Abstract
One of the main environmental issues affecting coastal marine environments is the accumulation of contaminants in sediments and their potential mobility. In situ benthic chamber experiments were conducted at two tourist ports (marinas) located in the Gulf of Trieste, one in Slovenia and one in Italy. The aim was to understand if and where recycling at the sediment-water interface (SWI) may affect metal(loid)s. Short sediment cores were also collected near the chamber to investigate the solid (sediments) and dissolved phases (porewaters). Both diffusive and benthic fluxes were estimated to elucidate the release of metal(loid)s at the SWI. Total element concentrations and their labile fractions were determined in sediments to quantify their potential mobility. The total element contents were found to be two orders of magnitude higher in the Italian marina than in the Slovenian one, especially for Hg (up to 1000 mg kg−1), whereas the labile fraction was scarce or null. The opposite occurred in the Slovenian marina. Metal(loid)s in porewaters showed a clear diagenetic sequence and a close dependence upon the suboxic/anoxic conditions of sediments. The results suggest that although the sediments of the Italian marina exhibit the highest total metal(loid) concentration, these elements are scarcely remobilisable. Conversely, in the Slovenian marina, sediments seem to be comparatively more prone to release metal(loid)s at the SWI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine coastal environments are often subjected to high pressure from human activities. Research conducted in port areas have investigated and assessed the need for remediation action in order to limit the contamination of waters and sediments (e.g. Islam and Tunaka 2004; Botwe et al. 2017a). These latter are often recognised as “reservoirs” of contaminants including potential toxic elements, due to a lack of management of diffusive sources such as industrial, urban and agricultural waste water discharge (Botwe et al. 2017b and references therein) and accidental spills from ships (Casado-Martínez et al. 2006). Two main factors contribute to the accumulation of contaminants in harbours (Guerra et al. 2000): they have been designed to minimise hydrodynamic energy from within and are the focus of industrial (shipping, loading and unloading, accidental spills) and urban (waste water emissions) activities.
Contaminants may occur in a variety of dissolved, colloidal and particulate forms depending on physico-chemical characteristics of the water column and sediment (Campbell and Tessier 1987). When contaminants are released into the water column in dissolved form, they can be adsorbed to the suspended particulate matter and can be rapidly transferred to bottom sediments. Thus, sediments can act both as a reservoir and a secondary source of contamination. Contaminants can be mobilised and transformed into more toxic forms as a result of chemico-physical alterations (i.e. Kelderman and Osman 2007), thus becoming available for benthic organisms (Jackson et al. 2005; Di Palma and Mecozzi 2007) and entering at the entire aquatic trophic chain (Hong et al. 1995; His et al. 1999; Botwe et al. 2017a). Moreover, contaminated harbour sediments may adversely impact other coastal marine and terrestrial ecosystems via the disposal of dredged materials (Caille et al. 2003; Schipper et al. 2010; Ho et al. 2012).
In the northern Adriatic Sea, both the Italian and Slovenian sector are affected by the presence of significant urban areas (Trieste, Koper and Monfalcone), several industrial settlements and tourism activities which are responsible for a high degree of local contamination. For example, it is well-known that mercury (Hg) is one of the main concerns (Horvat et al. 1999; Covelli et al. 2001; Faganeli et al. 2003), as a consequence of the secular Idrija mining activity, but also the presence of persistent organic pollutants (POPs) in the Port of Trieste has been found (Adami et al. 2000), along with other trace metals in dredged coastal sediments from the Bay of Koper (Šömen Joksič et al. 2005) and moderate contamination of sediments by organotin compounds in the Portorož marina and the dockyard in Izola (Ščančar et al. 2007).
Since sediments in these harbour areas are highly impacted by different contaminants (trace metals, policyclic aromatic hydrocarbons (PAHs), polychlorobiphenyls (PCBs), organotin compounds (OTC), etc.), understanding their behaviour and cycling at the sediment-water interface (SWI) is of great importance. Indeed, a net export of these substances from the sediment to the upper water column could result in a general deterioration of the water quality.
This work represents a first step toward understanding the role of the SWI in recycling a pool of trace elements (As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb and Zn) and nutrients in these anthropogenically modified coastal environments. The final aim was (1) to explore the behaviour of these species among porewaters, sediments and the water column, by estimating benthic and diffusive fluxes at two different marinas using an in situ deployed benthic chamber, which has successfully been employed in several studies as reported in Petranich et al. (2018a, b), and references therein, and (2) to evaluate possible remobilisation under natural conditions not perturbed by the physical removal of sediments (i.e. dredging operations or resuspension due to boat propellers). These results, together with those obtained for selected organic compounds, could be useful in providing the necessary scientific background to help manage these environments.
Materials and methods
Study area
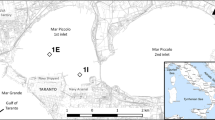
Selected sampling sites (Fig. 1) are located in the Gulf of Trieste, a shallow semi-enclosed basin (A = 500 km2) in the northernmost part of the Adriatic Sea (max depth = 25 m). In the Gulf, the main inputs of freshwaters and suspended sedimentary material originate from the Isonzo River (NW), whereas the Rosandra, Rižana and Dragonja streams (SE) are of minor importance. Tides and the prevalently N-NE wind pattern (Olivotti et al. 1986) result in an anticlockwise circulation. The semi-diurnal tidal oscillation is among the largest in the Mediterranean Sea, with an average tidal excursion equal to 86 cm in spring tide and 22 cm in neap tide (Marocco 1989). Tides along with wind action can significantly influence the spreading of contaminants (Bogunović and Malačič 2008). The oceanographic properties of the Gulf are highly variable due to the pronounced seasonal cycles of seawater temperature (5–26 °C) and the formation of salinity gradients (25–38 psu, using the practical salinity scale) originating from the contrasting effects of runoff and seawater exchange at the open boundary (Cozzi et al. 2012).
The marina of Lucija/Santa Lucia (site SL) near Portorož (Bay of Piran, Slovenia) was constructed in 1974 on part of the former salt ponds and currently has about 1000 berths in an area of 0.1 km2. Although the structure received several awards from the International Foundation for Environment Education (FFE) and introduced the ISO 9001 quality standard (Brebbia et al. 2007), it suffers from environmental problems due to human activities (i.e. construction of coastal infrastructure, discharges, habitat modification and intense boat traffic). Sediments, waters and mussels have been found to have high concentrations of organic compounds and trace metals (Milivojevič Nemanič et al. 2002; Ščančar et al. 2007). For example, in sediments, tributyltin (TBT) reaches up to 0.78 mg kg−1, comparable to 0.4–1.5 mg kg−1 values found in polluted harbour areas in Greece and Japan (Lalere et al. 1995; Harino et al. 1999). On the other hand, Cr and Zn reach up to 188 and 140 mg kg−1, respectively, exceeding the values of 52.3 and 124 mg kg−1 fixed by the Interim Sediment Quality Guidelines (ISQG), which correspond to the threshold level effects below which adverse biological effects are not expected (Ščančar et al. 2007).
