Abstract
Chromic oxide nanoparticles (Cr2O3 NPs) are widely used in commercial factories and can cause serious environmental problems. However, the mechanism behind Cr2O3 NP-induced phytotoxicity remains unknown. In this study, the effects of Cr2O3 NPs on the growth, chlorophyll fluorescence, SEM-EDS analysis, and chloroplast ultrastructure of soybean (Glycine max) were investigated to evaluate its phytotoxicity. The growth of soybean treated with various Cr2O3 NP suspensions (0.01, 0.05, 0.1, and 0.5 g L−1) was significantly inhibited. Specially, shoot and root biomass decreased by 9.9 and 46.3%, respectively. Besides, the maximum quantum yield of PSII (Fv/Fm) as well as the photochemical quenching (qP) decreased by 8–22 and 30–37%, respectively, indicating that the photosynthetic system was damaged when treated with Cr2O3 NPs. Moreover, the inhibition was confirmed by the reduction of Rubisco and MDH enzyme activity (by 54.5–86.4 and 26.7–96.5%, respectively). Overall, results indicated that the damage was caused by the destruction of chloroplast thylakoid structure, which subsequently reduced the photosynthetic rate. Our research suggests that Cr2O3 NPs can be transported and cause irreversible damage to soybean plants by inhibiting the activity of electron acceptors (NADP+) and destroying ultrastructure of chloroplasts, providing insights into plant toxicity issues.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal oxide nanoparticles (NPs) have smaller size (particles with at least one dimension of less than 100 nm) and larger surface area, which lead to the unique physico-chemical patterns and high reactivity. NPs have recently been the focus of intense research because of its wide application in industrial, agricultural, and household use (Meshram et al. 2012; Zhu et al. 2004). Previous studies have proved that NPs can enter the environment via waste water from industrial sites or through domestic sewage (Hernandez-Viezcas et al. 2013; Rico et al. 2011). Besides, NPs can be transported to soil via sewage sludge, then ultimately be absorbed by plants (Petersen et al. 2014), raising concerns to environmental safety and threat to plants (Hawthorne et al. 2014), living organisms, and even humans as a function of direct or indirect exposure (Rickerby and Morrison 2007; Ma et al. 2010).
The potential toxicity and bioaccumulation of NPs motivate the investigation of their fate and transportation in the uptake system. Plants are first produced in the ecosystem and may therefore easily act as intermediaries for the transfer of NPs to the organism (Hawthorne et al. 2014). It is reported that NPs can be species specific absorbed by various plants (Zhang et al. 2011; Wang et al. 2013; Ebbs et al. 2016a, b; Cui et al. 2014) and allocated to the roots, stems, and leaves (Harris and Bali 2008; Parsons et al. 2010; Navarro et al. 2008). Evidence showed that CeO2 cannot be absorbed into maize through root resorption (Birbaum et al. 2010), but ZnO and CeO2 can be accumulated into both shoot and root of soybean and corn. Besides, the accumulation of ZnO was higher than CeO2 (Zhu et al. 2004; Priester et al. 2012; Zhao et al. 2012). Also, the cumulative amount of Ag NPs in soybean plant exposed to Ag NPs was significantly lower than those in the plants exposed to CeO2 NPs and ZnO NPs (De La Torre-Roche et al. 2013). Therefore, it is necessary to verify the uptake of different NPs in the plants.
Some studies argue that the varying degrees of toxicity responding to nanoparticles is related to the migration process inside the plants. CuO NPs can be transported to the shoots through xylem sap and translocated back to roots trough phloem-based transport in maize (Wang et al. 2012), which is similar to Au NPs in woody poplar (Zhai et al. 2014), expressing a root-shoot-root distribution loop and providing evidence for the bioaccumulation and detoxification of NPs in plants. The migration process of NPs in plants may also be accompanied by ion release, change of redox state, combined with increased reactive oxygen species, thus resulting in irreversible damage to gene expression, causing root swelling and inhibition of root elongation (Wang et al. 2016; Ebbs et al. 2016a, b). In addition to morphology, changes in the valence of nanoparticles can be found in plants. The redox state of a small number of Cu were found to be changed from Cu(II) to Cu(I) in maize (Wang et al. 2012), and the redox state of a small part of Ce NPs were found to be reduced from Ce(IV) to Ce(III) in soybean (Hernandez-Viezcas et al. 2013); metal oxidation process is often accompanied with changes in plant organic metabolism, pH, and reactive oxygen species. CuO NPs were reported to raise reactive oxygen species (ROS) in rice (Shaw and Hossain 2013). In Arabidopsis thaliana, CuO NPs can cause the generation and accumulation of ROS in the chloroplasts, affecting the electron transfer, causing gene damage (Wang et al. 2016); Ag NPs were reported to induce the up-regulated expression of ROS-associated genes (Kohan-Baghkheirati and Geisler-Lee 2015).
