Abstract
The primary source of pharmaceuticals to the aquatic environment is the discharge of wastewater effluents. Pharmaceuticals are a large and diverse group of compounds. Among them, psychotropic substances are particularly interesting to study due to their specific known mode of action. The present study was performed to investigate the effects of wastewater effluents from a psychiatric hospital wastewater treatment plant (WWTP) on several aquatic organisms. All the analyzed pharmaceuticals (10 compounds) were detected in WWTP effluents as well as in the receiving river. Although the environmental concentrations were generally at trace levels (ng L−1 to μg L−1), induce toxic effects were observed. This study showed the effects of the WWTP effluents on the oogenesis and/or embryogenesis of amphipod crustacean Gammarus fossarum, Japanese fish medaka Oryzias latipes, mollusk Radix peregra, and planarian Schmidtea polychroa. A decrease of the number of oocytes and produced embryos was observed for G. fossarum and S. polychroa. Similarly, the hatching rate of R. peregra was affected by effluents. In the receiving river, the macroinvertebrate community was affected by the wastewater effluents discharge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceutical products (PPs) are a major group of chemical compounds being continuously released into the environment. Wastewater treatment plants (WWTPs) represent the main source of pharmaceuticals found in aquatic environments. On the one hand, this is owing to the strong occurrence of drugs in WWTP influents due to high drug consumption in human populations of industrialized countries, followed by an excretion of parent molecules or metabolites in urine and feces of consumers (Halling-Sorensen et al. 1998). The occurrence of PPs in urban wastewater has been widely documented (Pasquini et al. 2014; Togola and Budzinski 2007; Bartelt-Hunt et al. 2009, Zorita et al. 2009; Calisto and Esteves 2009; Santos et al. 2010; Sousa et al. 2011) as well as the importance of hospital effluent contribution to the presence in urban wastewater of several pharmaceutical classes (Kummerer 2009; Thomas and Langford 2007). On the other hand, WWTPs are not designed to specifically remove drug complex molecules leading to an incomplete or limited degradation of these compounds (Orias and Perrodin 2014). Thus, levels of removal depend on treatment processes, operational conditions, and substance properties and vary from on plant to another (Muter et al. 2017; Aubertheau et al. 2017; Bartelt-Hunt et al. 2009; Vieno et al. 2007). Moreover, concentration of drugs in WWTP effluents is not a criterium to evaluate sewage treatment plant efficiency. It is currently based on nitrogen, phosphorus, some pesticides and metal levels, biochemical oxygen demand, and total suspended solid (Directive 2000/60/EC) despite the presence of some pharmaceutical products on the Water Framework Directive watch list (diclofenac, 17 alpha-ethinylestradiol (EE2), and 17 beta-estradiol (E2). Chemical analyses of WWTP effluents described the occurrence of drugs at concentrations ranging from < 1 ng L−1 to few microgram per liter (Verlicchi and Zambello 2016; Yang et al. 2017). These relatively high levels of active and putatively toxic compounds discharged in surface waters could represent a major threat for aquatic ecosystems. Many studies have demonstrated the toxicity of wastewater effluents (Galus et al. 2013; Mendonça et al. 2009; Hernando et al. 2005; Manusadzianas et al. 2003) though just a few have focused on hospital wastewater treatment plants (Orias and Perrodin 2014) and a very limited number of studies have explored the impact of wastewater effluents in situ on freshwater macroinvertebrate communities (Muñoz et al. 2009; Ginebreda et al. 2010). The aim of this study is to evaluate effects of effluents released by a psychiatric hospital on aquatic organisms by two methods: in situ in the receiving river (macroinvertebrates) and in controlled condition (i.e., bioassays) context.
Materials and methods
Study site
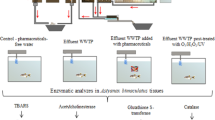
The study site is a regionally psychiatric hospital with 250 used beds and 120 places in ambulatory services (Dordogne, France). This center is used for children, adult, and geriatric psychiatry and addictive disorders. In 2010, approximately 25 kg of psychotropic drugs was consumed in this hospital (information given by the hospital). This hospital has a wastewater treatment plant (WWTP), which treats about 600 m3 of wastewater/day. This WWTP is constituted by a primary decanter, a bacterial filter, and a secondary decanter and does not operate continuously. When the volume in the lifting area reaches its maximum capacity, waters fall over by pour in the primary decanter (about 10 discharges of effluent per 24 h). This ensures mixing of wastewater effluents over 24 h. The effluents are released into the Isle river. This river is 255 km long and covers a catchment area of about 7500 km2. The flow of this river is characterized by a high variability controlled by seasonal rainfall (average flow 120 m3/s in winter and 16 m3/s in summer).
Wastewater samples
As a first step, chemical analysis has been performed to determine pollutants in WWTP effluent (24-h composite sample). Second, a first sampling campaign has been done to link chemistry and ecotoxicology (2011). Finally, a last sampling campaign has been carried out to assess the ecotoxicological effects of the effluents.
For the chemical characterization of effluents, five samples were collected between 23th May 2011 and 10th October 2011, whose characteristics are listed in Table 1. During the sampling period, the WWTP was confirmed to be under normal operations. The chemical characterization of these composite samples (pool of 6 discharges of effluent with equal volume of each discharge) was compared to one flow-proportional (24 h) composite sample collected using automatic samplers. Classical water analyses were also conducted on 10 February 2011 by the Departmental Analysis and Research Laboratory (approved by the Ministry of Agriculture) in the Isle at the effluent discharge point (Table 2).