The San Rocco marina (site SR), belonging to the Bay of Muggia (Italy), is close to the Trieste Port area (steel factory, oil pipeline, ex-refinery). Three breakwaters close the bay, acting as a barrier for the shallow waters (depth of 8–20 m) and promoting the accumulation of contaminants (metal(loid)s, PAHs and PCBs) in surface sediments (Adami et al. 1998, 2000). The whole bay was recognised as a Site of National Interest (SIN) by decree of the Italian Ministry of the Environment in 2003 and was intensively monitored to assess its chemical quality status (Cibic et al. 2017). The San Rocco marina area was used for shipbuilding from 1858 to 1970. In 1982, the whole area was assigned to a local firm which aimed to redevelop the area into a tourist port (Gellner and Valenti 2005). The project started in 1998 and involved the recovery and reconversion of the degraded shipyard area into a coastal resort with tourist facilities. Approximately 18,000 m3 of contaminated material coming from excavation activities were dumped in a nearby dredged disposal site and buried in a man-made hill near built-up areas (Giurastante 2010). In this marina, two sites (SR-1 and SR-2) were selected for core collection due to the different textural features of sediments. Site SR-1 was characterised by coarser sediments (sandy fraction) in comparison to SR-2 where the silty fraction prevailed. The benthic chamber experiment was only performed at SR-1, in agreement with information obtained on site by local inhabitants regarding the areas previously contaminated by former shipbuilding activities and later subject to excavation.
Sampling
An in situ transparent benthic chamber (30 × 50 × 30 cm polymethylmethacrylate box), open at the bottom and equipped with a stirring mechanism consisting of a rotating bar (30 cm long, speed of 5 rpm) inside the chamber, was carefully deployed by a scuba diver at the bottom, thus isolating an area of about 0.25 m2 of sediments together with an approximately 30 cm of water column. Two stopcocks were fastened on the top of the chamber for water sampling and to replace the volume removed during sample collection (Covelli et al. 1999). Water samples were collected with 50-mL polypropylene syringes at approximately 2-h time intervals (from T0 = 0 to T4 = 8 h), filtered through 0.45 mm pore size Millipore Millex HA membrane filters, transferred into vials and properly stored until final analysis. A detail of these operations is reported in Covelli et al. (2008) and De Vittor et al. (2012), except for dissolved metals which were acidified with HNO3 12 M (0.5 mL/50 mL sample). Ancillary water parameters (pH, redox potential, dissolved oxygen, temperature and conductivity) were recorded using a multiparameter probe (YSI Professional Plus Multiparameter Meter) inserted into the benthic chamber before submersion. The probe recorded all parameters during the experiment but only the values corresponding to the exact times of sample collection were considered.
In parallel, virtually undisturbed short sediment cores (30 cm; i.d. 16 cm in length) were collected along with the overlying water. These cores were transported to the laboratory and subsampled in a N2-filled chamber to preserve the original redox conditions. Firstly, supernatant water was collected after measuring the redox potential (pH 25, Crison Instrument, Barcelona, Spain), and the sediment core was then sectioned into discrete levels (0–1, 1–2, 2–3.5, 3.5–5 and 5–7 cm). Redox potential (Eh) was also measured in each sediment levels before extracting porewaters by centrifugation at in situ temperature (5000 rpm; t = 14 min). Porewaters were then filtered through membrane filters (0.45 μm pore size, Millipore Millex HA), collected and stored until analysis. Solid phase samples were divided into three subsamples for subsequent analyses.
Solid phase analyses
For grain-size analysis, approximately 15–20 g of fresh sediment was treated with H2O2 (3%) for a minimum of 24 h to eliminate most of the organic matter. Subsequently, the sediment was wet-sieved through a 2-mm sieve to remove coarse shell fragments. The resulting < 2 mm fraction was analysed using a laser granulometer (Malvern Mastersizer 2000).
Total and organic carbon (Ctot and Corg) and nitrogen (Ntot) content were determined in freeze-dried samples using an ECS 4010 Elemental Combustion System, Costech. Ctot and Ntot were determined at a combustion temperature of 1020 °C and Corg at 920 °C (Hedges and Stern 1984) after progressive acidification with 0.1–1.0 M HCl to remove carbonates.
Total Hg (THg) was determined using a Direct Mercury Analyser (DMA-80, Milestone) according to the US EPA 7473. The limit of detection (LOD) was approximately 0.005 ng. Accuracy of the method was checked with standard reference material (PACS-3-Marine Sediment, NRCC, Canada) and the relative standard deviation of at least three determinations was < 2%.
The US EPA Method 3052 was applied to determine total metal(loid) content (As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, V and Zn). Three subsamples of PACS-3 Marine Sediment Certified Reference Material (NRCC, Canada) were mineralised in the same batch with the sediment samples to verify the accuracy of the procedure. Recovery varied between 85 and 105%. The solutions obtained were determined by inductively coupled plasma optical emission spectrometry (ICP-OES) using an Optima 8000 Spectrometer, equipped with a S10 Autosampler, Perkin Elmer. The measurements were taken using calibration curves obtained by dilution (range 0–10 mg L−1) of the standard solutions for ICP analyses (Sigma-Aldrich, USA).
A weak extraction (HCl 0.5 M; Adami et al. 1999) was performed in order to assess the amount of the elements in association with Fe and Mn oxides and hydroxides, carbonates, phosphates as well as fractions adsorbed on the surface of fine sediment particles. In detail, 2 g of each sediment sample and 20 mL of the extraction solution were secured in a rotary extractor device and rotated at room temperature for 18 ± 2 h. Following the extraction process, the material in the extractor vessels was centrifuged at 3000 r/min for 10 min, separated into its liquid (labile chemical forms of Fe and Mn) and solid components by filtering through membrane filters (0.45 μm) and diluted with Milli-Q water up to a final volume of 25 mL before being analysed using an inductively coupled plasma mass spectrometry (ICP-MS). Analyses showed a variation coefficient of < 3%.
Dissolved phase analyses
Total dissolved Hg (DHg) determination was conducted via a pre-reduction using NH2OH-HCl (30%, 0.25 mL) until the yellow colour disappeared, followed by a reduction with SnCl2 2% in HCl 4%, as in EPA Method 1631e and AFS detection (Mercur, Analytic Jena) coupled with a gold trap pre-concentration system. NIST 3133 certified solution was used for calibration and ORMS-5 (CRM, Canada) was used for quality control. The detection limit calculated on the basis of three times the standard deviation of ten reagent blanks was 0.63 ng L−1 and the quantification limit (LQD), calculated on the basis of ten times the standard deviation of ten reagent blanks, was 2.11 ng L−1. The reproducibility of the method was 4%.
Excluding Hg, all total dissolved metal(loid) concentrations were determined using an ICP-MS (NexION 350X equipped with an ESI SC autosampler) working in kinetic energy discrimination (KED) mode, using ultra-high purity helium (flow rate of 4.0 mL/min) to minimise polyatomic interference. The analyses were carried out using the calibration curve method obtained by analysing seven standard solutions (range 0–10 μg L−1) prepared after dilution of a stock standard solution (1000 mg L−1). Analyses showed a variation coefficient < 3%.
Nutrient analyses were performed using a segmented flow autoanalyser (Bran + Luebbe QuAttro) following the methods reported in Grasshoff et al. (1999) and modified for the specific instrument. Certified standards (Inorganic Ventures Standard Solutions and MOOS-2, NRC) were used to ensure the accuracy of the procedures and performance was periodically checked using a proficiency test (QUASIMEME programmers AQ1 and AQ2).
