Abstract
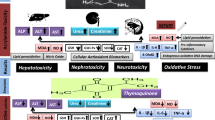
Fipronil (FPN) is a phenylpyrazole insecticide, widely used for agricultural and veterinary activities. Early reports indicated that FIP organ toxicity is primarily mediated by the induction of oxidative stress. Both thymoquinone (TQ) and diallyl sulfide (DAS) are natural antioxidants with established health benefits. This study investigated the potential ameliorative effects of DAS and TQ against FPN-induced toxicity in rats. Thirty-two male Wistar rats (150–180 g) were randomized into four treatment groups, receiving (I) saline, (II) FPN (10 mg/kg bw), (III) FPN with DAS (200 mg/kg bw), and (IV) FPN with TQ (10 mg/kg bw). All treatments were administered once daily for 28 days. The results showed that compared to the control rats, FPN-treated rats had significantly increased (p < 0.05) serum levels of uric acid, urea, creatinine, cholesterol, aspartate transferase, alanine transferase, alkaline phosphatase, lactate dehydrogenase, and γ-glutamyl transferase. Moreover, FPN significantly reduced (p < 0.05) the serum levels of total proteins, albumin, and triglycerides. In addition, compared with the control group, FPN-treated rats had significantly elevated (p < 0.05) malondialdehyde and nitric oxide levels, as well as significantly reduced glutathione concentration and activities of glutathione peroxidase, superoxide dismutase, and catalase enzymes in the hepatic, renal, and brain tissues. Cotreatment with DAS or TQ significantly ameliorated (p < 0.05) the FPN-induced alterations in all the previously mentioned parameters with more frequent restoration of normal control ranges in the TQ group. In conclusion, both DAS and TQ alleviated the oxidative injury of FPN, probably by enhancing tissue antioxidant defenses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fipronil (FPN) is a phenylpyrazole insecticide, widely used for agricultural and veterinary activities (Tingle et al. 2003). It acts by blocking the GABA-regulated chloride channels, causing central nervous system (CNS) depression and death in pests (Das et al. 2006). Toxicity may occur in humans due to inappropriate use or exceeding the dose, recommended by the manufacturer (Anadon and Gupta 2012). Several studies have reported that FPN induces oxidative stress and cellular DNA damage in rat pheochromocytoma cell culture (Lassiter et al. 2009), female rats (Leghait et al. 2009), Japanese quails (Ali et al. 2016), and tadpoles (Gripp et al. 2017). In addition, FPN disrupts the mitochondrial oxidative phosphorylation, leading to ATP exhaustion, glycolysis activation, and lactate accumulation (Vidau et al. 2011). This may later activate enzymes, involved in the apoptotic process, such as caspases 3 and 7 (Das et al. 2006).
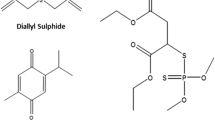
Thymoquinone (TQ) is a safe phytochemical compound (Fig. 1), extracted from the seeds of Nigella sativa L. (Darakhshan et al. 2015). Animal studies have shown the protective efficacy of TQ against xenobiotics-induced toxicity by normalizing the reduced glutathione (GSH) tissue concentrations and the activities of endogenous antioxidant enzymes (Ince et al. 2012; Ince et al. 2013). Other reports have demonstrated the hepatoprotective effects of TQ against carbon tetrachloride (Nagi et al. 1999) and cyclophosphamide (Alenzi et al. 2010). Moreover, TQ has been shown to protect the renal functions against cisplatin, ifosfamide, and mercuric chloride toxicities by minimizing the alterations in renal GSH concentration and lipid peroxidation (Badary 1999; Badary et al. 1997; Fouda et al. 2008).
Another interesting phytochemical compound is diallyl sulfide (DAS), an essential organosulfur component of garlic oil (Fig. 1) (Tamaki and Sonoki 1999) with established health benefits (Banerjee et al. 2003). Several animal studies have reported the hepatoprotective effects of DAS against aflatoxin B (Sheen et al. 2001) and thallium acetate (Abdel-Daim and Abdou 2015). Moreover, DAS has shown promising results in neuroprotection against ethanol (Huentelman et al. 1999) and ischemia-induced neuronal injuries (Lin et al. 2012). Further, it has well-documented anti-hyperlipidemic, anti-hypertensive, anti-diabetic, and anti-thrombotic activities (Jakubowski 2003; Sato and Miyata 2000).