However, most of the studies focused on the toxicity and crop yield reduction induced by NPs, only a few of them focused on the effects of nanoparticles on plant photosynthetic system. A life circle experiment on cucumber pointed that NPs showed no effect on chlorophyll and gas exchange (Zhao et al. 2013), while CuO NPs were reported to cause damage to Arabidopsis thaliana chloroplasts, inhibiting the electron transfer during the photosynthetic process (Wang et al. 2016). Besides, the chlorophyll content was reported to be significantly reduced in wheat once exposed to ZnO NPs and CuO NPs (Dimkpa et al. 2012) but not significantly changed in soybean plants exposed to CeO2 and ZnO (Zhao et al. 2013). Ag NPs only inhibited chlorophyll b content in rice (Mirzajani et al. 2013). In addition, TiO2, ZnO, and Au NPs were reported to enhance the chlorophyll content in some edible plants (Ma et al. 2015). To date, many studies only investigated the chlorophyll content and ROS damage, while only a small number of them have studied the changes in plant morphology and even less were focused on the ultrastructural changes. Photosynthesis-related organelles can be found by observing the subcellular structure of plants. Chlorophyll fluorescence imaging can visually show the damage of leaf photosynthesis system, including electron transportation, opening degree of light system, as well as dark reaction rate. These two methods could provide an insight into the way that nanoparticles affect the photosynthesis of different plants.
Cr2O3 is versatile as it is an excellent green dye material and stainless steel raw material (Farinati et al. 2011) and are used for industrial applications such as catalysts and pigments (Ondřej et al. 2014). In recent years, due to increasing technological requirements, the use of Cr2O3 NP inputs was rising, especially in magnetic materials, refractories, and environmental catalysis (Park et al. 2016), but little attention has been paid to the environmental risks that Cr2O3 NPs could pose to plants and even humans. The transportation form and the toxicity of Cr2O3 NPs could pose to the growth, and photosynthesis of plants still remains uncertain. Soybean plants, as one of the largest farm species around the world, were chosen because of its popularity as food and also economical crops, which have direct connections with human life. To get a comprehensive understanding of the direct effects of nanoparticles on plants, SEM/TEM and CF Imager were conducted in the present study. Experiments were performed and differences in Cr2O3 NP intensity were confirmed by various analyses. This study aimed to (1) confirm that Cr2O3 NPs can be absorbed and translocated by soybean plants; (2) measure the activity of photosynthetic enzymes and observe the substructure damage of chloroplasts with the addition of Cr2O3 NPs; (3) collect and analyze the photosynthetic data along with the chlorophyll fluorescence image to reveal the electron transport and the damage of dark reaction. The findings in this work will help to gain insight into the negative effects induced by Cr2O3 NPs and provide evidence to assess the risk.
Materials and methods
Cr2O3 nanoparticle suspension characterization
Cr2O3 NPs were purchased from Jianglai Biology Ltd., Shanghai, China. The morphology of the Cr2O3 NPs was examined by transmission electron microscopy, operated at 200 kV, and the characteristics of Cr2O3 NPs (0.05 μm) are shown in Fig. S1. Cr2O3 NPs of different concentrations were added to nutrient solution and agitated by ultrasonic vibration (100 W, 40 kHz) for 30 min to increase dispersion. The stability of NPs in the nutrient solution dispersion system was determined by Zeta potential.
Germination experiment
Soybean seeds were sterilized by 0.7% NaClO solution for 10 min, and then rinsed with deionized water for three times to ensure that the surface was clean. Then the seeds were immersed in Cr2O3 NP-water with different concentrations of suspension (CK, 0.01, 0.05, 0.1, and 0.5 g L−1), and subsequently placed in the dark at 25 °C, making sure that the seeds maintained humidity. After germination (3d), the germination rate was calculated.
Plant culture
Soybean seeds were rinsed with deionized water for three times after sterilizing by 0.7% NaClO solution for 10 min. Then the seeds were immersed in deionized water for germination, and subsequently placed in the dark at 25 °C, making sure the seeds maintained humidity. After 3 days, buds whose lengths surpassed half of the seed length were chosen and then placed to the nutrient solution with the addition of Cr2O3 NPs and cultured under natural conditions.
Uniform seedlings were selected and moved to plastic containers amended with strength of the following nutrient solution (mmol L−1): Ca(NO3)2, 2.0; KH2PO4, 0.1; MgSO4, 0.5; KCl, 0.1; K2SO4, 0.7; H3BO3,10 × 10−3; MnSO4, 5 × 10−4; ZnSO4, 5 × 10−4; CuSO4, 2 × 10−4; (NH4)6MoO24, 1 × 10−4; and Fe-EDTA, 20 × 10−3. Cr2O3 NPs were added to the nutrient solution, and five experimental treatment groups were initiated (g L−1): 0.01, 0.05, 0.1, 0.5, and control (CK). The pH of the nutrient solution was adjusted to 6.0. Each experiment had four replicates. The seedlings grew for 14 days under natural conditions: at 25–30/20–25 °C (day/night) with relative humidity at 60–70% and a light intensity at 15,000 lx. The suspensions with NPs were renewed every 3 days. The exposure time was 14 days; shoot and root tissue were washed by ultrasonic vibration (output frequency 53 kHz, power 500 W, SK20GT, Ishine, China) to remove the NPs on the surface, subsequently separated and dried by Bal-Tec CPD 030 a critical point dryer (Leica, Wetzlar, Germany) at 65 °C for 72 h. Fresh biomass of shoots and roots of tissues from all treatments were measured, and Cr content in plant tissues were analyzed by ICP-MS (Agilent 7500a, USA) after digestion with HNO3-HClO4.