Dissolved inorganic carbon (DIC) was calculated from carbonate alkalinity (CA), in situ temperature-corrected pH and salinity using the apparent acidity constants for CO2 and boric acid as reported by Lucker et al. (2000) and Dickson (1990), respectively. CA was computed from total alkalinity (TA) determined via Gran titration (Millero et al. 1993). Sulphide was analysed using the standard colorimetric method (Grasshoff et al. 1999). DOC was analysed using a high temperature catalytic method using a Shimadzu TOC 5000A analyser after acidifying the samples with 6 M HCl (Sugimura and Suzuki 1988).
Diffusive and benthic fluxes calculation
The mobility of the dissolved species across the SWI may be estimated by calculating the diffusive fluxes according to Fick’s first law (e.g. Emili et al. 2016; Covelli et al. 1999) applying the following formula:
where F is the instantaneous flux of a solute with concentration C at depth x, and δC/δx is the concentration gradient of chemical species between the porewater at a depth of 1 cm and the overlying water, ϕ sediment porosity and θ tortuosity (dimensionless) estimated from porosity using the following formula θ2 = 1 − ln (ϕ2) (Boudreau 1999).
The diffusion coefficient of each solute in water (Dw) in the absence of the sediment matrix at 18 °C was obtained from Li and Gregory (1974), whereas the Dw values proposed by Ullman and Aller (1982) and Alperin et al. (1994) were used for DIC, DOC, H2S and nutrients. Positive values indicate net fluxes from the porewaters to the overlying water (effluxes), whereas the negative values are representative of net influxes.
To calculate the benthic fluxes we used the method proposed by Santschi et al. (1990), after a careful evaluation of the different estimation procedures proposed by the literature, such as linear regression (Warnken et al. 2001) or integrating the area under the “concentration versus time” curve and normalising the results to 24 h (Covelli et al. 2008; Emili et al. 2016).
For instance, the linear regression method, which considers all the sampled points (one sample every 2 h for a total of 8 h), was discarded because even if it was exhaustive for SR-1 data, it was not very effective at site SL as it did not take the real trend into account.
According to the approach reported by Santschi et al. (1990), diurnal benthic fluxes (F) of dissolved species across the SWI reflect the difference between their final (Cf) and initial concentration (C0) as follows:
where tf and t0 are the final and starting times, respectively, V is the benthic chamber volume (52.5 L) and A (0.25 m2) is the covered sea-bottom area.
Results and discussion
Solid phase characteristics
Grain size was found to be different among the sites and rather homogeneous with depth (Fig. 2). According to Shepard’s (1954) classification, SL and SR-2 consist mostly of silty fraction (78 ± 1.78 and 68 ± 2.0%, respectively). Sandy and clay fractions are less represented. On the contrary, at SR-1 the sandy fraction (51%) is dominant on silt (43%) and clay (6%). Ctot, Corg and Ntot were also constant with depth (Fig. 3). On average, Ctot and Corg were highest at SR-1 (7.69 ± 0.24 and 4.06 ± 0.29%, respectively). Conversely, Ntot was found to be slightly higher at SR-2 (avg. 0.14 ± 0.02%). Generally, the values found in these areas vary in a wide range and with concentrations higher than those found by Emili et al. (2011) in the open Gulf of Trieste.
The Corg/Ntot molar ratio is useful in establishing the source (marine or terrestrial) of organic matter (OM). Goñi et al. (2003) recognised that values > 14 are typical of OM of terrestrial origin depleted in nitrogen-containing biochemicals and enriched in carbon (i.e. lignin and cellulose). Conversely, a molar ratio < 10 is usually associated with marine-derived organic matter (i.e. phytoplankton and bacterioplankton), characterised by higher nitrogen contents (Ogrinc et al. 2003). Thus, in our case, the OM is of terrestrial origin and is more marked at site SR-1, where the molar ratio, mostly constant for all profiles, reached the maximum value of 44.8 in the deepest layers. The scarce communication with the open sea prevents inputs of OM of marine origin, and most likely, the material disposed in the building and maintenance of the marinas can justify the high values compared to those found in the open Gulf (7.5–8.9, Emili et al. 2011).
Metal(loid)s in sediments: total and labile fraction
Total metal(loid) content in cores collected varied significantly among the investigated sites. Generally, the lowest values with rather constant trends with depth were detected at the Portorož marina (SL). Natural background concentrations for this site were taken from those previously reported by Ogorelec et al. (1981) regarding the borehole drilled in the nearby saltmarsh of the Sečovlje salt ponds (Table 1). A comparison reveals a marked enrichment in Cu and Zn in the marina sediments. On the contrary, increasing trends downcore were observed for most of the elements (As, Cd, Cu, Pb and Zn) at both sites of the San Rocco marina, with SR-1 clearly more contaminated than SR-2. Here, the vertical trends suggest a common source of contamination as already reported for sediments from Taranto’s Mar Piccolo port area (Cardellicchio et al. 2009; Emili et al. 2016). The simultaneous presence of elements such as Cd, Cr, Cu, Ni and Zn has already been observed in sediments contaminated by antifouling paints applied to ship hulls and to other submerged structures (Turner 2010). Moreover, the high Pb and Hg concentrations found at site SR-1 (up to 2500 and 1000 mg kg−1, respectively) are probably due to an excess of Pb oxide (i.e. Pb3O4) used as anti-rust paint treatment in the shipyard and to the accumulation of waste containing Hg from measuring instruments, electrical and electronic equipment, batteries or components used in motor vehicles. Regarding Hg, contamination at SR-1 ranges from two to three orders of magnitude higher (809–1010 mg kg−1) than the open Gulf of Trieste which has been affected by long-term mining activity (0.064–30.4 mg kg−1, Covelli et al. 2001). Similarly, As, Cd, Cu, Pb and Zn are highly enriched in comparison to concentrations observed in the surface sediments of the eastern sector of the Gulf of Trieste (Dolenec et al. 1998, Acquavita et al. 2010). The contamination is also clear with respect to the local background values obtained from the 2.2–2.6 m depth bottom layers of the two cores RF1 and RF2 collected in front of Muggia (Furlan et al. 1999), the city approximately 1 km further east from the San Rocco marina (Table 1). Nickel and chromium concentrations in the two marinas are similar to the surface samples of the easternmost sector of the Gulf of Trieste (Dolenec et al. 1998; Acquavita et al. 2010) and to the local background values. Indeed, chromites and Cr-bearing spinels deriving from flysch (Lenaz et al. 1996) characterise the heavy mineral fractions of the coastal sediments of the whole Gulf of Trieste.
The mobility of metals from sediments to porewaters and to the overlying water column depends upon several factors. The redox state of sediments and the bacterial degradation of OM are important, but a primary role is played by the chemical form in which the metals are present in the solid matrix. Metal sulphides are poorly soluble or mostly insoluble and tend to remain trapped in the sediment (Oliveri et al. 2016 and references therein), whereas less stable and more redox-sensitive compounds, such as metal oxy-hydroxides, are more easily released into the dissolved form (Turner et al. 2004).
In spite of presenting the lowest total metal(loid) concentrations compared to the Italian marina (Table S1), site SL is characterised by high percentages of the labile fraction for some metals (Cu > Pb > Zn > Mn). The only exception is represented by Cd, whose labile fraction is only apparent in the deepest level (about 45% of the total content at a depth of 5–7 cm).