To our knowledge, no studies have been published on the chemoprotective potency of DAS and TQ against FPN toxicity. Therefore, our objective was to investigate the antioxidant and cytoprotective effects of DAS and TQ against FPN-induced oxidative stress in a rat model.
Materials and methods
Chemicals and reagents
Fipronil (5-amino-1-(2,6-dichloro-α,α,α-triflouro-p-tolyl)-4-[(triflouromethyl)sulfinyl]pyrazole-3-carbonitrile) was purchased as a commercial product (Fipromex 20% SC) from MAC-GmbH (Sigmarszell, Germany). Thymoquinone (CAS Number 490–91-5; molecular weight 164.20 g/mol; purity ≥ 98.5%) and DAS (CAS Number 2179–57-9; molecular weight 146.27 g/mol; purity 80%) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Biochemical kits were purchased from Biodiagnostics Co. (Cairo, Egypt) except for lactate dehydrogenase (LDH) kits, which were provided by Randox Laboratories Ltd., UK. Other chemicals in this experiment were of analytical grade.
Animals
Thirty-two healthy male Wistar rats, weighing between 150 and 180 g, were housed in wire-mesh cages under controlled temperature (25 ± 2 °C) and a 12 h light/dark cycle. Rats had free access to a balanced rat chow and water and were acclimatized for 2 weeks prior to the experiment to restore normal behavior and growth. All animal investigations were performed as per the institutional and national rules for using animals in scientific research and were affirmed by the local ethics committee (Approval No. 201617).
Experimental design
Rats were divided into four groups (n = 8/group). Group (I) animals were used as a control and received oral physiological saline. Group (II) received oral FPN at a dose of 10 mg/kg bw (Badgujar et al. 2015). Groups (III and IV) received oral FPN at the same previous dose plus DAS (200 mg/kg bw) (Szutowski et al. 2002) or TQ (10 mg/kg bw) (Radad et al. 2014). All treatments were given once daily for 28 days.
Serum collection and tissue preparation
Twenty-four hours after the last FPN dose, individual blood samples were collected from rats under sodium pentobarbital anesthesia and the rats were then sacrificed by decapitation. The resulting serum samples were left to clot and were then centrifuged at 5000 rpm for 10 min and stored at − 20 °C until the biochemical parameters were assessed. Then, the liver, kidney, and brain tissues were homogenized in 10% (w/v) homogenizing buffer (0.1 M phosphate buffer, pH 7.4 + 150 mM KCl) and were later centrifuged at 9000 r/min at 4 °C for 20 min.
Lipid peroxidation and antioxidant assays
The concentration of the lipid peroxidation biomarker malondialdehyde (MDA) was assessed in the hepatic, renal, and brain tissues according to Mihara and Uchiyama (1978), while the nitric oxide (NO) concentration was measured according to Ridnour et al. (2000). We used the methods described by Beutler et al. (1963) to measure the tissue level of GSH, while the enzymatic activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured according to Nishikimi et al. (1972), Aebi (1984), and Paglia and Valentine (1967), respectively.
Serum biochemical assay
Serum liver and renal injury biomarkers were measured according to the manufacturer’s protocol. Serum alanine transferase (ALT) and aspartate transferase (AST) levels were evaluated according to Reitman and Frankel (1957), while alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (GGT) serum levels were measured according to Tietz et al. (1983) and Vázquez-Medina et al. (2011), respectively. Moreover, serum cholesterol and triglycerides were measured according to Allain et al. (1974), Richmond (1973), and Winartasaputra et al. (1980), respectively. Further, serum total proteins and albumin were evaluated following the methods of Lowry et al. (1951) and Hinton et al. (1990), respectively. In addition, serum lactate dehydrogenase (LDH) levels were determined according to Babson and Babson (1973), while serum creatinine, urea, and uric acid were measured according to Larsen (1972), Coulombe and Favreau (1963), and Whitehead et al. (1991), respectively.
Data analysis
All statistical analyses were performed using SPSS software (version 17.0 for Windows). All data were expressed as the mean ± standard deviation (SD) of the mean.We used the one-way ANOVA followed by Tukey’s multiple range tests to evaluate the significance of differences between means. When the probability value was lower than 0.05, the difference was considered statistically significant.