SEM-EDS and TEM observation
SEM-EDS was performed to observe the transport of nanoparticles in plants and their effects on plant morphology. TEM was used to analyze the structure change of chloroplast, thus revealing the reasons for inhibition of photosynthetic system.
Fresh soybean root and shoot tissues were separated, and the shoots were placed in 4 °C refrigerator to avoid light overnight for dark recovery. The samples were first fixed with 2.5% glutaraldehyde in phosphate buffer (0.1 M, pH 7.0) for more than 4 h, washed three times in the phosphate buffer (0.1 M, pH 7.0) for 15 min at each step, then post fixed with 1% OsO4 in phosphate buffer for 1–2 h and washed three times in the phosphate buffer (0.1 M, pH 7.0) for 15 min at each step. The samples were prepared for dehydration: the samples were first dehydrated by graded series of ethanol (30, 50, 70, 80, 90, 95, and 100%) for about 15 to 20 min at each step.
For SEM scanning, the samples were transferred to the mixture of alcohol and iso-amyl acetate (v:v = 1:1) for about 30 min, and then transferred to pure iso-amyl acetate for about 1 h. In the end, the samples were dehydrated in Hitachi Model HCP-2 critical point dryer with liquid CO2. The dehydrated samples were coated with gold-palladium in Hitachi Model E-1010 ion sputter for 4–5 min and observed in Hitachi Model TEM-1000 SEM and INCA100 EDS (Oxfordshire, UK).
For TEM scanning, the specimen was placed in 1:1 mixture of absolute acetone and the final Spurr resin mixture for 1 h at room temperature, then transferred to 1:3 mixture of absolute acetone and the final resin mixture for 3 h and lastly to the final Spurr resin mixture for overnight. The specimen was placed in Eppendorf contained Spurr resin and heated at 70 °C for more than 9 h. The specimen was sectioned in LEICA EM UC7 ultratome and sections were stained by uranyl acetate and alkaline lead citrate for 5 to 10 min, respectively, and observed in Hitachi Model H-7650 TEM.
Chlorophyll fluorescence measurement
After being cultured in complete nutrient solution with Cr2O3 NPs, the samples were picked and ready to be used to evaluate the effect of Cr2O3 NPs on PSII electron transport. Soybean leaves were placed in the dark for 30 min for dark adaptation, CF Imager (CF0056, TNC, America) was used to evaluate the dark reaction efficiency. Readings of dark-adapted minimum fluorescence (Fo), dark-adapted maximum fluorescence (Fm), variable fluorescence Fv (Fv = Fm-Fo) were recorded. Fo′ and Fm′ were measured by CF Imager after 30 min of light intensity of 1000 μmol (m2 s)−1. Data of potential capture efficiencies Fv/Fm (XE), non-photochemical quenching Fm/Fm′-1 (NPQ) and the capture rate of excitation energy of PSII reaction center Fv′/Fm (XE′), photochemical quenching coefficients Fq′/Fv′ (qP) and Fq′/Fm′ (φPSII) were recorded. State was ready for monitoring in leaves in vivo with CF Imager. All the leaves were measured under the same condition.
Photosynthesis rate, intercellular CO2 concentration, and stomatal conductance were measured by using the LI-6400/LI-6400XT Portable Photosynthesis System (LI-6400XT; Li-Cor Environmental, Lincoln, NE, USA).
Activity of the photosynthetic enzyme (MDH, Rubisco) and determination of chlorophyll content
This experiment adopted the tissue kit (Suzhou Comin Biotechnology Co. Ltd) and followed the protocol provided to measure the activity of Rubisco (Rubisco, EC 4.1.1.39) in soybean leaves.
Malate dehydrogenase (MDH) tissue kit was from Nanjing Jiancheng Bioengineering Institute, and the activity of MDH was examined according to the protocol provided (Lv et al. 2013).
Fresh soybean leaves were collected and washed for sampling. A mass of 0.1 g crushed leaves were put into 25 mL colorimetric tube and 15 mL of extract (acetone:ethanol = 1:1) were added. After 24-h of dark soaking, the leaves turned to be transparent. The extract was used as blank control. Chlorophyll was examined by spectrophotometer (721–100) at the wavelength of 649 and 665 nm.
Statistical analysis
Data were analyzed statistically using the SPSS package (version 11.0; SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was performed on the datasets. Means of significant difference were separated by t test or Duncan’s multiple range test at the p < 0.05 level.
Results
NP characterization
The characterization of Cr2O3 NPs was determined by Zeta potential and TEM. The stability of NP suspensions was increasing with the growing concentrations as shown in Table S1. Ion release of the Cr2O3 NP-nutrient solution was under 0.02 mg kg−1 before plant culture, and it was stable around 0.033 mg kg−1 at harvest. The NP aggregate morphology is shown in Fig. S1a, the size of which was around 500 nm, while the original size of Cr2O3 NPs was around 30–50 nm (Fig. S1b), and the original size of which meets the experimental requirements.
Seed germination, growth inhibition and metal accumulation
Seed germination and plant growth experiment were conducted. Cr2O3 NPs were not influential to soybean seed germination (p < 0.05) (Fig. S2).