For ecotoxicity tests, samples were collected within the same timeframe as mentioned above for the chemical characterization (from May to October 2011) for the first sampling program and between May 2012 and June 2012 for the second sampling program. Samples were refrigerated and used immediately for tests using freshwater amphipods and medaka or kept frozen (− 20 °C) for the other bioassays.
Chemical analyses
Metals, organic substances, and microbiological parameters in the Isle samples were investigated by the “Laboratoire Départemental d’Analyse et de Recherche de Dordogne,” approved by the Ministry of Agriculture (Table 1) in accordance with current standards. The physico-chemical characterization of effluents (2011 and 2012) was realized by IRSTEA (Table 2) in accordance with French standard operating procedures (AFNOR) as indicated in Table 2. For the analysis of pharmaceuticals, the extraction procedure has been already validated and detailed (Togola and Budzinski 2007, 2008, Dévier et al. 2013, Bonnafe et al. 2015). Pharmaceutical analysis was performed by ultra-performance liquid chromatography (UPLC) ACQUITY coupled to waters Quattro Premier XE triple quadrupole (Waters, Saint Quentin-en-Yvelines, France) mass spectrometer fitted with an electrospray ionization (ESI) source operated in both positive and negative ion mode. Regarding the nature of the hospital (psychiatric hospital) and top drug consumption rate, ten molecules were targeted: carbamazepine, diazepam, oxazepam, norfluoxetine, fluoxetine, sertraline, lorazepam, cyamemazine, citalopram, and hydroxyzine (Table 3).
Bioassays
For all bioassays, two campaigns were carried out to assess the toxicity of effluent samples collected in 2011 and 2012. The results presented here are an average of the two campaigns.
Freshwater snail model
Sampling and handling of organisms
Freshwater pulmonate snails Radix peregra were collected from the Caussel River (Tarn, France) in slow waters. The snails were maintained in covered 40-L aquariums during 1 month at 20 ± 1 °C with a 10/14-h light/dark photoperiod and in reconstituted water (OECD 2004). Organisms were fed ad libitum with green lettuce leaves (“Bio” without pesticide) every 3 days.
Assessment of toxic effects on snail embryos
Embryos in cocoon were used to investigate the embryotoxicity of the hospital wastewater effluents. The eggs were deposited in egg masses and each containing about 150–250 eggs per capsule. The extremities of the laying were removed. Egg mass was gently dislodged and each egg was separated with the cup tip of a 200-μL pipette. All the embryos selected were at the same stage and in good developmental conditions. The unfertilized eggs or immobilized embryos were removed. Twenty eggs for each condition were placed in 96-well plates with one egg/well in a total volume of 300 μL at 22 ± 1 °C with the same photoperiod as employed for culturing until hatching. Organisms are kept in the same waters until they hatch. At the end of the test, the delay of development, the hatching rate, and number of malformed and dead embryos were recorded using an inverted microscope. The experiment was performed in triplicate with two samples of wastewater effluent (September 2011 and May 2012).
Freshwater planarian model
Sampling and handling of organisms
The sexual diploid Schmidtea polychroa freshwater planarian population used in this study was collected from the Caussel River (Tarn, France). The organisms were kept during a 15-day acclimatization period under constant aeration with a temperature of 18 °C (± 1 °C) and a 10/14-h light/dark photoperiod. Organisms were maintained in 10-L tanks with dechlorinated water and fed with living ancylidae mollusks once every 2 days.
Assessment of reproductive effects on planarian
The reproduction performance was evaluated with 40 randomly selected adult planarians. They were exposed to wastewater effluent for 5 weeks at a temperature of 21 ± 1 °C and under a 12/12-h light/dark photoperiod (to stimulate breeding behavior) in a 500-mL glass beaker while controls were maintained in the same conditions in dechlorinated water. Water and wastewater were renewed biweekly and planarians were fed at the same time. Cocoons produced by sexual reproduction were counted biweekly and transferred to separated beaker. Fecundity was evaluated as the number of cocoons produced by planarian. The experiment was performed in triplicate with two samples of wastewater effluent (September 2011 and May 2012).
Freshwater amphipod model
Sampling and handling of organisms
Crustaceans (Gammarus fossarum) were collected at La Tour du Pin, a known unpolluted upstream part of the Bourbre River (Isère, France). Sexually mature gammarids were used. The organisms were kept during a 15-day acclimatization period under constant aeration and with a temperature of 12 °C (± 1 °C). A 10/14-h light/dark photoperiod was maintained. Organisms were fed with alder leaves. The protocol was described by Besse et al. (2013).
Assessment of reprotoxic effects on amphipod females
Reproductive toxicity tests were performed using the method described by Geffard et al. (2010). Twenty-one females were exposed to wastewater effluent (at concentrations of 100, 33, 11, and 0% of effluent) for 21 days at a temperature of 12 ± 1 °C and under a 16/8-h light/dark photoperiod (to stimulate breeding behavior) in a 500-mL glass beaker. Water and wastewater were renewed twice a day. At the end of exposure, the number of oocytes per female was determined by in vivo observation of the two ovaries under the binocular microscope. To assess the number of embryos per female, embryos were manually recovered from the marsupium and counted under a binocular microscope. Abnormal embryos were defined as embryos with aberrations and malformations as described by Lawrence and Poulter (2001). The last analyzed parameter was the duration of molt stages. The molt stages of Gammarus fossarum females were defined using criteria described by Charniaux-Cotton and Payen (1985) in the marine amphipod Orchestia gammarellus and by Geffard et al. (2010).