Results
Protective effects of DAS and TQ against FPN-induced serum biochemical alterations
Compared to the control rats, FIP-intoxicated rats showed significantly elevated (p < 0.05) serum concentrations of hepatic (ALT, AST, ALP, and GGT) and renal (urea, creatinine, and uric acid) injury biomarkers, as well as elevated serum LDH and cholesterol concentrations in comparison to control rats. Moreover, FPN induced significant reductions (p < 0.05) in serum albumin, total proteins, and triglycerides levels. Cotreatment of FPN-intoxicated rats with DAS or TQ significantly ameliorated all serum biochemical changes with more frequent restoration of normal control ranges in the TQ group (Table 1).
Antioxidant activity in the hepatic tissue
Fipronil-treated rats showed significant drops (p < 0.05) in hepatic tissue GSH concentrations (by 59.4%) and activities of GPx (by 56%), SOD (by 60.6%), and CAT (by 58%) enzymes, compared to the control rats. Concomitant treatment by DAS or TQ significantly increased GSH concentration (by 55 and 106%, respectively) and the activities of the aforementioned antioxidant enzymes (GPx by 62 and 117%, SOD by 105 and 145%, and CAT by 75 and 114%, respectively) in the liver, compared to FPN-treated rats. In addition, the hepatic tissue concentrations of MDA and NO were significantly increased (p < 0.05) following FPN treatment (by 163 and 99%, respectively), compared to control rats. These elevations were significantly ameliorated by joint treatment with DAS (MDA by 48% and NO by 39%) or TQ (MDA by 48.4% and NO by 48.3%), restoring the normal control levels (Fig. 2).
The protective effects of diallyl sulfide (200 mg/kg bw) and thymoquinone (10 mg/kg bw) against fipronil (10 mg/kg bw) on hepatic tissue malondialdehyde, nitric oxide, and antioxidant biomarkers (n = 8). MDA malondialdehyde, NO nitric oxide, GSH reduced glutathione, GPx glutathione peroxidase, SOD superoxide dismutase, CAT catalase, TQ thymoquinone, DAS diallyl sulfide. Columns (means ± SD) with different superscripts indicate significant differences (p < 0.05) between groups
Antioxidant activity in the renal tissue
In comparison to the control rats, FIP intoxication caused significant elevations (p < 0.05) in renal tissue MDA (by 93%) and NO (by 83%) concentrations, as well as significant decreases in renal tissue GSH concentration (by 47%) and activities of GPx (by 55.5%), SOD (by 53.4%), and CAT (by 62.5%) enzymes. However, treatment of FPN-intoxicated rats by DAS or TQ significantly decreased MDA (by 34.4 and 45.4%, respectively) and NO (by 36.6 and 43%, respectively) concentrations, while it significantly increased GSH concentration (by 53 and 77%, respectively) and activities of GPx (by 52 and 99%, respectively), SOD (by 60.7 and 94.6%, respectively), and CAT (by 96 and 144%, respectively) in the renal tissue, restoring the normal ranges of these parameters (Fig. 3).
The protective effects of diallyl sulfide (200 mg/kg bw) and thymoquinone (10 mg/kg bw) against fipronil (10 mg/kg bw) on renal tissue malondialdehyde, nitric oxide, and antioxidant biomarkers (n = 8). MDA malondialdehyde, NO nitric oxide, GSH reduced glutathione, GPx glutathione peroxidase, SOD superoxide dismutase, CAT catalase, TQ thymoquinone, DAS diallyl sulfide. Columns (means ± SD) with different superscripts indicate significant differences (p < 0.05) between groups
Antioxidant activity in the brain tissue
Compared to the control rats, we observed significant increases (p < 0.05) in MDA (by 104%) and NO (by 119%) concentrations, as well as significant decreases in GSH concentration (by 45%) and activities of GPx (by 47.4%), SOD (by 57%), and CAT (by 65%) enzymes in the brain tissue following FPN administration. On the other hand, treatment of FPN intoxication by DAS or TQ significantly reduced the brain tissue concentrations of MDA (by 34.7 and 49%, respectively) and NO (by 27 and 46.6%, respectively) and increased its GSH concentration (by 43 and 60%, respectively) and activities of GPx (by 46 and 76%, respectively), SOD (by 98 and 125%, respectively), and CAT (by 109 and 151%, respectively) enzymes in comparison to normal control levels (Fig. 4).