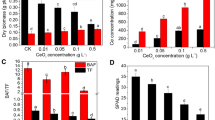
Plant growth was inhibited significantly when exposed to Cr2O3 NPs, and leaf malformation was observed when Cr2O3 NP level ≥ 0.1 g L−1 (Fig. 1a). Comparing with control treatment, shoot fresh weights at Cr2O3 NP treatments (0.01, 0.05, 0.1, and 0.5 g L−1) were decreased by 9.6, 20.9, 33, and 40.1%, respectively, and the corresponding values for root fresh weight were 39.1, 37.1, 42.6, and 46.4%, respectively. Plant morphology changes were visible. At high concentrations (0.1 and 0.5 g L−1), the soybean foliage became slender and more wrinkles appeared. Root growth was inhibited as well. Under the high concentration of nanoparticle solution, the roots were swollen with root elongation inhibited, and the number of lateral roots was less than that of the control group.
Effect of Cr2O3 NPs on plant growth (a), biomass (b), and Cr contents in shoot and root (c). Soybean plants were treated with different Cr2O3 NP levels. The image was taken on the 14th day of treatment, and biomass and Cr concentration were analyzed. Data are means ± SD (n = 4). Bars with different letters are significantly different at p < 0.05
The Cr content in shoots and roots of soybean increased along with the Cr2O3 NPs level (Fig. 1c). Cr content of the roots was significantly higher than that of the shoots. In the low concentration fraction, there was little difference in the uptake of metals in the shoots, but when the concentration reached 0.1 g L−1, the metal content of the shoots was quite different. At 0.5 g L−1, the Cr content was much higher than the content of 0.01 g L−1. And the root Cr concentration also increased with the increase of the concentration of the nanoparticle dispersion.
Xylem transport of Cr2O3 NPs and root morphology changes
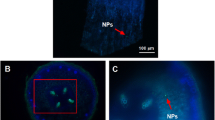
Scanning electron microscopy along with EDS was conducted to determine the root and stem growth of the group exposed to 0.5 g L−1 Cr2O3 NPs. The root surface of the control group was texture clear, and the lateral roots and root hairs could be seen under the SEM view. The cortical, endocortical, and xylem ducts were found from the section view of the stem of the soybean (Fig. 2a), and the root size was normal. As for the high Cr2O3 NPs exposure group, a large number of NPs can be seen under high power lens (Fig. 3d). These nanoparticles were gathered and remained on the surface, the size of which were hundred and thousand times of their original size. The roots and lateral roots of the experimental group were swollen (Fig. 3), the surface of which were covered by Cr2O3 NPs, and the root hair can hardly be seen at high magnification. The stem section diagram of the high exposure group is shown in Fig. 2b, in which the xylem catheters were attached by a large number of particles (Fig. 2c, d); the massive particles adhered to the xylem catheter and the phloem catheter were proved to be Cr2O3 NPs by EDS.
Chlorophyll fluorescence
After 14 days of growth in the nutrient solutions containing five different Cr2O3 NPs concentrations, the chlorophyll fluorescence parameters of soybean leaves were measured (Fig. 4). When the leaf was illuminated, the darker part of the image provided evidence for leaf damage in the Fq′/Fm′, reflecting an inhibition of both photochemical and non-photochemical PSII processes (Fig. 4b); the data reflected a downward trend except for the 0.1-g L−1 group. After 30 min dark recovery (Fig. 4a), the maximum quantum yield of the controlled group of PSII (Fv/Fm) was 0.854, while the test group results were all lower than it (p value < 0.05.), which indicated that non-photochemical quenching has reversed and decreased during the dark period with the growing concentration of NPs. As compared with CK, the qP decreased by 30, 30, 32, and 37%, respectively. The NPQ were increased gradually, which indicated the heat dissipation of leaves increased with the rise of the NP concentrations.
Effect of different Cr2O3 NP-level treatment on the maximum quantum yield of PSII (Fv/Fm), photochemical quenching (qP), and non-photochemical quenching (NPQ) of intact leaves. The images were taken by CF Imager on the 14th day of treatment. a Leaf damage evaluation mapping based on potential energy capture efficiency of the reaction center after dark recovery for 30 min. The specific color band from red to dark blue stated the leaf damage from slight to severe. b Leaf damage evaluation mapping based on non-photochemical quenching NPQ under illumination. The specific color band from light green to red stated the leaf damage from slight to severe. c Leaf damage evaluation mapping based on photosynthesis quenching coefficiency qP. The specific color band from red to light green stated the efficiency from high to low. Data are means ± SD (n = 3). Data with different letters are significantly different at p < 0.05
Photosynthetic parameters and activity photosynthetic enzymes (MDH and Rubisco)
Intercellular CO2 concentration (Fig. 5) decreased gradually from 335 to 268 μmol CO2 mol−1, and the reduction rate was 6.9, 9.4, 15.8, and 19.7%, respectively, indicating the available CO2 was lowered due to the stronger respiration (NPQ, Fig. 4). As shown in Fig. 5, leaf conductance to H2O decreased (except the stomatal conductance of group 0.01 g L−1), and this result was closely related to photosynthetic rate, which showed a downslope similarly (Fig. S2), ranging from the control group 11.46 μmol CO2 m−2 s−1 to the experimental group 9.59, 9.41, 8.73, and 7.68 μmol CO2 m−2 s−1, respectively. Strong reduced activity of both MDH and Rubisco were observed in soybean groups grown in the presence of NPs compared with the control treatment. As shown in Fig. 5b, MDH activity decrease began from 4.62 to 3.39 U mg−1 prot−1, then reduced by 77.4, 90.8, and 96.5% (1.04, 0.42, and 0.16 U mg−1 prot−1, respectively). This downtrend became slow when the NP concentration reached 0.05 g L−1, meanwhile Rubisco activity (Fig. 5a) similarly showed a rapid decline with the addition of NPs (CK, 95.3 nmol min−1 g−1; reduction rate, 54.5, 72.7, 81.8, and 86.4%, respectively) and then kept closely to 10 nmol min−1 g−1. The effect that NPs posed to photosynthetic enzymes was debased after the NP concentration rose to a certain degree.