Fish model: assessment of toxic effects on Japanese medaka embryos
Japanese medaka embryos (CAB line) at 24 h post fertilization (hpf) were supplied by UMS Amagen (Gif-sur-Yvette). On arrival, embryos were carefully examined under a stereomicroscope (Leica MZ75, Leica Microsystems) to remove unfertilized, dead, or non-synchronized embryos. After sorting, 25 embryos were randomly distributed in plastic Petri dishes and then incubated with 0.2 μm filtered wastewater or river water at two different concentrations 100 or 30%. Dilutions were performed with spring water (Cristalline). Water was renewed every day, just after dissolved oxygen measurement (PA2000, Unisense, Aarhus, DK). Exposure was carried out in a climate chamber (Economic Delux, Snijders Scientific, Tilburg, NL) at 26 ± 0.3 °C, under a 5000-lx white light and a 12/12-h light/dark photoperiod until total resorption of yolk sac (13–14 days). Three independent replicates were performed for each condition.
Viability was checked daily under the stereomicroscope at embryonic and larval stages during the whole experiment. Dead embryos or larvae were counted and removed.
Cardiac activity was assessed at day 7 (pf) as described previously by Barjhoux et al. (2012). Five randomly selected embryos per replicate were used. Heart beats were counted in three 20-s intervals per individual using a Leica MZ75 stereomicroscope and a cold light source. These three measurements were then added to obtain cardiac activity in beats per minute.
At hatching, a whole-body picture of 15 larvae per replicate was taken and total body length was determined through image analysis system (Leica Application Suite v2.8.1).
Development abnormalities were observed on 15 newly hatched larvae per replicate. Six different categories of abnormalities were recorded including pericardial, yolk sac, and cranial oedemata; spinal deformities (scoliosis, lordosis, and tail malformations); craniofacial deformities; eye anomalies; cardiovascular anomalies; and yolk sac anomalies as previously described (Barjhoux et al. 2012).
Macroinvertebrate analysis
To assess potential environmental effects of wastewater effluent on aquatic environment, we need sensitive bioindicators. Macroinvertebrates are known to be good bioindicators of aquatic ecosystem health.
Four sampling sites from the Isle river were selected near the psychiatric hospital. The first site (1) was the discharge point of the wastewater effluent. This point was at approximately 10 m from the bank at a depth of 1.6 m. To sample as precisely as possible at its close vicinity, fluorescein was added to the wastewater. The second site (2) was located upstream, 50 m far from the discharge point. Sites 3 and 4 were located respectively at 40 and 90 m downstream of the discharge (Fig. 1). All these sites were selected with comparable water depths (between 1 and 1.7 m), stream velocity (less than 5 cm/s), and substrate dominance (silt and leaf litter) to limit sampling bias due to habitat heterogeneity.
For all sites, benthic macroinvertebrates were collected during each season, using a polyvinyl sand corer (internal diameter 45 mm). Due to the water depth (over 1 m deep), the sand corer was not directly used on the sampling point. Therefore, the sediment was firstly collected from a boat with an Ekman bottom grab sampler; this sample was later used with the sand corer. On each site, five sand corer samples were performed on the first 10 or 15 cm of the top sediment layer, where 90% of the benthic fauna is present (Sherfy et al. 2000; Kajak and Dusoge 1971). Thus, a total of 80 samples were collected during the entire study period. Samplings were performed during the four seasons (May 2011, September 2011, November 2011, and February 2012).
Macroinvertebrates were fixed with 4% formaldehyde, sorted, counted, and identified at the laboratory under a stereoscopic microscope. Identification was at species level for oligochaetes and at the genus or family level for other remaining present groups (mollusks, crustaceans, insects, etc.).
Macroinvertebrate communities can be affected by a multitude of natural or anthropic factors and to assess the effects of a specific stressor can be a hard task. This problem can be solved using biological and physiological traits (Statzner and Beche 2010). Indicators such as “SPEcies At Risk” (SPEAR), based on this concept, have been developed for the retrospective ecological risk assessment (Liess and Von Der Ohe 2005) and most specifically “SPEARorganic Indicator” is able to detect community disturbance by organic toxicants, from mountain streams to lowland rivers (Beketov and Liess 2008). The higher the value of SPEARorganic, the higher the proportion of taxa that are sensitive to continuously present organic toxicants.
SPEAR index was calculated using the SPEAR calculator (available online at https://www.ufz.de/index.php?en=38122).
Statistical analysis
Data were given as mean ± standard deviation. Statistical analyses were performed with R software program version 2.14 on untransformed data (www.r-project.org). The equality of variances and normality were tested (using Bartlett test and Shapiro-Wilk normality test respectively). To compare means effects, one-way analysis of variance (ANOVA), followed by Tukey’s test for multiple comparisons, was used. Whenever the normality was not respected, the non-parametric Mann-Whitney test was performed. The level of significance was set at 0.05.
Results
Chemical analyses
The results of the analysis of the wastewater effluent and surface water samples are shown in Tables 1, 2, and 3. All the parameters of treated effluent were within the discharge limits. All the parameters analyzed in Isle rwere below the quality limits.