The protective effects of diallyl sulfide (200 mg/kg bw) and thymoquinone (10 mg/kg bw) against fipronil (10 mg/kg bw) on brain tissue malondialdehyde, nitric oxide, and antioxidant biomarkers (n = 8). MDA malondialdehyde, NO nitric oxide, GSH reduced glutathione, GPx glutathione peroxidase, SOD superoxide dismutase, CAT catalase, TQ thymoquinone, DAS diallyl sulfide. Columns (means ± SD) with different superscripts indicate significant differences (p < 0.05) between groups
Discussion
Our results showed that FPN treatment induced oxidative injuries in the liver, kidney, and brain tissues of male rats and promoted the intracellular lipid peroxidation (as evidenced by increased tissue MDA concentrations) and thereby disturbing the cellular membrane function in the examined tissues (Badgujar et al. 2015). Antioxidant enzymes, such as SOD, CAT, and GPx, are recognized as the first line of defense for cellular macromolecules against oxidative breakdown. In accordance with previous studies (Gill and Dumka 2016; Ki et al. 2012), FPN significantly reduced GSH concentration and the activities of antioxidant enzymes in animal tissues. The malfunction of the cellular antioxidant machinery increases the cellular sensitivity to the detrimental effects of free radicals, whose production is augmented by FPN (Mohamed et al. 2004).
The liver and kidneys play a vital role in the biotransformation of insecticides, resulting in chemically-induced hepatorenal injuries and disturbances in the oxidant-antioxidant system (Mansour and Mossa 2010). The elevations in AST, ALT, ALP, and GGT following FPN treatment in our study can be attributed to hepatocellular membrane damage and outflow of these enzymes into the blood (Goel et al. 2005). Moreover, De Oliveira and colleagues showed that FPN induces swelling and hypertrophy of hepatocytes, causing bile duct obstruction and elevation of serum ALP and GGT levels (De Oliveira et al. 2012).
The increases in serum creatinine, uric acid, and urea may be due to degradation of purines and pyrimidines (DNA breakdown) and the deterioration of renal function in excreting catabolic by-products (Hovind et al. 2009; Sun et al. 2013). In addition, we observed significant reductions in serum total proteins, albumin, and triglycerides, as well as a significant elevation in serum cholesterol levels. This may be attributed to the FPN-induced hepatic injury.
Our results revealed that DAS and TQ ameliorated the oxidative and biochemical alterations, induced by FPN. Our proposed mechanism for such effects in this study is the ability of both compounds to ameliorate lipid peroxidation and enhance the cellular antioxidant defenses. Our data are in agreement with the findings of former studies on the antioxidant/anti-inflammatory effects of DAS (Lawal and Ellis 2011; Nasr and Saleh 2014) and TQ (Fouda et al. 2008; Oguz et al. 2012; Woo et al. 2012).
The literature suggests the following antioxidant and cytoprotective mechanisms for DAS: (1) suppressing the enzymatic activity of cytochrome P450-2E1 and thereby reducing the generation of reactive oxygen species (Khanum et al. 2004) and (2) inducing the mRNA expression of the nuclear factor (erythroid-derived 2)-like 2 [Nrf2] and heme-oxygenase 1 enzyme (Gong et al. 2004). Similarly, former studies have concluded that TQ can (1) directly scavenge for superoxide (O2−) and hydroxyl radicals (OH·) (Badary et al. 2003; Kruk et al. 2000), (2) suppress mRNA expression of the inducible nitric oxide synthase (iNOS) enzyme (El-Mahmoudy et al. 2002), and (3) enhance the expression of antioxidant enzymes’ genes, such as CAT and glutathione-S-transferase in the liver (Ismail et al. 2010).
Considering the antioxidant effects of both agents in the brain tissue (shown in our study), several studies have reported the neuroprotective effects of TQ against irradiation (Ahlatci et al. 2014), toluene exposure (Kanter 2008), and allergic encephalitis (Mohamed et al. 2003). Similarly, other studies have reported that DAS can protect the neuronal cells against xenobiotics (Huentelman et al. 1999) and transient focal ischemia (Lin et al. 2012). Along with the established connection between pesticides exposure and neurodegeneration (Ahmed et al. 2017), these findings make DAS and TQ promising candidates for experimental research on neuroprotection (Al-Majed et al. 2006).