Effect of Cr2O3 NPs on rubisco activity (a), MDH activity (b), intercellular CO2 concentration (c), and stomatal conductance(d) of soybean leaves. Soybean plants were treated with different Cr2O3 NP levels. Data are means ± SD (n = 4). Bars with different letters are significantly different at p < 0.05
Photosynthetic pigments and TEM observation
Soybean grown in Cr2O3 NPs had a significant reduction in chlorophylls a and b compared with those grown in the nutrient solution (Table 1). With the increase of the concentration, the chlorophyll content of plants decreased and the growth of plants became weaker, with shorter root elongation and relatively smaller and narrower leaves (Fig. 1c), providing evidence that Cr2O3 NPs had negative effect on the photosynthesis of soybean leaves.
Transmission electron microscopy was used to analyze the ultrastructure structures of the high-concentration group (Cr2O3 NPs, 0.5 g L−1). As for the control group (Fig. 6a), the cells observed under transmission electron microscope were complete, the grana stacking in the chloroplasts were clear as well, and starch granules were evenly distributed between the grana. As for the high Cr2O3 NP concentration exposure group (Fig. 6b), the vacuole in the cell was relatively larger than the control group. The grana stacking was broken and relatively more starch granules can be observed. Under the TEM, one special cell was found in which the chloroplast particles were completely broken and the chloroplasts were completely encapsulated by the starch granules (Fig. 6e).
Effect of Cr2O3 NPs on chloroplast ultrastructure of leave cells of soybean treated with or without Cr2O3 NPs. The TEM images were taken on the 14th day of treatment. a, b Control; c, d treated by 500 mg L−1 Cr2O3 NPs. e, f chloroplast of treated group full of starch grains and destroyed grana. Red narrow-pointed starch grains. Blue narrow-pointed thylakiod membrane
Discussion
In recent years, several investigators have shown impact of nanoparticles on plant growth and their accumulation in food source (Anshu et al. 2017), but few studies addressing the toxic potential of these NPs have been reported in the literature (Puerari et al. 2016). The problems caused by industrial wastewater discharge have been constant issues under the present circumstances. Once the crops were contaminated by industrial wastewater with large quantity of high NPs (such as Cr2O3) in a short term, our research will provide insights for the research on the hazards of Cr2O3 NP pollution.
Cr2O3 NPs were found to adhere and aggregate in soybean plants (Figs. 2 and 3), and this is a direct evidence that Cr2O3 NPs can be absorbed and transported by soybean plants, which also indicated the potential problems caused by NPs. Also, soybean phloem catheter a small amount of nanoparticles, indicating that nanoparticles can be transferred down through the phloem (Fig. 2c), which is consistent with the previous report (Ma et al. 2015). The cross-section showed that NPs are gathered in the xylem and phloem in the stem, indicating an up-and-down transfer inside the plants. This mechanism may contribute to protection of plants from heavy metal toxicity. To determine the uptake and accumulation of NPs by plants that show consistency with previous studies (Ma et al. 2015), we examined photosynthetic and morphology changes that NPs posed to plants as well.
Soybean plant growth were inhibited under the exposure of Cr2O3 NPs
Consistently with previous research (Ma et al. 2015), seed germination was not sensitive to Cr2O3 NPs under all levels in this study. This may have related to the seed coat, which has selective permeability and can protect the embryo from external toxicity (Dimkpa et al. 2012). However, the influence on mature plants is different after being exposed to Cr2O3 NPs for 14 days of treatment. According to ICP-MS data, the uptake of Cr2O3 NPs in roots showed a positive correlation with the concentration, which indicate that the root elongation of soybean is affected by the content of Cr2O3 NPs absorbed by plants. Besides, roots growth showed an inhibition in early growth stage at a low level of NPs, and tissue morphology changes were visible. As for the roots, our study found that the roots were shorter and swelled with less lateral roots. Liu et al. (2010) found that NPs can induce swollen roots by affecting isotropic growth of epidermal cells, auxin disruption, and aberrant microtubule arrangement as well as cell division, causing root morphology changes. On the other hand, the uptake of Cr2O3 NPs in shoots was only significant at high concentrations. In agreement with root morphology changes, leaf areas were shrunk with the growing concentration of Cr2O3 NPs. The estimation of total leaf areas indicated that the plant health was affected by the water stress (Gutiérrez-Boem and Thomas 2001) and the metal exposure (Weryszko-Chmielewska and Chwil 2005). Previous research supported our findings that nanoparticles can be gathered into a group in the surface of plant, clogging root transport channels of water and ion, inhibiting root hydraulic conductivity and nutrient absorption (Adani et al. 2010). These findings are consistent with our observations that NPs can directly lead to the shorter root elongation and the shrunken leaf area of the plant.