Several studies conducted on marine sediments report that some elements can be mostly bound to the organic matter fraction (70–80%) in highly polluted environments (Rapin et al. 1983; Li et al. 2000; Belzunce Segarra et al. 2008), for instance Cu, due to the high formation constants of organic Cu compounds (Stumm and Morgan 1995). Lead and zinc partitioning is also similar, but they are prevalently adsorbed by Fe and Mn oxy-hydroxides (Ramirez et al. 2005; Petronio et al. 2008, 2012; Belzunce Segarra et al. 2008). Reduction of Mn oxy-hydroxides in anoxic conditions may affect metal remobilisation and redistribution (Hellali et al. 2015), thus favouring the availability of Pb and Zn previously associated to sediment particles in porewaters. On the other hand, Fe and Ni seem to be mainly present in an unavailable form, showing limited mobility equal to, on average, 19 and 18% of the total content respectively, followed by As (avg. 9%), Hg (avg. 7%) and Cr (avg. 5%).
At site SR-1, the mobility is null for Cr, Cu, Hg, Pb and V or scarce for Cd and Zn (Table S1). This could be explained by the formation of sulphides (Emili et al. 2016), which are stable in reduced conditions, or by the occurrence of detrital metallic particles coming from historical shipyard activities.
Although the bottom sediments of this marina are significantly contaminated, the very low concentration of the labile fraction seems to limit their role as a potential secondary source of contamination. We cannot, however, exclude the hypothesis that if these sediments are perturbed, changes in redox conditions might partially favour the release of metals in the water column.
Dissolved phase and partitioning of metal(loid)s
According to Warnken et al. (2001), when the sediment becomes depleted of oxygen, the release of metals is driven by the reduction of Fe and Mn oxy-hydroxides, which are efficient scavengers for many soluble trace metals in oxidising conditions (Daye et al. 2015). They are, however, promptly released when microbial degradation of OM occurs in oxygen depleted conditions. Thus, the increasing porewater concentrations with depth of almost all metal(loid)s (excluding Pb and Zn at SL and SR-1, Ni only at SR-1, Cd and Cu at SR-2) for all experimental sites (Fig. 4) could be related to the solubility changes in Mn and Fe oxy-hydroxides (Warnken et al. 2001), as already observed in the Gulf of Trieste (Covelli et al. 1999 and references therein; Hines et al. 1997). The following increase in metal(loid) concentrations at a greater depth at site SR-2, more marked for Fe, Pb, Hg and Zn, could conversely be attributed to sulphate-reduction conditions which could be explained by the increase in H2S concentration with depth (Fig. 4). In addition, the variation of trends with depth can also be influenced by exchange reactions with solid phase, diffusion and complexation with OM and products of local sulphide oxidation resulting from bioturbation (Gagnon et al. 1996).
Generally, the highest metal(loid) concentrations in porewaters were found at site SR-1, with the exception of some elements mostly found at SR-2. The “Fe-Mn reduction” zone usually occurs in the first centimetres of sediment (Oliveri et al. 2016). The reduction of Mn is expected to occur before the reduction of Fe according to the early diagenetic sequence (Froelich et al. 1979). This is confirmed in our study where the peaks appear in the first centimetres (clearer for Mn), followed by a sharp decrease with depth (excluding Fe at SR2) probably due to the precipitation of metals as sulphides (Ni et al. 2017). As reported by Emili et al. (2016) in Taranto’s Mar Piccolo, the parallel increase in H2S at the same depth of Fe at SL and SR-2 (Fig. 4) would suggest that sulphate-reduction is ongoing. Moreover, the following decrease in Fe concentration at the deepest levels, occurring only at SL, could be related to the formation of sulphides that consequently precipitate in sediment (see Oliveri et al. 2016).
The role of Fe is well depicted in the 1–2 cm level at SR-1, where the maximum peak occurred just before that of Hg (2–3.5 cm level) and where Pb and Zn increase from this level to the supernatant water, thus determining a net influx into sediment. The simultaneous reduction of S and Fe oxidised compounds (Rigaud et al. 2013), for instance, may be responsible for the well-defined increase in almost all metal(loid)s in the deepest porewaters at site SR-2. On the contrary, the rapid decrease in all metal(loid)s in the last level at SL could be due to very fast reactions between reactive iron (and metal(loid)s) and sulphur arising from the reduction of SO42− under anoxic conditions, which leads to the formation of sulphides (i.e. FeS, HgS, PbS) (Hines et al. 1997; Middelburg and Levin 2009).
The distribution coefficient KD ([metal]solid/[metal]dissolved, expressed in L kg−1) calculated between sediment (solid phase) and porewaters (dissolved phase) (Hammerschmidt and Fitzgerald 2004) is an important parameter in estimating the potential for the adsorption of dissolved contaminants in contact with particles (EPA 1999). The highest log KD values (Table S2) were observed at site SR-1, especially for As (3.73 ± 0.03), Cd (3.99 ± 0.38), Hg (5.30 ± 0.88) and Pb (5.59 ± 0.36), in accordance with their scarce mobility from sediments found in this site (Table S1). Generally, most metal(loid)s show quite constant trends in terms of log KD with depth, thus suggesting a steady equilibrium between the dissolved and solid phase.
The partitioning also depends on the chemical, physical and biological features of the sedimentary column (i.e. pH, temperature, Eh, oxygen, OM content, microbial activity). In this study, Eh values rapidly decreased just below the SWI, reaching extremely negative values in the deepest levels which could have strongly influenced the partition found.
The redox condition of the system was apparent also taking into consideration the vertical distribution of nutrients and H2S. Ammonium and H2S increasing trends confirm the active mineralisation of OM downcore (De Vittor et al. 2016) and the role of SO42− as a final electron-acceptor in the system. This is coupled by the release of PO43− and SiO44− as a result of the biogenic silica dissolution occurring in sediments (Ogrinc and Faganeli 2006; Zhang et al. 2013). Increasing trends downcore were also found for DIC, which is the last product of Corg oxidation (Alperin et al. 1999), and DOC, slightly higher at SL than at SR-2, whereas data regarding site SR-1 have not been reported due to the low amount of porewaters extracted.
The behaviour of DOC is less clear because its concentration reflects either a balance between production and consumption reactions (Alperin et al. 1999) and/or the presence of OM with low mobility (Burdige et al. 1992).
Diffusive fluxes at the SWI
In general, a net efflux involved most of the considered solutes (Table 2), especially at the Portorož marina (SL). Influxes were estimated for Pb and Zn both at SL (− 52.2 and − 3.36 μg m−2 day−1, respectively) and at SR-1 (− 62.7 and − 90.7 μg m−2 day−1, respectively). In addition, Cu also showed a high influx at the two sites of the San Rocco marina (− 17.7 and − 155 μg m−2 day−1 at SR-1 and SR-2, respectively). The highest effluxes were estimated for Fe and Mn in all investigated sites along these gradients: 92.6 > 283 > 932 μg m−2 day−1 for Fe at SR-2, SR-1 and SL respectively and 40.9 > 499 > 6980 μg m−2 day−1 for Mn at SR-1, SL and SR-2 respectively. Compared to the results obtained from Taranto’s Mar Piccolo by Emili et al. (2016) (Table S3), the Fe and Mn fluxes were high—up to two orders of magnitude—thus indicating a greater mobility at the SWI. Significant Hg efflux was estimated at both sites (1465, 966 and 33 μg m−2 day−1 at SR-1, SR-2 and SL respectively), reflecting the elevated metal concentrations in the corresponding sediments, especially for SR. In addition, Hg diffusive fluxes were significantly higher than those reported for the Gulf of Trieste (Covelli et al. 1999) and the Grado and Marano Lagoon (Covelli et al. 2008; Emili et al. 2012).