Of note, the antioxidant and cytoprotective effects against FPN were consistently higher in the TQ group than those in the DAS group (as evidenced by the more frequent restoration of normal control levels after FPN intoxication in the TQ group than in the DAS group). This may be related to the potent direct scavenging activity of TQ. Moreover, the selection of the DAS dose in this study was based on the work by Szutowski et al. (Szutowski et al. 2002); therefore, increasing the dose of DAS could achieve better results.
In conclusion, treatment with TQ or DAS ameliorated the FPN-induced cerebral and hepatorenal injuries in rats, probably through enhancing tissue antioxidant defenses. Further experimental and clinical research is needed to translate these findings into therapeutic applications.
Abbreviations
- ALP:
-
alkaline phosphatase
- ALT:
-
alanine transferase
- AST:
-
aspartate transferase
- CAT:
-
catalase
- DAS:
-
diallyl sulfide
- GGT:
-
γ-glutamyl transferase
- GPx:
-
glutathione peroxidase
- GSH:
-
reduced glutathione
- FPN:
-
fipronil
- MDA:
-
malondialdehyde
- NO:
-
nitric oxide
- SOD:
-
superoxide dismutase
- TQ:
-
thymoquinone
References
Abdel-Daim MM, Abdou RH (2015) Protective effects of diallyl sulfide and curcumin separately against thallium-induced toxicity in rats. Cell J (Yakhteh) 17:379
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahlatci A, Kuzhan A, Taysi S, Demirtas OC, Alkis HE, Tarakcioglu M, Demirci A, Caglayan D, Saricicek E, Cinar K (2014) Radiation-modifying abilities of Nigella sativa and thymoquinone on radiation-induced nitrosative stress in the brain tissue. Phytomedicine 21:740–744
Ahmed H, Abushouk AI, Gabr M, Negida A, Abdel-Daim MM (2017) Parkinson’s disease and pesticides: a meta-analysis of disease connection and genetic alterations. Biomed Pharmacother 90:638–649
Al-Majed AA, Al-Omar FA, Nagi MN (2006) Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur J Pharmacol 543:40–47
Alenzi F, El-Bolkiny YE-S, Salem M (2010) Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br J Biomed Sci 67:20–28
Ali SA, Mohamed AA-R, Ali H, Elbohi KM (2016) Sublethal effect of fipronil exposure on liver and kidney tissues with evaluation of the recovery ability of Japanese quail (Coturnix japonica). Jpn J Vet Res 64:S131–S138
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
Anadon AN, Gupta RC (2012) Fipronil. In: Gupta RC (ed) Veterinary toxicology: basic and clinical principles. Academic press/Elsevier, Amsterdam, pp 604–608
Babson SR, Babson AL (1973) An improved amylase assay using dyed amylopectin. Clin Chim Acta 44:193–197
Badary OA, Nagi MN, al-Shabanah OA, al-Sawaf HA, al-Sohaibani MO, al-Bekairi AM (1997) Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol 75:1356–1361
Badary OA (1999) Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J Ethnopharmacol 67:135–142
Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH (2003) Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol 26:87–98
Badgujar PC, Pawar NN, Chandratre GA, Telang A, Sharma A (2015) Fipronil induced oxidative stress in kidney and brain of mice: protective effect of vitamin E and vitamin C. Pestic Biochem Physiol 118:10–18
Banerjee SK, Mukherjee PK, Maulik SK (2003) Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res 17:97–106
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Coulombe JJ, Favreau L (1963) A new simple semimicro method for colorimetric determination of urea. Clin Chem 9:102–108
Darakhshan S, Bidmeshki Pour A, Hosseinzadeh Colagar A, Sisakhtnezhad S (2015) Thymoquinone and its therapeutic potentials. Pharmacol Res 95;138–58
Das PC, Cao Y, Cherrington N, Hodgson E, Rose RL (2006) Fipronil induces CYP isoforms and cytotoxicity in human hepatocytes. Chem Biol Interact 164:200–214
De Oliveira PR, Bechara GH, Denardi SE, Oliveira RJ, Mathias MI (2012) Cytotoxicity of fipronil on mice liver cells. Microsc Res Tech 75:28–35