However, the reason for the decrease of plant biomass and the narrower shape of leaves with increasing concentration has not been fully understood yet and needs further study.
Photosynthetic damage of soybean plants exposed to Cr2O3 NPs based on chlorophyll fluorescence
Chlorophyll fluorescence analysis has become one of the most powerful technique to estimate the photochemical activities of PSII in leaves and the operating quantum efficiency of electron transport through PSII (Baker and Rosenqvist 2004), which can provide insight into intra-cellular photosynthetic responses to abiotic stress (Seaton and Walker 1990).
Consistently with Perreault et al. (2010), NPs induced photosynthetic decrease was remarkable according to the photosynthetic parameters taken by a CF imager. The maximum quantum yield of PSII (Fv/Fm) can reflect a dissociation of light harvesting pigment systems of PSII from PSII core (Murchie and Lawson 2013). According to Fig. 4a, b, the dark response of the plant was inhibited and the light system was also reduced with the addition of Cr2O3 NPs and showed a reduction along with the concentration, which was similar to CuO NPs induced by photosynthetic inhibition (Perreault et al. 2010). The process of NPQ is regulated by the acidification of the thylakoid lumen due to the accumulation of protons in the thylakoid lumen (during linear and cyclic electron flow) that forms a ΔpH (Ruban et al. 2012). The outcome of both qP and NPQ suggested that the respiration intensity was promoted while the access to the photochemical center was suppressed (Oxborough and Baker 1997). Stomatal conductance and intercellular CO2 were measured to confirm the hypothesis. In conclusion, plant respiration increased along with the concentration of Cr2O3 NPs, which indicated a consumption of plant energy and a reduction of CO2 content, thereby affecting the photosynthesis.
Results showed that Cr2O3 NPs can inhibit the ability of plant to produce chlorophylls a and b, thus the photosynthetic procedure could be reduced by the lack of raw materials (Anderson and Boardman 1966). Aside from the chlorophyll content, plant respiration, and the capacity for photochemistry, electron transportation, on the other hand, also plays an essential role in the photosynthetic system. PSII quantum efficiency (Fq′/Fm′) theoretically measured the proportion of the light absorbed by photosystem II that is used in photochemistry, and it could indicate the mass level of electron acceptors (NADP+) at the acceptor side of PSI (Murchie and Lawson 2013). According to chlorophyll fluorescence mapping, we can infer that electron transportation was inhibited in photosynthesis, which was connected with the existence of Cr2O3 NPs, but no concentration-dependent relation was being showed. The interruption of energy transfer can be determined by the activity of the enzyme. Rubisco and Matate dehydrogenase are related to the abundance of photosynthesis-related proteins (Murchie and Lawson 2013). Ribulose-1,5-bisphosphate carboxylase/oxygenase is a key enzyme in the plant photosynthetic procedure (Lin et al. 2014), controlling the fixing ability of CO2 fixation, meanwhile, restricting and splitting carbon to the Calvin cycle and photorespiration cycle (Buchanan and Balmer 2005), the activity of which directly influences the rate of photosynthesis. In photorespiration, MDH provides NAD+ for oxidation of Gly (glycine) (Hatch et al. 1982; Moreadith and Lehninger 1984; Gietl 1992). According to Fig. 5a, b, the activity of the enzymes confirmed that Cr2O3 NPs could inhibit the electron transportation during the optoelectronic circulation of PSI, causing damage to the photosynthetic system, thus reducing the photosynthesis rate of soybean plants. Since the enzyme activity of photosynthetic system is affected, chloroplasts of soybean plants may also be affected or even destroyed (Redondo-Gómez et al. 2010), thus we took chloroplasts into further consideration.
Chloroplasts were observed under TEM. Starch grains are produced during the photoreaction stage to store the energy, and they will be delivered to dark reaction for the Calvin cycle (Slack et al. 1969). Thus, the amount of starch granules can be used to assess the circulation of the cycle system. From Fig. 6, the exposure group showed more starch grains and less grana, and a deformed chloroplast was observed under TEM, whose structure was totally destroyed, suggesting the damage of the structure of chloroplast was induced by Cr2O3 NPs. Based on the previous data of Rubisco and MDH activity and TEM observation, the conversion process from C5 to C3 of Calvin cycle was influenced with the inhibition electron transportation.
It can be said that once Cr2O3 NP-contaminated water are absorbed by plants, the plants will show different degrees of inhibition on growth and photosynthesis at different concentrations. The growth and morphology changes happened mainly through the blockage of plant nutrient absorption channel as well as by reducing the enzyme activity which to the reduce and even suspend the energy conversion during Calvin cycle, so that the starch granules cannot be decomposed in the chloroplast thus destroying the chloroplast ultrastructure, resulting in reduced photosynthetic efficiency.