The analgesic paracetamol presented the highest concentrations detected among all pharmaceuticals in effluents and Isle river (9800 ng L−1 in river to 38,000 ng L−1 in effluent). Caffeine was also detected at high concentrations in effluent and discharge water (4900 and 900 ng L−1 respectively). All ten psychiatric pharmaceuticals were detected in the hospital effluent. High concentrations of oxazepam (7239 ng L−1) and carbamazepine (2114 ng L−1) were detected. Citalopram (104 ng L−1), diazepam (115 ng L−1), cyamemazine (232 ng L−1), and lorazepam (408 ng L−1) were mainly found at concentrations above 100 ng L−1. Far lower concentrations were observed for fluoxetine (25 ng L−1), hydroxyzine (20 ng L−1), sertraline (12 ng L−1), and norfluoxetine (3 ng L−1). In the Isle river at the discharge point of effluent, oxazepam and carbamazepine were detected at the highest levels with concentrations of 816 and 165 ng L−1 respectively. The concentrations of these two molecules were approximately ten times lower in this river than in the hospital effluent. Lorazepam, fluoxetine, and norfluoxetine were not detected in the river sample. Hydroxyzine, sertraline, diazepam, citalopram, and cyamemazine were found at concentrations below 10 ng L−1.
Bioassays
Hatching rate of Radix balthica
Hatching time ranged from the 10th–12th day of exposure. The Isle river sample (at the psychiatric hospital effluent discharge) did not cause significant impact on hatching success as compared to the control group (Fig. 2). While the effluent-treated group showed a significant decrease of cumulative hatching after 12 days.
Reproductive performance evaluation of Schmidtea polychroa
Cumulative numbers of cocoons produced by individual flatworm from each treatment group are shown in Fig. 3. Mortality was not significantly different between controls and tested conditions (results not shown). No effect on the number of egg capsules produced per Schmidtea polychroa was obtained with Isle river water. In contrast, effluent dramatically impaired the reproduction of this species, with cocoon production close to zero.
Reproductive performance evaluation of Gammarus fossarum
No significant mortality was observed in amphipods exposed 3 weeks to 11 and 33% of effluent, with values higher to 80% and similar to the ones observed in controls. At the concentration of 100% effluent, mortality increased significantly with value reaches 33%.
The fecundity rates observed are presented in Fig. 4. No effect was observed in females exposed to 11 or 33% of effluent. Decrease observed at the concentration of 100% is not significant. Results for the fertility are presented in Fig. 5. With the lower concentration of effluent (11%), the number of embryos per female was not significantly different from the controls. In contrast, at 33 and 100% effluent concentrations, the production of embryos per female decreased significantly to reach 0% at the highest concentration. In the same way, percentages of abnormal embryos (Fig. 6) significantly increased through tested concentrations, reaching more than 40% at the concentration of 33%.
Effects on embryos of Japanese medaka Oryzias latipes
No significant increases of embryonic and larval mortalities were observed for medaka exposed either to effluent or to Isle river (at the psychiatric hospital effluent discharge) samples compared to controls with embryo or larval viability values higher than 94% (Table 4).
No significant modification of cardiac rhythm was observed in response to both treatments (river or effluent). In the same way, the exposure to effluent or river water samples did not cause significant increase of embryonic abnormalities (Table 4).
The Isle river water and effluent samples did not induce significant changes in hatching success when compared to the control group although a decrease trend was observed for effluent-treated larvae (Fig. 7). Neither river water nor effluent exposure induced modifications in larvae body length (Table 4).
Effects of wastewater discharge on the macroinvertebrate assemblages in the Isle river
Considering all collected organisms, more than 90% of them were Oligochaeta and Hexapoda Diptera. These communities of benthic invertebrates composed of 66 different taxa are characteristics of a perturbed fluvial system. Focusing on the community composition in the spring period (where the highest diversity was found; Fig. 8a), the taxonomic richness decreased from site 2 to sites 1 and 3 (at the vicinity of the discharge and 40 m downstream). This general pattern was also observed during summer and winter (Fig.8a).
During the studied period, SPEARorganic index varied from − 0.031 to − 0.277 (Fig.8b). The lowest values were recorded for site 1 or 3 (i.e., sites close to the effluent discharge point) during the four seasons. Then, macroinvertebrates, present close to discharge point or directly downstream the discharge, are less sensitive to organic pollutants than organisms located upstream or 90 m downstream of the discharge. Moreover, this observation is particularly true during autumn and winter where SPEAR index values were very low (− 0.243 and − 0.277 respectively).
Discussion
In regard to regulation of WWTP effluents, based mainly on ionic charge, pH, and organic matter contents, the results of the present study (Table 2) do not show any problem, none of the 18 studied parameters exceed regulatory standard, throughout sampling dates. Acceptable values were observed mainly for ammonium, nitrites, and nitrates that are known to be primary problems for WWTP effluents, given their toxicity toward aquatic organisms. Physico-chemical parameters observed in this study were comparable to the ones reported in other studies (Giannakis et al. 2015; Chonova et al. 2016; Knopp et al. 2016; Diaz-Garduno et al. 2017).
