ 24), [h];
y 0 = reference intensity, [μmol m−2 s−1];
A [μmol m−2], w [h], x c [h] = fitting parameters characteristic of daily irradiation profiles for each season.
These profiles were simulated by the LED lamp system. In Fig. 2 a comparison between experimental and simulated day–night irradiation curves is given.
The photoconversion, a measure of the fraction of the PAR energy that is converted to biomass in a PBR, was calculated as:
where
E fix bio = energy converted into biomass per year (MJ year−1),
E PAR year = PAR energy hitting the PBR per year (MJ year−1);
P bio = biomass productivity per unit area (kg m−2 year−1)
E bio = energy content of biomass (MJ kg−1)
A = PBR surface (m2)
Simulation model
Equations
In a PBR three phases coexist: a liquid (the culture medium), a gas (CO2, N2, Air) and a solid suspension (the microalgae); liquid and gas phases provide the nutrients to the solid suspension.
Photosynthesis, the process by which the cells grow, also is driven by light energy. If nutrients are supplied in non-limiting conditions, photosynthesis is related to the availability and penetrability of light inside the reactor only.
So, as light is converted inside the culture volume, it is also necessary to apply a suitable model to represent the light energy transfer within the PBR. Light attenuation conditions can be well represented using radiative transfer models which include a number of assumptions, as absorption, scattering and self-shading phenomena are responsible of the reduction of light along with the reactor depth [21].
In this work the two flux model of Cornet, as generalized by Pruvost et al. [21] was used, under the hypothesis of an isotropic radiative field where the scattered part of light is assumed to be parallel to the main direction of radiation [22]. Even if it refers to artificial light, this model can be used also in the case of solar radiation. In our experimental set up the LED lamp is not moving according to the day time, so the model is applied taking into account only the direct light contribution which is delivered to the PBR at an incidental angle of 0°. Using a fixed lamp is a rough approximation of the natural conditions, so the results obtained cannot be directly compared to field experimental measurements.
For a continuous reactor without recycle, assuming perfect mixing, the mass balance can be written as:
where
c x,out = outlet biomass concentration (kg m−3);
r x,mean,out = mean growth rate at the outlet biomass concentration (kg m−3 s−1);
τ = residence time, given by the ratio of the reactor volume to the inlet volumetric flowrate (day).
To solve Eq. 3, the value of r x,mean,out is required [21]. It can be calculated by the integration of the local growth rate r x,z along the reactor depth h, whose coordinate is indicated with z:
Note that the subscript “out” has been dropped, as the reactor is assumed to be perfectly mixed.
The equation for r x,z is written as [21]:
where
ρ m = maximum energetic yield for photon conversion (−);
K = half saturation constant for the photosynthesis (μmol m−2 s−1);
Φ = mass quantum yield for Z-scheme of photosynthesis (kg μmol−1);
I(z) = direct PAR at depth z (μmol m−2 s−1);
c x = biomass concentration at depth z (g L−1);
E a = absorption mass coefficient (m2 kg−1);
μ e = maintenance term, including both respiration and death of cells (s−1).
Note that in Eq. 5 the contributions of both biomass growth and maintenance due to cells respiration and death are considered.
Finally, direct PAR at depth z is given by:
where
I dir(0) = direct PAR at z = 0 within the PBR (μmol m−2 s−1);
α, δ dir = model parameters (−);
b = backscattering fraction (−);
E s = scattering mass coefficient (m2 kg−1).
Concerning the experimental system used in this work, a reduction of 11 % of the direct PAR was measured, due to the light absorbed by the front polycarbonate sheet of the flat panel PBR.
Parameters
The parameters in Eqs. 5–8 were defined and quantified as follows. In particular, we focused on absorption (E a), back scattering (bE s) and maintenance term (μ e), that play a key role in the model sensitivity and affect the simulation results. The maximum energy yield for photon dissipation in antennae (ρ m) can be considered as a moderately species-independent factor [21] and was set equal to 0.8. According to S. obliquus elemental analysis and reaction stoichiometry, the mass quantum yield for Z-scheme of photosynthesis Φ resulted 2.77 × 10−9 kg μmol−1.
For S. obliquus, the maximum exponential growth rate was reached at 150 μmol m−2 s−1 and the half saturation constant K was 75 μmol m−2 s−1 [23].
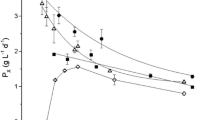
Absorption and back scattering parameters (E a, bE s) were evaluated measuring, by means of the photoradiometer, the light attenuation across the reactor depth, that is the irradiance on the back side of the reactor (“back irradiance”) as a function of the concentration in the reactor during the continuous flow experiments. These measurements were performed at different constant incident irradiation intensities, as shown in Fig. 3 for two cases. The best fit values of E a and bE s were correlated to these data. Indeed, they depend on light intensity, but the model sensitivity to its values was found to be low. So, we have used absorption and back scattering coefficients obtained at 150 μmol m−2 s−1 for all our simulations.