DIC, DOC and H2S showed similar diffusive effluxes between the sites, with very low values especially of H2S (0.001 and 0.006 mmol m−2 day−1) with respect to DIC (0.62 and 0.64 mmol m−2 day−1) and DOC (0.51 and 0.39 mmol m−2 day−1) at SR-2 and SL, respectively. Nevertheless, the effluxes of DIC (up to 0.64 mmol m−2 day−1) and DOC (up to 0.51 mmol m−2 day−1) were found to be lower than those found by De Vittor et al. (2012) (up to 8.76 and 0.60 mmol m−2 day−1, respectively). In our case, similar DIC and DOC effluxes could suggest that they are utilised as fast as they are produced (De Vittor et al. 2012, 2016).
Inorganic nitrogen forms showed effluxes at all sites such as SiO44− and PO43−. This latter showed a weak influx at SR-1 (− 0.00013 mmol m−2 day−1), thus indicating the importance of benthic nutrient regeneration. On the contrary, SiO44− showed high flux values, likely due to the dissolution of the protective coatings of diatom siliceous structures accumulated in sediment and then released in dissolved form into the porewaters (Belias et al. 2007).
In situ benthic chamber experiments
The highest metal(loid) concentrations during benthic chamber experiments were found at the Portorož marina (SL), excluding Pb and Hg which were the highest at SR-1 (Fig. 5). This is in agreement with the labile fraction found in sediments at this site (Table S1). Generally, the maximum metal(loid) peaks at SL occurred in samples taken at T1 (after 2 h) and T2 (after 4 h), and a decrease was observed until the end of the experiment (T4, after 8 h), with the exception of Cr which decreased until T2 and then remained constant. This behaviour may be explained by the opposite trends observed for dissolved O2 and Eh, which slightly decrease from T0 to T1 (Fig. 5). Under these conditions, the desorption of metal(loid)s following the weak reduction of Fe and Mn oxy-hydroxides could be promoted (Gagnon et al. 1997; Hellali et al. 2015) in this time interval. This hypothesis is also supported by the Fe (159 μg L−1) and Mn (10.2 μg L−1) peaks revealed at T1. Following this, when the oxygen increased a reprecipitation of these species as oxy-hydroxides may have occurred, seeing as lower Fe (16.2 μg L−1) and Mn (8.29 μg L−1) concentrations were measured at T2. These processes were very well observed in incubation experiments (Covelli et al. 2008; Emili et al. 2014), where the transition from oxic to hypoxic and anoxic conditions play a significant role in the formation/reduction of Fe and Mn oxy-hydroxides, which in turn influence the remobilisation of Hg at the SWI.
Surprisingly, at SR metal(loid)s showed opposite trends, especially Pb which markedly increased from T0 to T4. Mercury concentrations varied in a wide range and were one order of magnitude lower than other metal(loid)s. Moreover, a decrease from T0 to T1 parallel to Fe suggests the precipitation of this toxic element as oxy-hydroxides when dissolved O2 increases as a consequence of photosynthetic processes. In fact, the remobilisation (dissolution and precipitation) of Hg can be influenced by several factors and processes, including adsorption or co-precipitation with Fe sulphides and Fe-Mn oxy-hydroxides (Gagnon et al. 1997; Gobeil and Cossa 1993).
Regarding the nutrients, the last sample at SR-1 is missing. In both sites, a general daily increase of O2 was recorded and NH4+ was almost < LOD (limit of detection, 0.02 μM). Conversely, oxidised nitrogen forms showed variable trends and reached their maximum, because of active nitrification, at the end of the experiment together with O2. Phosphate was quite constant, whereas SiO44− increased at SR-1, due to the dissolution of biogenic Si, and decreased at SL, possibly due to Si uptake by benthic microalgae. DIC varied in a narrow range with similar values at the two sites and remained quite constant during the day. DOC concentrations also remained quite constant.
In situ benthic fluxes
Metal(loid) benthic fluxes were higher than the diffusive fluxes (Table 2). This is commonly observed as reported by Emili et al. (2016) and can be due to intense sediment bioirrigation (Berelson et al. 2003) and/or insufficient resolution in the characterisation of the concentration gradient close to the sediment-water interface in calculating the diffusive fluxes (e.g. Covelli et al. 1999; Point et al. 2007). However, a comparison with results obtained in these studies cannot be made, due to the different method of flux calculation (by integrating the area under the concentration versus time curve and normalising the results to 24 h; Covelli et al. 2008). Nevertheless, a purely indicative comparison is reported in Table S4, taking into account different methods for the calculation of the benthic fluxes.
In most cases, effluxes were estimated at both sites for As, Mn, Ni, Pb, Zn and, only at SL, Cu (6800 μg m−2day−1) (Table 2), were they also found to be significantly higher than those reported by Emili et al. (2016) for a similar environment (Table S4). Conversely, slight benthic influxes were calculated for Cd (− 20 μg m−2day−1) whereas the influx was more significant for Cr (− 3310 μg m−2day−1) at SL. Iron and Hg exhibited a net influx at both sites (− 1760 μg m−2day−1 of Fe and − 0.14 μg m−2day−1 of Hg; − 2330 μg m−2day−1 of Fe and − 7.84 μg m−2day−1 of Hg, for SL and SR-1, respectively) (Table 2). H2S also showed similar and weak influxes, due to oxic conditions found in the chamber (Fig. 5). Precipitation of metal(loid)s either with Fe and Mn oxy-hydroxides (and/or sulphides) or as sulphates, and complexation with the OM at the SWI (Muresan et al. 2007) may have occurred over a longer period of time, thus sequestering them from the water column.
DIC and DOC also showed benthic effluxes, thus indicating the continuous production by the phytobenthic, benthic fauna and bacteria activities (McGlathery et al. 2001), with the exception of a DIC influx at SR-1, which is coupled with an O2 efflux as result of consumption by benthic autotrophic organisms (i.e. microphytobenthos).
Nutrients showed weak and opposite fluxes between the sites. The low concentrations and variability found and the subsequent low calculated fluxes suggest that in these areas, biological processes at the SWI (i.e. photosynthesis/respiration, nitrification/denitrification, OM degradation), are less active than those found in environments which are open and less subject to anthropic pressures (De Vittor et al. 2012; Petranich et al. 2018b).
Conclusions
The bottom sediments in two noted tourist harbours subject to anthropic pressures were investigated in order to understand the biogeochemical behaviour of selected metal(loid)s at the SWI and to verify their possible remobilisation to the water column in undisturbed conditions. The main results are summarised as follows:
-
Among the two investigated marinas, site SR-1 at Porto San Rocco is the most contaminated by metal(loid)s of anthropogenic sources (i.e. Pb, Cu, As, Zn and Hg), with concentrations that greatly exceed the local background values for sediments. However, the metal(loid) labile forms were found to be negligible suggesting their association with the residual fraction and a scarce bioavailability. The Portorož marina (SL) is clearly less contaminated (except for Cu and Zn), but almost all metal(loid)s have a higher mobility.