El-Mahmoudy A, Matsuyama H, Borgan MA, Shimizu Y, El-Sayed MG, Minamoto N, Takewaki T (2002) Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Int Immunopharmacol 2:1603–1611
Fouda AM, Daba MH, Dahab GM, Sharaf El-Din OA (2008) Thymoquinone ameliorates renal oxidative damage and proliferative response induced by mercuric chloride in rats. Basic Clin Pharmacol Toxicol 103:109–118
Gill KK, Dumka VK (2016) Antioxidant status in oral subchronic toxicity of fipronil and fluoride co-exposure in buffalo calves. Toxicol Ind Health 32:251–259
Goel A, Dani V, Dhawan DK (2005) Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem Biol Interact 156:131–140
Gong P, Hu B, Cederbaum AI (2004) Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch Biochem Biophys 432:252–260
Gripp HS, Freitas JS, Almeida EA, Bisinoti MC, Moreira AB (2017) Biochemical effects of fipronil and its metabolites on lipid peroxidation and enzymatic antioxidant defense in tadpoles (Eupemphix nattereri: Leiuperidae). Ecotoxicol Environ Saf 136:173–179
Hinton RJ, Mallon BS, Morris KD, Miller J, Atkinson T, Hammond PM, Price CP (1990) Colorimetric determination of human albumin. J Clin Pathol 43:610
Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH (2009) Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 58:1668–1671
Huentelman MJ, Peters CM, Ervine WE, Polutnik SM, Johnson P (1999) Ethanol has differential effects on rat neuron and thymocyte reactive oxygen species levels and cell viability. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 124:83–89
Ince S, Kucukkurt I, Demirel HH, Turkmen R, Sever E (2012) Thymoquinone attenuates cypermethrin induced oxidative stress in Swiss albino mice. Pestic Biochem Physiol 104:229–235
Ince S, Kucukkurt I, Demirel HH, Turkmen R, Zemheri F, Akbel E (2013) The role of thymoquinone as antioxidant protection on oxidative stress induced by imidacloprid in male and female Swiss albino mice. Toxicol Environ Chem 95:318–329
Ismail M, Al-Naqeep G, Chan KW (2010) Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med 48:664–672
Jakubowski H (2003) On the health benefits of Allium sp. Nutrition 19:167–168
Kanter M (2008) Nigella sativa and derived thymoquinone prevents hippocampal neurodegeneration after chronic toluene exposure in rats. Neurochem Res 33:579–588
Khanum F, Anilakumar KR, Viswanathan KR (2004) Anticarcinogenic properties of garlic: a review. Crit Rev Food Sci Nutr 44:479–488
Ki YW, Lee JE, Park JH, Shin IC, Koh HC (2012) Reactive oxygen species and mitogen-activated protein kinase induce apoptotic death of SH-SY5Y cells in response to fipronil. Toxicol Lett 211:18–28
Kruk I, Michalska T, Lichszteld K, Kladna A, Aboul-Enein HY (2000) The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere 41:1059–1064
Larsen K (1972) Creatinine assay in the presence of protein with LKB 8600 reaction rate Analyser. Clin Chim Acta 38:475–476
Lassiter TL, MacKillop EA, Ryde IT, Seidler FJ, Slotkin TA (2009) Is fipronil safer than chlorpyrifos? Comparative developmental neurotoxicity modeled in PC12 cells. Brain Res Bull 78:313–322
Lawal AO, Ellis EM (2011) The chemopreventive effects of aged garlic extract against cadmium-induced toxicity. Environ Toxicol Pharmacol 32:266–274
Leghait J, Gayrard V, Picard-Hagen N, Camp M, Perdu E, Toutain PL, Viguie C (2009) Fipronil-induced disruption of thyroid function in rats is mediated by increased total and free thyroxine clearances concomitantly to increased activity of hepatic enzymes. Toxicology 255:38–44
Lin X, Yu S, Chen Y, Wu J, Zhao J, Zhao Y (2012) Neuroprotective effects of diallyl sulfide against transient focal cerebral ischemia via anti-apoptosis in rats. Neurol Res 34:32–37
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mansour SA, Mossa A-TH (2010) Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic Biochem Physiol 96:14–23