The aim of the present study was to assess the contamination levels by pharmaceuticals compounds of an effluent from a psychiatric hospital and then its toxicity for aquatic organisms. At the discharge point of psychiatric hospital wastewater effluent, all searched compounds were detected and quantified with values ranging from nanogram per liter to microgram per liter. The occurrence of pharmaceuticals in wastewater effluents from hospital at such high concentrations results mainly from their low adsorption coefficients and their low degradability which favors their mobility through the wastewater plant (Ohlenbusch et al. 2000). Highest concentrations were obtained for the paracetamol with values ranged from 4.8 to 38 μg L−1, as previously observed by Kosma et al. (2010) and Chonova et al. (2016) in wastewaters from hospitals (until 12 μg L−1). For the oxazepam, mean concentrations in effluent samples were seven to eight times higher than the values measured in European WWTP effluents. In the present study, the concentration reached 7 μg L−1 whereas concentrations in the range from 0.25 to 1.1 μg L−1 were reported in the literature for Chinese or European hospital effluents (Calisto and Esteves 2009; Orias and Perrodin 2013; Yuan et al. 2013). The use of oxazepam at Montpon psychiatric hospital is very significant (± 7000 kg an−1) and the WWTP is an older generation of plant. However, in the frame of their risk assessment study of pharmaceutical compounds focusing on wastewater of hospitals, Escher et al. (2011) predicted environmental concentrations (PEC) that can reach values of 7.24 μg L−1, detection and more specifically for the oxazepam, a concentration of 7 μg L−1.
The occurrence of carbamazepine at high concentrations (2 μg L−1 in effluent) is in accordance with data from the literature on wastewater effluents and surface waters (Calisto and Esteves 2009; Orias and Perrodin 2013; Yuan et al. 2013; Gonzalez Alonso et al. 2010). This result can be explained by the low removal efficiency of this molecule by WWTP, in relation to its specific molecular characteristics (Ternes et al. 2007). The contamination levels and the characteristics of the hospital effluents are directly related to pharmaceutical compounds used and the molecular specificity of them. The prescription and the choice of the molecule may vary from one institution to another and from one country to another, making it difficult the proposal of a generalizable methodology for their risk assessment (Yuan et al. 2013). For example, cyamemazine is mainly used in French psychiatric hospitals but much less used in other countries, leading lack of available data to compare. In the present study, this compound was quantified to concentrations up to 320 ng L−1. At last, caffeine, which is not a pharmaceutical compound in the same way as those previously discussed, is however found at very high concentration reaching 5 μg L−1, as reported in earlier studies (Muter et al. 2017), and this despite the fact this compound is known to be highly degradable (Muter et al. 2017).
The concentrations of pharmaceuticals in the receiving river (Isle river, site 1) are consistent with literature data (Calisto and Esteves 2009). They ranged from 0.4 to 900 ng L−1 for the caffeine. Depending on the compounds, the dilution factors varied from 4 for the paracetamol to 12 for the carbamazepine (5 for caffeine, 8.5 for oxazepam). These values are consistent with the fixed standard dilution factor of 10 used for ecological risk assessment of many chemical regulations (ECHA 2016). Link et al. (2017) determined median dilution factor of 5 to 14.5 in Germany.
Quality of receiving waters were investigated using three species from different trophic levels, the planarian Schmidtea polychroa, the mollusk Radix balthica, and the fish Oryzias latipes. In all cases, no effect was observed on the development and the reproduction of these species. On contrary, with effluent, the effects observed were much more marked. Psychiatric hospital effluents caused detectable effects on reproductive performance (including viability and development of offprints) of all test organisms. The raw effluent (100%) led to a delay in hatching of the mollusk R. balthica and the fish O. latipes, with a decrease of 20% for the two cases. Gust et al. (2013) reported the occurrence of pharmaceuticals and estrogenic compounds in municipal wastewater which alter the immune status of the pond of Lymnaea stagnalis. Moreover, Sanchez-Arguello et al. (2012) demonstrated the embryotoxicity of fluoxetine in the freshwater snail Physa acuta. In fish, the observed delay of hatching suggests that embryos were exposed to contaminants despite the presence of chorion which acts as a barrier to chemical penetration. Pruvot et al. (2012) described teratogenic effects such as hatching retardation and axial malformations in zebrafish after pharmaceutical exposure such as valproic acid, carbamazepine, pentobarbital, caffeine, and theophylline. These compounds were found in Montpon hospital effluent. Canadian wastewater effluent containing a pharmaceutical mixture composed of carbamazepine, gemfibrozil, venlafaxine, and acetaminophen caused a significant reduction in the average cumulative number of viable zebrafish embryos produced per female, relative to the controls (Galus et al. 2013). These same authors also reported developmental abnormalities in zebrafish embryos exposed to pharmaceutical mixture or effluent. At last, in one of our previous studies, it was demonstrated that a 72-h exposure to six different psychotropic drugs, isolated or in mixture, at concentrations ranged from 10 to 1000 μg L−1 could affect the viability and swimming activity of medaka larvae (Chiffre et al. 2016). Although this study focused solely on the occurrence of pharmaceuticals, WWTP effluents did not contain only pharmaceutical compounds and detergents can also be detected (Montes-Grajales et al. 2017) and can participate in the toxicity of the effluent. In the same way, Chen et al. (2008) also showed that caffeine, present at high concentrations (5 μg L−1) in our studied effluent, caused disturbances of early neurogenesis during early embryogenesis of zebrafish.