On the other hand, the simulated day–night cycle growth was found to be heavily affected by the maintenance term μ e. Accordingly, we used this parameter to tune our model to experimental data.
Results and discussion
Steady-state biomass productivity under seasonal irradiations
Seasonal PAR irradiation data and experimental results of different runs are summarized in Table 2. The outlet concentrations reported refer to steady-state conditions. For April and October, continuous experiments both with and without recycle were carried out to measure the effect of recycle on productivity, by considering that in a PBR the ratio between biomass amount and light available is crucial looking at the productivity of the system. In general, using a recycle with a CSTR does not improve the reactor performance. However, in the case of PBR it is essential to absorb all the light energy available and this can be done only by optimizing the microalgae concentration within the PBR. In addition, under fluctuating light regimes, where intensity in some cases reaches high irradiances, an increased concentration could lead to a lower photosaturation effect. On the other hand, the self-shading effect could affect the light capture when irradiance is not saturating, leading to an overall decreased photoconversion efficiency.
In fact, as reported in Table 2, the recycle increased the biomass concentration, but resulted also in lower biomass productivity. This is confirmed by looking at the energy conversion efficiency, which is lower with recycle, as the effect of the increased self-shading of biomass, enhanced by higher concentrations.
By comparing productivities obtained in runs without recycle, it is observed that the highest value was achieved with July irradiation. An acceptable productivity was also obtained in January even if the irradiation is much lower. Looking at the energy conversion, it is clear that at lower irradiations algae can exploit light much better, suggesting that no photosaturation nor photoinhibition events occurred at this irradiation. In view of an industrial application, such a result suggests that an acceptable biomass production is achievable even with winter irradiation, if the reactor temperature is properly controlled as can be done in a closed PBR.
PBR dynamic and steady-state simulation
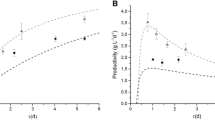
Experimental runs without recycle were simulated by the model outlined in Sect. “Equations”. In all cases, the continuous PBR was initially operated under transient conditions, and subsequently reached the steady state. Accordingly, we have simulated also the initial transient behavior. Depending on the energy supplied, a cycled steady-state concentration was eventually found as can be seen in Fig. 4 for the winter season, as an example. The curves reported correspond to different days and it can be seen that a cyclic steady-state is achieved after about 20 days of operation. In addition, the biomass concentration within each day ranges between a minimum in the early morning and a maximum in the late afternoon, and the fluctuations are within ±10 %.
In Fig. 5, the experimental data obtained in the different seasons (winter, spring, summer and autumn respectively) are presented and compared to simulations. Note that the simulated results are the daily average values. As reported in Sect. “Parameters”, each curve was fitted to experimental concentration profiles by adjusting the value of the maintenance term. Table 3 shows the best fit values of μ e.
It is clear that in all the cases considered the simulation is able to satisfactorily reproduce experimental data, with respect to the simple model developed. However, the sensitivity of the simulated steady-state biomass concentration to the maintenance term parameter is high, as can be seen in Table 4, where the steady-state calculated concentrations for three different μ e’s in the October season are reported. This occurred in all simulations, suggesting that the predicted cellular growth and productivity calculated by the model under different light regimes are strongly depending on maintenance term. Presumably, this is a consequence of the inaccuracy of the model, that needs to be improved by including in it photosaturation and inhibition effects. Work is in progress to measure directly μ e values as a function of the PBR operating parameter and to improve the model by taking into account also the photosaturation and photoinhibition terms.
In summary, our results demonstrate that the presently proposed model, with a simple calibration of the absorption and scattering parameters, can be applied as a first approximation to predict the performances of a flat-plate PBR, in terms of biomass concentration and energy conversion, provided that a sound value of the maintenance parameter is measured. In this work, a perfectly stirred reactor was considered, according to the experimental evidence of the laboratory-scale unit used. To apply it to the design of a large-scale PBR with similar geometry, the model must be improved by accounting for the extent of mixing in the axial reactor direction (i.e. the Peclet number).
Conclusions
The effect of seasonal irradiation on S. obliquus growth in a flat-plate PBR operated continuously was investigated experimentally at laboratory-scale. Four months representative of each season of the year were simulated with respect to light irradiation energy, and outlet biomass concentration, productivity and energy conversion efficiency were measured. The maximum energy conversion was obtained in winter, probably due to the low average light intensity impinging the panel, that allowed to avoid photosaturation, photoinhibition and energy dissipation. Anyway, a remarkable productivity was obtained in all seasons, even if at higher irradiation the energy conversion was lower. When recycling part of the biomass to increase its concentration in the reactor a reduction in productivity was always achieved probably due to a predominant self-shading effect. The results of the experimental runs were simulated with a mathematical model derived from the literature. The simulations reproduced well the experimental results, provided that the maintenance parameter value was adjusted to the data. In fact, the cyclic steady-state biomass concentration in the PBR is heavily affected by this parameter. The model presented in this work is ready to be extended to large-scale PBRs of similar geometry and different extent of axial mixing, but the direct measure of the maintenance term is needed, to use the proposed approach for PBR design.