-
Heavily reduced conditions occur below the SWI. Peaks of Mn, Fe and H2S were easily identified in specific depth layers, as a consequence of the active OM degradation, and clearly defined the respective reduction zones of oxidised forms. DIC, DOC and nutrients in porewaters confirm the presence of these processes. Metal(loid) profiles in porewaters are strictly dependent on these processes that, in turn, drive their release and precipitation under reductive conditions.
-
The diffusive fluxes estimated are an instantaneous measure of the flux of solutes diffusing along a concentration gradient from the sediment porewaters to the overlying water column and were almost always found to be positive for metal(loid)s and extremely low for H2S, nutrients, DIC and DOC. More information derives from in situ daily benthic fluxes, as they represent the variability of chemical species over time and take into consideration certain significant features such as bioturbation and bioirrigation. These latter, which show mostly effluxes for both sites, were higher than the corresponding diffusive fluxes. SR-1 showed a general increasing trend especially for Cd and Cr. A general release from sediment to overlying water and a reprecipitation may be responsible for the active recycling of metal(loid)s at the two marinas.
These results demonstrate how port areas, or marinas in particular, may be critical in terms of biogeochemical processes at the SWI. Bottom sediments, in fact, can act as a sink or as a secondary source of contaminants. Possible perturbations of the current state of sediments (i.e. inadvertent disturbances due to storm or dredging) and chemical and physical variation (Eh, pH, dissolved oxygen and so on) could modify the basic behaviour found in this study. Further investigations such as a mesocosm approach could be planned in the future to verify how the effects of resuspension and the resulting chemical and physical changes may affect metal(loid) mobility in these areas.
References
Acquavita A, Predonzani S, Mattassi G, Rossin P, Tamberlich F, Falomo J, Valic I (2010) Heavy metal contents and distribution in coastal sediments of the Gulf of Trieste (northern Adriatic Sea, Italy). Water Air Soil Poll 211:99–111
Adami G, Barbieri P, Piselli S, Predonzani S, Reisenhofer E (1998) New data on organic pollutants in surface sediments in the harbour of Trieste. Ann Chim 88:745–754
Adami G, Barbieri P, Reisenhofer E (1999) A comparison on five sediment decomposition procedures for determining anthropogenic trace metal pollution. Int J Environ Anal Chem 75(3):251–260
Adami G, Barbieri P, Piselli S, Predonzani S, Reisenhofer E (2000) Detecting and characterising sources of persistent organic pollutants (PAHs and PCBs) in surface sediments of an industrialized area (harbor of Trieste, northern Adriatic Sea). J Environ Monitor 2:261–265
Alperin MJ, Albert DB, Martens CS (1994) Seasonal variations in production and consumption rates of dissolved organic carbon in an organic-rich coastal sediment. Geochim Cosmochim Acta 58:4909–4930
Alperin MJ, Martens CS, Albert DB, Suayah IB, Benninger LK, Blair NE, Jahnke RA (1999) Benthic fluxes and porewater concentration profiles of dissolved organic carbon in sediments from the North Carolina continental slope. Geochim Cosmochim Acta 63:427–448
Belias C, Dassenakis M, Scoullos M (2007) Study of the N, P and Si fluxes between fish farm sediment and seawater. Results of simulation experiments employing a benthic chamber under various redox conditions. Mar Chem 103:266–275
Belzunce Segarra MJ, Prego R, Wilson MJ, Bacon J, Santos-Echeandía J (2008) Metal speciation in surface sediments of the Vigo Ria (NW Iberian Peninsula). Sci Mar 72:119–126
Berelson W, McManus J, Coale K, Johnson K, Burdige D, Kilgore D, Colodner D, Chavez F, Kudela R, Boucher J (2003) A time series of benthic measurements from Monterey Bay, CA. Cont Shelf Res 23:457–481
Bogunović B, Malačič V (2008) Circulation in the Gulf of Trieste: Measurements and model results. Nuovo Cimento 31:301–326
Botwe BO, Kelderman P, Nyarko E, Lens PNL (2017a) Assessment of DDT, HCH and PAH contamination and associated ecotoxicological risks in surface sediments of coastal Tema Harbour (Ghana). Mar Pollut Bull 115:480–488
Botwe BO, Alfonso L, Nyarko E, Lens PNL (2017b) Metal distribution and fractionation in surface sediments of coastal Tema Harbour (Ghana) and its ecological implications. Environ Earth Sci 76:514
Boudreau BP (1999) Metals and models: diagenetic modelling in freshwater lacustrine sediments. J Paleolimnol 22:227–251
Brebbia CA, Tiezzi E, Conti ME (2007) Management of natural resources, sustainable development and ecological hazards. Southampton: Wit Press. 837 pp.
Burdige DJ, Alperin MJ, Homstead J, Martens CS (1992) The role of benthic fluxes of dissolved organic carbon in oceanic and sedimentary carbon cycling. Geophys Res Lett 19:1851–1854
Caille N, Tiffreau C, Leyval C, Morel JL (2003) Solubility of metals in an anoxic sediment during prolonged aeration. Sci Total Environ 301:239–250
Campbell PGC, Tessier A (1987) Metal speciation in natural waters: influence of environmental acidification. In: Sources and fates of aquatic pollutants. American Chemical Society, Washington DC, pp 185–207
Cardellicchio N, Buccolieri A, Di Leo A, Librando V, Minniti Z, Spada L (2009) Methodological approach for metal pollution evaluation in sediments collected from the Taranto Gulf. Toxicol Environ Chem 91:1273–1290
Casado-Martínez MC, Buceta JL, Belzunce MJ, DelValls TA (2006) Using sediment quality guidelines for dredged material management in commercial ports from Spain. Environ Int 32:388–396
Cibic T, Franzo A, Nasi F, Auriemma R, Del Negro P (2017) The port of Trieste (northern Adriatic Sea)—a case study of the “ecosystem approach to management”. Front Mar Sci 4(OCT):336
Covelli S, Horvat M, Faganeli J, Brambati A (1999) Porewater distribution and benthic flux of mercury and methylmercury in the Gulf of Trieste (northern Adriatic Sea). Estuar Coast Shelf Sci 48:415–428
Covelli S, Faganeli J, Horvat M, Brambati A (2001) Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic Sea). Appl Geochem 16(5):541–558
Covelli S, Faganeli J, De Vittor C, Predonzani S, Acquavita A, Horvat M (2008) Benthic fluxes of mercury species in a lagoon environment (Grado Lagoon, northern Adriatic Sea, Italy). Appl Geochem 23(3):529–546
Cozzi S, Falconi C, Comici C, Čermeljc B, Kovac N, Turk V, Giani M (2012) Recent evolution of river discharges in the Gulf of Trieste and their potential response to climate changes and anthropogenic pressure. Estuar Coast Shelf Sci 115:14–24
Daye M, Kadlecova M, Ouddane B (2015) Biogeochemical factors affecting the distribution, speciation, and transport of Hg species in the Deule and Lys rivers (Northern France). Environ Sci Pollut R 22(4):2708–2720
De Vittor C, Faganeli J, Emili A, Covelli S, Predonzani S, Acquavita A (2012) Benthic fluxes of oxygen, carbon and nutrients in the Marano and Grado lagoon (northern Adriatic Sea, Italy). Estuar Coast Shelf Sci 113:57–70
De Vittor C, Relitti F, Kralj M, Covelli S, Emili A (2016) Oxygen, carbon, and nutrient exchanges at the sediment–water interface in the Mar Piccolo of Taranto (Ionian Sea, Southern Italy). Environ Sci Pollut Res 23:12566–12581
Dickson AG (1990) Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep-Sea Res. Part A 37:755–766
Di Palma L, Mecozzi R (2007) Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J Hazard Mater 147:768–775
Dolenec T, Faganeli J, Pirc S (1998) Major, minor and trace elements in surficial sediments from the open Adriatic Sea: a regional geochemical study. Geol Croat 51:47–58
Emili A, Koron N, Covelli S, Faganeli J, Acquavita A, Predonzani S, De Vittor C (2011) Does anoxia affect mercury cycling at the sediment–water interface in the Gulf of Trieste (northern Adriatic Sea)? Incubation experiments using benthic flux chambers. Appl Geochem 26:194–204
Emili A, Acquavita A, Koron N, Covelli S, Faganeli J, Horvat M, Žižek S, Fajon V (2012) Benthic flux measurements of Hg species in a northern Adriatic lagoon environment (Marano and Grado Lagoon, Italy). Estuar Coast Schelf S 113:71–84
Emili A, Carrasco L, Acquavita A, Covelli S (2014) A laboratory-incubated redox oscillation experiment to investigate Hg fluxes from highly contaminated coastal marine sediments (Gulf of Trieste, northern Adriatic Sea). Environ Sci Pollut Res 21:4124–4133
Emili A, Acquavita A, Covelli S, Spada L, Di Leo A, Giandomenico S, Cardellicchio N (2016) Mobility of heavy metals from polluted sediments of a semi-enclosed basin: in situ benthic chamber experiments in Taranto’s Mar Piccolo (Ionian Sea, Southern Italy). Environ Sci Pollut Res 23:12582–12595
EPA (1999) Understanding variation in partition coefficient, Kd, values. EPA 402-R-99-004B, Vol II, 341 pp.