In the planarian and the gammarids, raw effluent exposure had dramatic impact on their reproduction since the production of cocoon and embryos was null respectively. Schirling et al. (2005) observed abnormal structure of oocytes in Gammarus pulex and Gammarus fossarum collected from streams impacted by sewage treatment plant effluents. Henry et al. (2004) showed that antidepressants (citalopram, fluoxetine, sertraline found in hospital effluents) negatively affected reproduction of the crustacean Cerodaphnia dubia and reduced the number of neonates per female. Fong and Ford (2014) found that any chemicals in the environment and particularly the antidepressants can modulate neurohormones (serotonin and dopamine) involved in reproduction, growth, maturation, and larval development of crustaceans. These pharmaceuticals can disrupt the normal endocrine and biological function in exposed organisms. Recently, Mazzitelli (2016) and Mazzitelli et al. (2017) showed a decrease in the number of cocoons by the flatworm following exposure to carbamazepine, oxazepam, sertraline, and cyamemazine with concentrations ranged from 1 to 100 ng L−1. In Gammarus fossarum, dilution of the effluent was also tested in order to better understand the impact of effluent on aquatic systems. In the Isle river, the dilution factor varies through the seasons, according to flow rate (from 16 m3 s−1 in August to 120 m3 s−1 in February). The ecological risk assessment of many chemical regulations, e.g., for medicinal products for human use or for biocidal products, employs a fixed standard dilution factor of 10 when evaluating chemical exposure of running waters via the WWTP route (ECHA 2016). The present study showed that the effluent concentration of 11% leads to deleterious effects on the embryonic development of G. fossarum, a key life history trait. The used of multiple bioassays highlighted a possible impact on the populations of organisms through reprotoxic or embryotoxic effects. Reproduction and development impairment can affect population dynamics leading to potential long-term consequences on the population success and survival.
In the Isle river, macroinvertebrate communities were dominated by pollution tolerant taxa such as Oligocheta and hexapod Diptera. This observation is a generalizable for most of the European lowland streams and rivers, running in populated areas. These running waters are affected by multiple stressors, such as organic pollution and morphological changes (Nijboer et al. 2004) affecting natural communities. Then, impact of hospital effluent on such ecosystems can be minimized or less detectable than in controlled condition and bioassays studies. Nevertheless, in this study, the total abundance, taxonomic richness, and SPEAR index were lower in sampled points where the effluent was strongly present than in upstream or downstream of these points. Ginebreda et al. (2010) also observed a correlation between anthropogenic pressures and pharmaceutical discharge and density or diversity of macroinvertebrates in Spanish rivers. Thus, the discharge of hospital effluents could alter the macroinvertebrate community structure. Most of the observed changes in the composition of macroinvertebrate community were due to the disappearance of some less tolerant taxa. These changes in diversity and taxonomic composition may alter the between-taxa interactions (Jarvis et al. 2014). However, in this study, the macroinvertebrate community was impacted only when close to the discharge points, i.e., sites 1 and 3, and this over the four seasons. Differences in indices (SPEAR index and taxonomic diversity) calculated during the four seasons can be due to sampling constraints. Indeed, despite fluorescein being added into the effluent, it was very difficult to sample at site 1 (in the close vicinity of the discharge point) due to the water depth and boat instability. In some cases, sampling occurred a few meters upstream the effluent discharges explaining variability of the site 1 results. Nevertheless, this tendency was also observed on site 3 located 39 m downstream from the discharge point. Though this study was only conducted on a single river, our results point out that the effluent discharge can locally impact river communities, while most likely not modifying the general ecological status of the river.
References
Aubertheau E, Stalder T, Mondamert L, Ploy MC, Dagot C, Labanowski J (2017) Impact of wastewater treatment plant discharge on the contamination of river biofilms by pharmaceuticals and antibiotic resistance. Sci Total Environ 579:1387–1398
Barjhoux I, Baudrimont M, Morin B, Landi L, Gonzalez P, Cachot J (2012) Effects of copper and cadmium spiked-sediments on embryonic development of Japanese medaka (Oryzias latipes). Ecotoxicol Environ Saf 79:272–282
Bartelt-Hunt S, Snow D, Damon T, Shockley J, Hoagland K (2009) The occurrence of illicit and therapeutic pharmaceuticals in wastewater effluent and surface waters in Nebraska. Environ Pollut 157:786–791
Beketov MA, Liess M (2008) An indicator for effects of organic toxicants on lotic invertebrate communities: independence of confounding environmental factors over an extensive river continuum. Environ Pollut 156(3):980–987
Besse JP, Coquery M, Lopes C, Chaumot A, Budzinski H, Labadie P, Geffard O (2013) Caged Gammarus fossarum (Crustacea) as a robust tool for the characterization of bioavailable contamination levels in continental waters: towards the determination of threshold values. Water Res 47:650–660