References
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232. doi:10.1016/j.rser.2009.07.020
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2013) Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl Energy 103:444–467. doi:10.1016/j.apenergy.2012.10.004
Singh J, Gu S (2010) Commercialization potential of microalgae for biofuels production. Renew Sustain Energy Rev 14:2596–2610. doi:10.1016/j.rser.2010.06.014
Suali E, Sarbatly R (2012) Conversion of microalgae to biofuel. Renew Sustain Energy Rev 16:4316–4342. doi:10.1016/j.rser.2012.03.047
Slegers PM, Wijffels RH, Van Straten G, Van Boxtel AJB (2011) Design scenarios for flat panel photobioreactors. Appl Energy 88:3342–3353. doi:10.1016/j.apenergy.2010.12.037
Ghasemi Y, Rasoul-Amini S, Naseri AT et al (2012) Microalgae biofuel potentials (Review). Appl Biochem Microbiol 48:126–144. doi:10.1134/S0003683812020068
González-López CV, Acién Fernández FG, Fernández-Sevilla JM et al (2012) Development of a process for efficient use of CO2 from flue gases in the production of photosynthetic microorganisms. Biotechnol Bioeng 109:1637–1650. doi:10.1002/bit.24446
Gutierrez-Wing MT, Benson BC, Rusch KA (2012) Impact of light quality and quantity on growth rate kinetics of Selenastrum capricornutum. Eng Life Sci 12:79–88. doi:10.1002/elsc.201000217
Tang H, Chen M, Ng KYS, Salley SO (2012) Continuous microalgae cultivation in a photobioreactor. Biotechnol Bioeng 109:2468–2474. doi:10.1002/bit.24516
Zijffers J-WF, Schippers KJ, Zheng K et al (2010) Maximum photosynthetic yield of green microalgae in photobioreactors. Mar Biotechnol 12:708–718. doi:10.1007/s10126-010-9258-2
Janssen M, Tramper J, Mur LR, Wijffels RH (2003) Enclosed outdoor photobioreactors: light regime, photosynthetic efficiency, scale-up, and future prospects. Biotechnol Bioeng 81:193–210. doi:10.1002/bit.10468
Quinn JC, Turner CW, Bradley TH (2012) Scale-up of flat plate photobioreactors considering diffuse and direct light characteristics. Biotechnol Bioeng 109:363–370. doi:10.1002/bit.23324
Gebremariam AK, Zarmi Y (2012) Synchronization of fluid-dynamics related and physiological time scales and algal biomass production in thin flat-plate bioreactors. J Appl Phys 111:034904. doi:10.1063/1.3678009
Huang Q, Yao L, Liu T, Yang J (2012) Simulation of the light evolution in an annular photobioreactor for the cultivation of Porphyridium cruentum. Chem Eng Sci 84:718–726. doi:10.1016/j.ces.2012.09.017
Li X, Yang N (2013) Modeling the light distribution in airlift photobioreactors under simultaneous external and internal illumination using the two-flux model. Chem Eng Sci 88:16–22. doi:10.1016/j.ces.2012.11.015
Belarbi E, Molina E, Chisti Y (2000) A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzym Microb Technol 26:516–529
Hindersin S, Leupold M, Kerner M, Hanelt D (2013) Irradiance optimization of outdoor microalgal cultures using solar tracked photobioreactors. Bioprocess Biosyst Eng 36:345–355. doi:10.1007/s00449-012-0790-5
El-Sheekh M, Abomohra AE-F, Hanelt D (2013) Optimization of biomass and fatty acid productivity of Scenedesmus obliquus as a promising microalga for biodiesel production. World J Microbiol Biotechnol 29:915–922. doi:10.1007/s11274-012-1248-2
Sforza E, Enzo M, Bertucco A (2013) Design of microalgal biomass production in a continuous photobioreactor: an integrated experimental and modeling approach. Chem Eng Res Des. doi:10.1016/j.cherd.2013.08.017
Pruvost J, Van Vooren G, Le Gouic B et al (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102:150–158. doi:10.1016/j.biortech.2010.06.153
Cornet J, Dussap C, Gros J (1995) A simplified monodimensional approach for modeling coupling between radiant light transfer and growth kinetics in photobioreactors. Chem Eng Sci 50:1489–1500
Gris B, Morosinotto T, Giacometti GM, Bertucco A, Sforza E (2013) Cultivation of Scenedesmus obliquus in photobioreactors: effects of light intensities and light-dark cycles on growth, productivity, and biochemical composition. Appl Biochem Biotechnol. doi:10.1007/s12010-013-0679-z
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertucco, A., Beraldi, M. & Sforza, E. Continuous microalgal cultivation in a laboratory-scale photobioreactor under seasonal day–night irradiation: experiments and simulation. Bioprocess Biosyst Eng 37, 1535–1542 (2014). https://doi.org/10.1007/s00449-014-1125-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1125-5