Faganeli J, Horvat M, Covelli S, Fajon V, Logar M, Lipej L, Cermelj B (2003) Mercury and methylmercury in the Gulf of Trieste (northern Adriatic Sea). Sci. Tot. Environ. 304:315–326
Froelich PN, Klinkhammer GP, Bender NA, Luedtke GR, Heath GR, Cullen C, Dauphin P, Hammond D, Hartman B, Maynard V (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochim Cosmochim Acta 43:1075–1090
Furlan N, Fontolan G, Sartore L, Milani B, Mosca R, Meriani S (1999) Caratterizzazione chimico-fisica dei sedimenti del porto di Trieste e problematiche derivanti dall’eventuale dragaggio e smaltimento a mare. Bollettino della Società Adriatica di Scienze LXXIX:3–26
Gagnon C, Pelletier E, Mucci A, Fitzgerald WF (1996) Diagenetic behavior of methylmercury in organic-rich coastal sediments. Limnol Oceanogr 41(3):428–434
Gagnon C, Pelletier E, Mucci A (1997) Behaviour of anthropogenic mercury in coastal marine sediments. Mar Chem 59:159–176
Gellner E, Valenti P (2005) San Rocco. Storia di un cantiere navale. Luglio editore 196 pp.
Giurastante R (2010) Tracce di legalità. Napoli: Autorinediti, 536 pp (in Italian)
Gobeil C, Cossa D (1993) Marcury in sediments and sediment pore water in the Laurentian trough. Can J Fish Aquat Sci 50:1794–1800
Goñi MA, Teixeira MJ, Perkey DW (2003) Sources and distribution of organic matter in a river-dominated estuary (Winyah Bay, SC, USA). Estuar Coast Shelf Sci 57:1023–1048
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis, 3rd edn. Wiley-VCH, Weinheim
Guerra RM, Martín R, Vila J, Romo J (2000) Informe de Caracteritzacío dels sediments en el Port de Barcelona. Servei de Medi Ambient de l’Autoritat Portuària de Barcelona
Hammerschmidt CR, Fitzgerald WF (2004) Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ Sci Technol 38:1487–1495
Harino H, Fukushima M, Kawai S (1999) Temporal trends of organotin compounds in the aquatic environment of the Port of Osaka. Japan Environ Pollut 105:1–7
Hedges JI, Stern JH (1984) Carbon and nitrogen determinations of carbonate–containing solids. Limnol Oceanogr 29:657–663
Hellali MA, Zaaboub N, Oueslati W, Added A, Aleya L (2015) Diagenetic processes and sediment-water exchanges of heavy metals in the Mejerda River Delta (Gulf of Tunis). Environ Earth Sci 74:6665–6679
Hines ME, Faganeli J, Planinc R (1997) Sedimentary anaerobic microbial biogeochemistry in the Gulf of Trieste, northern Adriatic Sea: influences of bottom water oxygen depletion. Biogeochem 39:65–86
His E, Beiras R, Seaman MNL (1999) The assessment of marine pollution—bioassays with bivalve embryos and larvae. Adv Mar Biol 38:178–371
Ho HH, Swennen R, Cappuyns V, Vassilieva E, Van Gerven T, Van Tran T (2012) Potential release of selected trace elements (As, Cd, Cu, Mn, Pb and Zn) from sediments in Cam River-mouth (Vietnam) under influence of pH and oxidation. Sci Total Environ 435:487–498
Hong H, Xu L-J, Zhang L, Chen J, Wong Y, Wan T (1995) Special guest paper: environmental fate and chemistry of organic pollutants in the sediment of Xiamen and Victoria harbours. Mar Pollut Bull 31(4):229–236
Horvat M, Covelli S, Faganeli J, Logar M, Mandić V, Rajar R, Širca A, Žagar D (1999) Mercury in contaminated coastal environments; a case study: the Gulf of Trieste. Sci Tot Environ 237/238:43–56
Islam Md I, Tanaka M (2004) Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar Pollut Bull 48:624–649
Jackson RN, Baird D, Els S (2005) The effect of the heavy metals lead (Pb2+) and zinc (Zn2+) on the brood and larval development of the burrowing crustacean, Callianassa kraussi. Water SA 31:107–116
Kelderman P, Osman A (2007) Effect of redox potential on heavy metal binding forms in polluted canal sediments in Delft (The Netherlands). Water Res 41:4251–4261
Lalere B, Szpunar J, Budzinski H, Garrigues P, Donard OFX (1995) Speciation analysis for organotin compounds in sediments by capillary gas chromatography with flame photometric detection after microwave-assisted acid leaching. Analyst 120:2665–2673
Lenaz D, Kamenetski VS, Princivalle F (1996) Cr-spinel supply in the Brkini, Istrian and Krk Island flysch basins (Slovenia, Italy and Croatia). Geol Mag 140:335–342
Li Y-H, Gregory S (1974) Diffusion if ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38:703–714
Li X, Shen Z, Way OWH, Li Y-S (2000) Chemical partitioning of heavy metals contaminants in sediments of the Pearl River Estuary. Chem Speciat Bioavailab 12:17–25
Lucker TJ, Dickson AG, Keeling CD (2000) Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equation for K1 and K2—validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar Chem 70:105–119
Marocco R (1989) Lineamenti geomorfologici della costa e dei fondali del Golfo di Trieste e considerazioni sulla loro evoluzione tardo-quaternaria. Int J Speleol 18:87–110 (In Italian)
McGlathery KJ, Anderson IC, Tyler AC (2001) Magnitude and variability of benthic and pelagic metabolism in a temperate coastal lagoon. Mar Ecol Prog Ser 216:1–15
Middelburg JJ, Levin LA (2009) Coastal hypoxia and sediment biogeochemistry. Biogeosciences 6:1273–1293
Milivojevič Nemanič T, Leskovšek H, Horvat M, Vrišer B, Bolje A (2002) Organotin compounds in the marine environment of the Bay of Piran, northern Adriatic Sea. Jour Environ Monit 4:426–430
Millero FJ, Zhang J-Z, Lee K, Campbell DM (1993) Titration alkalinity of seawater. Mar Chem 44(2–4):153–165
Muresan B, Cossa D, Jézéquel D, Prévot F, Kerbellec S (2007) The biogeochemistry of mercury at the sediment-water interface in the Thau lagoon. 1. Partition and speciation. Estuar Coast Shelf Sci 72:472–484
Ni Z, Zhanga L, Yua S, Jianga Z, Zhanga J, Wua Y, Zhaoa C, Liua S, Zhoua C, Huanga X (2017) The porewater nutrient and heavy metal characteristics in sediment cores and their benthic fluxes in Daya Bay, South China. Mar Pollut Bull 124(1):547–554