Bonnafe E, Sroda S, Budzinski H, Valière A, Pedelluc J, Marty P, Geret F (2015) Responses of cytochrome P450, GST and MXR in the mollusk Corbicula fluminea to the exposure to hospital wastewater effluent. Environ Sci Pollut Res 22:11033–11046
Calisto V, Esteves VI (2009) Psychiatric pharmaceuticals in the environment—a review. Chemosphere 77(10):1257–1274
Charniaux-Cotton H, Payen G (1985) Sexual differentiation. In: Integument, pigments, and hormonal processes, pp 217–299
Chen YH, Huang YH, Wen CC, Wang YH, Chen WL, Chen LC, Tsay HJ (2008) Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol Teratol 30:440–447
Chiffre A, Clérandeau C, Dwoinikoff C, Le Bihanic F, Budzinski H, Geret F, Cachot J (2016) Psychotropic drugs in mixture alter swimming behaviour of Japanese medaka (Oryzias latipes) larvae above environmental concentrations. Environ Sci Pollut Res 23:4964–4977
Chonova T, Keck F, Labanowski J, Montuelle B, Rimet F, Bouchez A (2016) Separate treatment of hospital and urban wastewater: a real scale comparison of effluents and their effect on microbial communities. Sci Total Environ 542:965–975
Dévier MH, Le Menach K, Viglino L, Di Gioia L, Lachassagne P, Budzinski H (2013) Ultra-trace analysis of hormones, pharmaceutical substances, alkylphenols and phthalates in two French natural mineral waters. Sci Total Environ 443:621–632
Diaz-Garduno B, Pintado-Herrera MG, Biel-Maeso M, Rueda-Marquez JJ, Lara-Martin PA, Perales JA, Manzano MA, Garrido-Perez C, Martin-Diaz ML (2017) Environmental risk assessment of effluents as a whole emerging contaminant: efficiency of alternative tertiary treatments for wastewater depuration. Water Res 119:136–149
ECHA (2016) Guidance on information Requirements and Chemical Safety Assessment: Chapter R16: Environmental Exposure assessment. (Helsinki)
Escher BI, Baumgartner R, Koller M, Treyer K, Lienert J, McArdell CS (2011) Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res 45:75–92
Fong PP, Ford AT (2014) The biological effects of antidepressants on the molluscs and crustaceans: a review. Aquat Toxicol 151:4–13
Galus M, Jeyaranjaan J, Smith E, Li H, Metcalfe C, Wilson JY (2013) Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat Toxicol 132-133:212–222
Geffard O, Xuereb B, Chaumot A, Geffard A, Biagianti S, Noël C, Abbaci K, Garric J, Charmantier G, Charmantier-Daures M (2010) Ovarian cycle and embryonic development in Gammarus fossarum: application for reproductive toxicity assessment. Environ Toxicol Chem 29:2249–2259
Giannakis S, Gamarra Vives FA, Grandjean D, Magnet A, De Alencastro LF, Pulgarin C (2015) Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res 84:295–306
Ginebreda A, Munoz I, Lopez de Alda M, Brix R, Lopez-Doval J, Barcelo D (2010) Environmental risk assessment of pharmaceuticals in rivers: relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain). Environ Int 36:153–162
Gonzales Alonso S, Catala M, Romo Maroto R, Rodriguez Gil J, Gil de Miguel A, Valcarcel Y (2010) Pollution by psychoactive pharmaceuticals in the Rivers of Madrid metropolitan area (Spain). Environ Int 36:195–201
Gust M, Fortier M, Garric J, Fournier M, Gagné F (2013) Immunotoxicity of surface waters contaminated by municipal effluents to the snail Lymnaea stagnalis. Aquat Toxicol 126:393–403
Halling-Sorensen B, Nielsen SN, Lanrky PF, Ingerslev F, Holten Lützhoft HC, Jorgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment. A review. Chemosphere 36:357–393
Henry TB, Kwon JW, Armbrust KL, Black MC (2004) Acute and chronic toxicity of five selective serotonin reuptake inhibitors in Ceriodaphnia dubia. Environ Toxicol Chem 23:2229–2233
Hernando MD, Fernandez-Alba AR, Tauler R, Barcelo D (2005) Toxicity assays applied to wastewater treatment. Talanta 65:358–366
Jarvis AL, Bernot MJ, Bernot RJ (2014) Relationships between the psychiatric drug carbamazepine and freshwater macroinvertebrate community structure. Sci Total Environ 496:499–509
Kajak Z, Dusoge K (1971) The regularities of vertical distribution of benthos in bottom sediments of three masurian lakes. Ekologia Polska 32:485–499
Knopp G, Prasse C, Ternes TA, Cornel P (2016) Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters. Water Res 100:580–592
Kosma C, Lambropoulo DA, Albanis TA (2010) Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J Hazard Mater 179:804–817
Kummerer K (2009) The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. J Environ Manag 90:2354–2366
Lawrence AJ, Poulter C (2001) Impact of copper, pentachlorophenol and benzo[a]pyrene on the swimming efficiency and embryogenesis of the amphipod Chaetogammarus marinus. Mar Ecol Prog Ser 223:213–223
Liess M, Von der Ohe PC (2005) Analyzing effect of pesticides on invertebrate communities in stream. Environ Toxicol Chem 24(4):954–965
Link M, Von der Ohe PC, Bob K, Schafer RB (2017) Comparison of dilution factors for German wastewater treatment plant effluents in receiving streams to the fixed dilution factor from chemical risk assessment. Sci Total Environ 598:805–813
Manusadzianas L, Balkelyte L, Sadauskas K, Blino I, Pollumaa L, Kahru A (2003) Ecotoxicological study of Lithuanian and Estonian wastewaters: selection of the biotests, and correspondence between toxicity and chemical-based indices. Aquat Toxicol 63:27–41
Mazzitelli J-Y., 2016. Evaluation de la toxicité de molecules médicamenteuses par une étude des réponses comportementales, physiologiques et transcriptomiques d’un mollusque dulçaquicole (Radix balthica) et d’un plathelminthe (Schmidtea polychroa). Thèse de doctorat, Ecotoxicologie, Toulouse