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Mohamed A, Shoker A, Bendjelloul F, Mare A, Alzrigh M, Benghuzzi H, Desin T (2003) Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomed Sci Instrum 39:440–445
Mohamed F, Senarathna L, Percy A, Abeyewardene M, Eaglesham G, Cheng R, Azher S, Hittarage A, Dissanayake W, Sheriff MH, Davies W, Buckley NA, Eddleston M (2004) Acute human self-poisoning with the N-phenylpyrazole insecticide fipronil--a GABAA-gated chloride channel blocker. J Toxicol Clin Toxicol 42:955–963
Nagi MN, Alam K, Badary OA, Al-Shabanah OA, Al-Sawaf HA, Al-Bekairi AM (1999) Thymoquinone protects against carbon tetrachloride hetatotoxicity in mice via an antioxidant mechanism. IUBMB Life 47:153–159
Nasr AY, Saleh HA (2014) Aged garlic extract protects against oxidative stress and renal changes in cisplatin-treated adult male rats. Cancer Cell Int 14:92
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Oguz S, Kanter M, Erboga M, Erenoglu C (2012) Protective effects of thymoquinone against cholestatic oxidative stress and hepatic damage after biliary obstruction in rats. J Mol Histol 43:151–159
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Radad K, Hassanein K, Al-Shraim M, Moldzio R, Rausch W-D (2014) Thymoquinone ameliorates lead-induced brain damage in Sprague Dawley rats. Exp Toxicol Pathol 66:13–17
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem 19:1350–1356
Ridnour LA, Sim JE, Hayward MA, Wink DA, Martin SM, Buettner GR, Spitz DR (2000) A spectrophotometric method for the direct detection and quantitation of nitric oxide, nitrite, and nitrate in cell culture media. Anal Biochem 281:223–229
Sato T, Miyata G (2000) The nutraceutical benefit, part iv: garlic. Nutrition 16:787–788
Sheen L-Y, Wu C-C, Lii C-K, Tsai S-J (2001) Effect of diallyl sulfide and diallyl disulfide, the active principles of garlic, on the aflatoxin B 1-induced DNA damage in primary rat hepatocytes. Toxicol Lett 122:45–52
Sun YM, Su Y, Li J, Wang LF (2013) Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem Biophys Res Commun 433:359–361
Szutowski MM, Zalewska K, Jadczak M, Marek M (2002) In vivo effect of diallyl sulfide and cimetidine on phenacetin metabolism and bioavailability in rat. Acta Biochim Pol 49:249–256
Tamaki T, Sonoki S (1999) Volatile sulfur compounds in human expiration after eating raw or heat-treated garlic. J Nutr Sci Vitaminol 45:213–222
Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, Shuey D, Zygowicz ER (1983) A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem 29:751–761
Tingle CC, Rother JA, Dewhurst CF, Lauer S, King WJ (2003) Fipronil: environmental fate, ecotoxicology, and human health concerns. Rev Environ Contam Toxicol 176:1–66
Vázquez-Medina JP, Zenteno-Savín T, Forman HJ, Crocker DE, Ortiz RM (2011) Prolonged fasting increases glutathione biosynthesis in postweaned northern elephant seals. J Exp Biol 214:1294–1299
Vidau C, Gonzalez-Polo RA, Niso-Santano M, Gomez-Sanchez R, Bravo-San Pedro JM, Pizarro-Estrella E, Blasco R, Brunet JL, Belzunces LP, Fuentes JM (2011) Fipronil is a powerful uncoupler of oxidative phosphorylation that triggers apoptosis in human neuronal cell line SHSY5Y. Neurotoxicology 32:935–943
Whitehead TP, Bevan EA, Miano L, Leonardi A (1991) Defects in diagnostic kits for determination of urate in serum. Clin Chem 37:879–881
Winartasaputra H, Mallet VN, Kuan SS, Guilbault GG (1980) Fluorometric and colorimetric enzymic determination of triglycerides (triacylglycerols) in serum. Clin Chem 26:613–617
Woo CC, Kumar AP, Sethi G, Tan KH (2012) Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol 83:443–451
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Abdel-Daim, M.M., Shaheen, H.M., Abushouk, A.I. et al. Thymoquinone and diallyl sulfide protect against fipronil-induced oxidative injury in rats. Environ Sci Pollut Res 25, 23909–23916 (2018). https://doi.org/10.1007/s11356-018-2386-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2386-3