Conclusion
The findings of this research revealed that Cr2O3 NPs can be absorbed by soybean plants through xylem, causing root swelling and affecting the growth of plants by (1) inhibiting the activity of photosynthetic enzymes and causing a low conversion efficiency of C5 to C3 in Calvin cycle; (2) increasing the starch grains and breaking the thylakoid membrane, consequently destructing the substructure of chloroplasts of soybean plants; (3) blocking the level of electron acceptors (NADP+) at the acceptor side of PSI transport during photosynthetic process, reducing the formation and activity of chlorophyll, and posing irreversible damage to plants. However, a further study of Cr2O3 NPs and its fate in soil is needed to understand the behavioral differences between the changes in the external environment. The information obtained from this continuation will help to understand the fate and transport of Cr2O3 NPs and assess the potential food safety risks associated with the consumption of soybean or other edible plants grown in the presence of Cr2O3 nanoparticle-contaminated industrial wastewater.
References
Adani F, Papa G, Schievano A, Cardinale G, D’Imporzano G, Tambone F (2010) Nanoscale structure of the cell wall protecting cellulose from enzyme attack. Environ Sci Technol 45(3):1107–1113
Anderson JM, Boardman N (1966) Fractionation of the photochemical systems of photosynthesis I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Biochim Biophys Acta 112(3):403–421
Anshu R, Marek Z, Oksana S, Kalaji HM, He X, Sonia M et al (2017) Impact of metal and metal oxide nanoparticles on plant: a critical review. Front Chem 5:78
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55(403):1607–1621
Birbaum K, Brogioli R, Schellenberg M, Martinoia E, Stark WJ, Günther D, Limbach LK (2010) No evidence for cerium dioxide nanoparticle translocation in maize plants. Environ Sci Technol 44(22):8718–8723
Buchanan BB, Balmer Y (2005) Redox regulation: a broadening horizon. Annu Rev Plant Biol 56:187–220
Cui D, Zhang P, Ma Y, He X, Li Y, Zhang J, Zhang Z (2014) Effect of cerium oxide nanoparticles on asparagus lettuce cultured in an agar medium. Environ Sci Nano 1(5):459–465
De La Torre-Roche R, Hawthorne J, Musante C, Xing B, Newman LA, Ma X, White JC (2013) Impact of Ag nanoparticle exposure on p,p′-DDE bioaccumulation by Cucurbita pepo (zucchini) and Glycine max (soybean). Environ Sci Technol 47(2):718–725
Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, Anderson AJ (2012) CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14(9):1–15
Ebbs SD, Bradfield SJ, Kumar P, White JC, Ma X (2016a) Projected dietary intake of zinc, copper, and cerium from consumption of carrot (Daucus carota) exposed to metal oxide nanoparticles or metal ions. Front Plant Sci 7
Ebbs SD, Bradfield SJ, Kumar P, White JC, Musante C, Ma X (2016b) Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ Sci Nano 3(1):114–126
Farinati S, DalCorso G, Panigati M, Furini A (2011) Interaction between selected bacterial strains and Arabidopsis halleri modulates shoot proteome and cadmium and zinc accumulation. J Exp Bot 62(10):3433–3447
Gietl C (1992) Malate dehydrogenase isoenzymes: cellular locations and role in the flow of metabolites between the cytoplasm and cell organelles. BBA-Biomembranes 1100(3):217–234
Gutiérrez-Boem FH, Thomas GW (2001) Leaf area development in soybean as affected by phosphorus nutrition and water deficit. J Plant Nutr 24(11):1711–1729
Harris AT, Bali R (2008) On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopart Res 10(4):691–695
Hatch MD, Tsuzuki M, Edwards GE (1982) Determination of NAD malic enzyme in leaves of C4 plants effect of malate dehydrogenase and other factors. Plant Physiol 69(2):483–491
Hawthorne J, De la Torre RR, Xing B, Newman LA, Ma X, Majumdar S, Gardea-Torresdey J, White JC (2014) Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain. Environ Sci Technol 48:13102–13109
Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Gardea-Torresdey JL (2013) In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 7(2):1415–1423
Kohan-Baghkheirati E, Geisler-Lee J (2015) Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nano 5(2):436–467
Lin MT, Occhialini A, Andralojc PJ, Parry MA, Hanson MR (2014) A faster Rubisco with potential to increase photosynthesis in crops. Nature 513(7519):547–550
Liu Q, Zhao Y, Wan Y, Zheng J, Zhang X, Wang C, Lin J (2010) Study of the inhibitory effect of water-soluble fullerenes on plant growth at the cellular level. ACS Nano 4(10):5743–5748
Lv Y, Liu Z, Tian Y, Chen H (2013) Effect on morphology, oxidative stress and energy metabolism enzymes in the testes of mice after a 13-week oral administration of melamine and cyanuric acid combination. Regul Toxicol Pharmacol 65(2):183–188
Ma X, Geiser-Lee J, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408(16):3053–3061
Ma C, White JC, Dhankher OP, Xing B (2015) Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol 49(12):7109–7122
Meshram SP, Adhyapak PV, Mulik UP, Amalnerkar DP (2012) Facile synthesis of CuO nanomorphs and their morphology dependent sunlight driven photocatalytic properties. Chem Eng J 204:158–168
Mirzajani F, Askari H, Hamzelou S, Farzaneh M, Ghassempour A (2013) Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol Environ Saf 88:48–54