Mazzitelli J-Y, Bonnafe E, Klopp C, Escudier F, Geret F (2017) De novo transcriptome sequencing and analysis of freshwater snail (Radix balthica) to discover genes and pathways affected by exposure to oxazepam. Ecotoxicology 26:127–140
Mendonça E, Picado A, Paixao SM, Silva L, Cunha MA, Leitao S, moura I, Cortez C, Brito F (2009) Ecotoxicity tests in the environmental analysis of wastewater treatment plants: case study in Portugal. J Hazard Mater 163:665–670
Montes-Grajales D, Fennix-Agudelo M, Miranda-Castro W (2017) Occurrence of personal care products as emerging chemicals of concern in water resources: a review. Sci Total Environ 595:601–614
Muñoz I, López-Doval JC, Ricart M, Villagrasa M, Brix R, Geiszinger A, Ginebreda A, Guasch H, de Alda MJ, Romaní AM, Sabater S, Barceló D (2009) Bridging levels of pharmaceuticals in river water with biological community structure in the Llobregat river basin (northeast Spain). Environ Toxicol Chem 2:2706–2714
Muter O, Perkons I, Selga T, Berzins A, Gudra D, Radovica-Spalvina I, Fridmanis D, Bartkevics V (2017) Removal of pharmaceuticals from municipal wastewaters at laboratory scale by treatment with activated sludge and biostimulation. Sci Total Environ 584:402–413
Nijboer RC, Verdonschot PFM, Johnson RK, Sommerhäuser M, Buffagni A (2004) Establishing reference conditions for European streams. Hydrobiologia 516:93–107
OECD (2004) OECD guideline for the testing of chemicals No. 202. Daphnia sp. Acute Immobil. Test. https://doi.org/10.1787/9789264069947-en
Ohlenbusch G, Kumke MU, Frimmel FH (2000) Sorption of phenols to dissolved organic matter investigated by solid phase microextraction. Sci Total Environ 253:63–74
Orias F, Perrodin Y (2013) Characterisation of the ecotoxicity of hospital effluents: a review. Sci Total Environ 454-455:250–276
Orias F, Perrodin Y (2014) Pharmaceuticals in hospital wastewater: their ecotoxicity and contribution to the environmental hazard of the effluent. Chemosphere 115:31–39
Pasquini L, Munoz JF, Pons MN, Yvon J, Dauchy X, France X, Dinh Le N, France-Lanord C, Gorner T (2014) Occurrence of eight household micropollutants in urban wastewater and their fate in a wastewater treatment plant. Statistical evaluation. Sci Total Environ 481:459–468
Pruvot B, Quiroz Y, Voncken A, Jeanray N, Piot A, Martial JA, Muller M (2012) A panel of biological tests reveals developmental effects of pharmaceutical pollutants on late stage zebrafish embryos. Reproductive Toxicol 34:568–583
Sanchez-Arguello P, Aparicio N, Fernandez C (2012) Linking embryo toxicity with genotoxic responses in the freshwater snail Physa acuta: single exposure to benzo(a)pyrene, fluoxetine, bisphenol A, vinclozolin and exposure to binary mixtures with benzo(a)pyrene. Ecotoxicol Environ Saf 80:152–160
Santos LHMLM, Araújo AN, Fachini A, Pena A, Delerue-Matos C, Montenegro MCB (2010) Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J Hazard Mater 175(1–3):45–95
Schirling M, Jungmann D, Ladewig V, Nagel R, Triebskorn R, Kohler HR (2005) Endocrine effects in Gammarus fossarum (Amphipoda): influence of wastewater effluents, temporal variability, and spatial aspects on natural populations. Arch Environ Contam Toxicol 49:53–61
Sherfy MH, Kirkpatrick Roy L, Richkus Kenneth D (2000) Benthos core sampling and chironomid vertical distribution: implications for assessing shorebird food avaibility. Wildl Soc Bull 18:124–130
Sousa MA, Gonçalves C, Cunha E, Hajšlová J, Alpendurada MF (2011) Cleanup strategies and advantages in the determination of several therapeutic classes of pharmaceuticals in wastewater samples by SPE-LC-MS/MS. Anal Bioanal Chem 399(2):807–822
Statzner B, Beche L (2010) Can biological invertebrate traits can resolve effects of multiple stressor on running water ecosystems? Freshw Biol 55:80–119
Ternes TA, Bonerz M, Herrmann N, Teiser B, Andersen HR (2007) Irrigation of treated wastewater in Braunschweig, Germany: an option to remove pharmaceuticals and musk fragrances. Chemosphere 66:894–904
Thomas KV, Langford K (2007) Occurrence of pharmaceuticals in the aqueous environment. Comprehensive Anal Chem 50:337–359
Togola A, Budzinski H (2007) Development of polar organic integrative samplers for analysis of pharmaceuticals in aquatic systems. Anal Chem 79:6734–6741
Togola A, Budzinski H (2008) Multi-residue analysis of pharmaceutical compounds in aqueous samples. J Chromatogr A 1177:150–158
Verlicchi P, Zambello E (2016) Predicted and measured concentrations of pharmaceuticals in hospital effluents. Examination of the strengths and weaknesses of the two approaches through the analysis of a case study. Sci Total Environ 565:82–94
Vieno N, Tuhkanen T, Kronberg L (2007) Elimination in sewage treatment plants in Finland. Water Res 41:1001–1012
Yang Y, Sik OKY, Kim K-H, Kwon E, Tsang YF (2017) Occurences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ 596-597:303–320
Yuan S, Jiang X, Xia X, Zhang H, Zheng S (2013) Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere 90:2520–2525
Zorita S, Mårtensson L, Mathiasson L (2009) Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci Total Environ 407:2760–2770
Acknowledgments
We would like to thank Maria Gonzales for English proofreading.
Funding
This work was supported by ANSES, the Adour-Garonne Water Agency, and the Midi-Pyrénées region.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Mazzitelli, JY., Budzinski, H., Cachot, J. et al. Evaluation of psychiatric hospital wastewater toxicity: what is its impact on aquatic organisms?. Environ Sci Pollut Res 25, 26090–26102 (2018). https://doi.org/10.1007/s11356-018-2501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2501-5