Ogorelec B, Misic M, Sercelj A, Cimerman F, Faganeli J, Stegnar P (1981) Sediment of the salt marsh of Sečovlje. Geologija 24:179–216
Ogrinc N, Faganeli J, Pezdic J (2003) Determination of organic carbon remineralization in near shore marine sediments (Gulf of Trieste, northern Adriatic) using stable carbon isotopes. Org Geochem 34:681–692
Ogrinc N, Faganeli J (2006) Phosphorus regeneration and burial in near-shore marine sediments (the Gulf of Trieste, northern Adriatic Sea). Estuar Coast Shelf Sci 67:579–588
Oliveri E, Salvagio Manta D, Bonsignore M, Cappello S, Tranchida G, Bagnato E, Sabatino N, Santisi S, Sprovieri M (2016) Mobility of mercury in contaminated marine sediments: biogeochemical pathways. Mar Chem 189:1–10
Olivotti R, Faganeli J, Malej A (1986) Impact of ‘ organic’ pollutants on coastal waters, Gulf of Trieste. Water Sci Technol 18:57–68
Petranich E, Covelli S, Acquavita S, Faganeli J, Horvat M, Contin M (2018a) Evaluation of mercury biogeochemical cycling at the sediment-water interface in anthropogenically modified lagoon environments. J Environ Sci 68:5–23
Petranich E, Covelli S, Acquavita A, De Vittor C, Faganeli I, Contin M (2018b) Benthic nutrient cycling at the sediment-water interface in a lagoon fish farming system (northern Adriatic Sea, Italy). Sci Tot Environ 644:137–149
Petronio BM, Calace N, Cardellicchio N, Ciardullo S, Pietrantonio M, Pietroletti M (2008) Metal distribution in marine sediments of the Mar Piccolo in Taranto (Ionic Sea, southern Italy). Toxicol Environ Chem 90:549–564
Petronio BM, Cardellicchio N, Calace N, Pietroletti M, Pietrantonio M, Caliandro L (2012) Spatial and temporal heavy metal concentration (Cu, Pb, Zn, Hg, Fe, Mn, Hg) in sediments of the Mar Piccolo in Taranto (Ionian Sea, Italy). Water Air Soil Pollut 223:863–875
Point D, Monperrus M, Tessier E, Amouroux D, Chauvaud L, Thouzeau G, Jean F, Amice E, Grall J, Leynaert A, Clavier J, Donard OFX (2007) Biological control of trace metal and organometal benthic fluxes in a eutrophic lagoon (Thau Lagoon, Mediterranean Sea, France). Estuar Coast Shelf Sci 72:457–471
Ramirez M, Massolo S, Frache R, Correa JA (2005) Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Mar Pollut Bull 50:62–72
Rapin F, Membrine GP, Förstner U, Gracia JL (1983) Heavy metals in marine sediment phases determined by sequential chemical extraction and their interaction with interstitial water. Environ Technol Lett 4:387–396
Rigaud S, Radakovitch O, Couture RM, Deflandre B, Cossa D, Garnier C, Garnier J-M (2013) Mobility and fluxes of trace elements and nutrients at the sediment-water interface of a lagoon under contrasting water column oxygenation conditions. Appl Geochem 31:35–51
Santschi P, Hohener P, Benoit G, Bucholtz-Ten Brink M (1990) Chemical processes at the sediment-water interface. Mar Chem 30:315–369
Ščančar J, Zuliani T, Turk T, Milacic R (2007) Organotin compounds and selected metals in the marine environment of northern Adriatic Sea. Environ Monit Assess 127:271–282
Schipper C, Rietjens I, Burgess R, Murk A (2010) Application of bioassays in toxicological hazard, risk and impact assessments of dredged sediments. Mar Pollut Bull 60:2026–2042
Shepard FP (1954) Nomenclature based on sand–silt–clay ratios. J Sediment Petrol 24:151–158
Sömen Joksič A, Katz SA, Horvat M, Milai R (2005) Comparison of single and sequential extraction procedures for assessing metal leaching from dredged costal sediments. Water Air Soil Pollut 162:265–283
Stumm W, Morgan JJ (1995) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. JohnWiley & Sons Inc., New York, p 1046
Sugimura Y, Suzuku Y (1988) A high temperature catalytic oxidation method for the determination of non-volatile dissolved organic carbon in seawater by direct injection of liquid samples. Mar Chem 24:105–131
Turner A (2010) Marine pollution from antifouling paint particles. Mar Pollut Bull 60:159–171
Turner A, Millward GE, Le Roux SM (2004) Significance of oxides and particulate organic matter in controlling trace metal partitioning in a contaminated estuary. Mar Chem 88:179–192
Ullman WJ, Aller RC (1982) Diffusion coefficients in nearshore marine sediments. Limnol Oceanogr 27:552–556
Warnken KW, Gill GA, Griffin LL, Santschi PH (2001) Sediment-water exchange of Mn, Fe, Ni and Zn in Galveston Bay, Texas. Mar Chem 73:215–231
Zhang L, Wang L, Yin K, Lü Y, Zhang D, Yang Y, Huang X (2013) Pore water nutrient characteristics and the fluxes across the sediment in the Pearl River estuary and adjacent waters, China. Estuar Coast Shelf Sci 133:182–192
Acknowledgements
The research activity was partially supported by the University of Trieste (Finanziamento di Ateneo per progetti di ricerca scientifica - FRA 2014, ref. Stefano Covelli). We are grateful to the management of the Portorož and Porto San Rocco marinas for their availability and hospitality. Karry Close is warmly acknowledged for proofreading the manuscript and Alessandro Acquavita for his help in preparing the manuscript. We are also grateful to the anonymous reviewers who provided useful comments and criticisms which improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Severine Le Faucheur
Electronic supplementary material
ESM1
(DOC 152 kb)
Rights and permissions
About this article
Cite this article
Petranich, E., Croce, S., Crosera, M. et al. Mobility of metal(loid)s at the sediment-water interface in two tourist port areas of the Gulf of Trieste (northern Adriatic Sea). Environ Sci Pollut Res 25, 26887–26902 (2018). https://doi.org/10.1007/s11356-018-2717-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2717-4