Moreadith RA, Lehninger AL (1984) The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem 259(10):6215–6221
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64(13):3983–3998
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao JA, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17(5):372–386
Ondřej J, Sedmidubský D, Sofer Z, Luxa J, Bartůněk V (2014) Simple synthesis of Cr2O3, nanoparticles with a tunable particle size. Ceram Int 41(3):4644–4650
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components–calculation of qP and Fv-/Fm-; without measuring Fo. Photosynth Res 54(2):135–142
Park S, Kheel H, Sun GJ, Kim HW, Ko T, Lee C (2016) Room-temperature hydrogen gas sensing properties of the networked Cr2. Met Mater Int 22(4):730–736
Parsons JG, Lopez ML, Gonzalez CM, Peralta-Videa JRJL (2010) Toxicity and biotransformation of uncoated and coated nickel hydroxide nanoparticles on mesquite plants. Environ Toxicol Chem 29(5):1146–1154
Perreault F, Oukarroum A, Pirastru L, Sirois L, Gerson Matias W, Popovic R (2010) Evaluation of copper oxide nanoparticles toxicity using chlorophyll fluorescence imaging in Lemna gibba. Aust J Bot
Petersen EJ, Henry TB, Zhao J, MacCuspie RI, Kirschling TL, Dobrovolskaia MA, HackleycV XB, White JC (2014) Identification and avoidance of potential artifacts and misinterpretations in nanomaterial ecotoxicity measurements. Environ Sci Technol 48:4226–4246
Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Schimel JP (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. PNAS 109(37):E2451–E2456
Puerari RC, Costa CHD, Vicentini DS, Fuzinatto CF, Melegari SP, Schmidt ÉC et al (2016) Synthesis, characterization and toxicological evaluation of Cr2O3, nanoparticles using Daphnia magna, and Aliivibrio fischeri. Ecotoxicol Environ Saf 128(June 2016):36–43
Redondo-Gómez S, Mateos-Naranjo E, Moreno FJ (2010) Physiological characterization of photosynthesis, chloroplast ultrastructure, and nutrient content in bracts and rosette leaves from glaucium flavum. Photosynthetica 48(4):488–493
Rickerby DG, Morrison M (2007) Nanotechnology and the environment: a European perspective. Sci Technol Adv Mater 8(1):19–24
Rico CM, Majumdar S, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL (2011) Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem 59(8):3485–3498
Ruban AV, Johnson MP, Duffy CD (2012) The photoprotective molecular switch in the photosystem II antenna. BBA-Bioenergetics 1817(1):167–181
Seaton GGR, Walker DA (1990) Chlorophyll fluorescence as a measure of photosynthetic carbon assimilation. Proc R Soc B Biol Sci 242(242):29–35
Shaw AK, Hossain Z (2013) Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 93(6):906–915
Slack CR, Hatch MD, Goodchild DJ (1969) Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J 114(3):489–498
Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B (2012) Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol 46(8):4434–4441
Wang P, Menzies NW, Lombi E, McKenna BA, Johannessen B, Glover CJ, Kopittke PM (2013) Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ Sci Technol 47(23):13822–13830
Wang Z, Xu L, Zhao J, Wang X, White JC (2016) Xing B (2016) CuO nanoparticle interaction with Arabidopsis thaliana: toxicity, parent-progeny transfer, and gene expression. Environ Sci Technol 50(11):6008–6016
Weryszko-Chmielewska E, Chwil M (2005) Lead-induced histological and ultrastructural changes in the leaves of soybean (Glycine max (L.)). Merr Soil Sci Plant Nutr 51(2):203–212
Zhai G, Walters KS, Peate DW, Alvarez PJ, Schnoor JL (2014) Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ Sci Technol lett 1(2):146–151
Zhang Z, He X, Zhang H, Ma Y, Zhang P, Ding Y, Zhao Y (2011) Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 3(8):816–822
Zhao L, Peralta-Videa JR, Ren M, Varela-Ramirez A, Li C, Hernandez-Viezcas JA, Gardea-Torresdey JL (2012) Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: electron microprobe and confocal microscopy studies. Chem Eng J 184:1–8
Zhao L, Sun Y, Hernandez-Viezcas JA, Servin AD, Hong J, Niu G, Gardea-Torresdey JL (2013) Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: a life cycle study. J Agric Food Chem 61(49):11945–11951
Zhu J, Li D, Chen H, Yang X, Lu L, Wang X (2004) Highly dispersed CuO nanoparticles prepared by a novel quick-precipitation method. Mater Lett 58(26):3324–3327
Acknowledgements
The authors are grateful to Ms. Si Li of the technician of 985-Institute of Agrobiology and Environmental Sciences of Zhejiang University for her contribution in carrying through the in situ hybridization.
Funding
This study was financially supported by the National Natural Science Foundation of China (No. 41271333, No. 21477104, No. 41671315) and National Key Research and Development Projects of China (No. 2016YFD0800802).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yi-ping Chen
Electronic supplementary material
ESM 1
(DOCX 2167 kb)
Rights and permissions
About this article
Cite this article
Li, J., Song, Y., Wu, K. et al. Effects of Cr2O3 nanoparticles on the chlorophyll fluorescence and chloroplast ultrastructure of soybean (Glycine max). Environ Sci Pollut Res 25, 19446–19457 (2018). https://doi.org/10.1007/s11356-018-2132-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2132-x