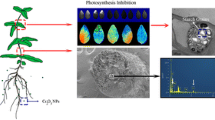
Abstract
Cerium oxide nanoparticles (CeO2 NPs) are widely used in industries and have caused environmental problems. However, the phytotoxicity induced by CeO2 NPs lacks detailed information on phytotoxicity. In this research, the effect of CeO2 NPs on soybean plants (Glycine max) was studied. Scanning electron microscopy with the energy dispersion spectroscopy was used to characterize the NPs form in soybean. The growth of the root was increased, whereas the growth of shoot was inhibited. Besides, Chlorophyll Fluorescence Imager (CF Imager) showed that chlorophyll synthesis was inhibited: the maximum quantum yield of Photosystem II complex (PSII) (Fv/Fm) and photochemical quenching (qP) decreased. Moreover, transmission electron microscopy revealed that the chloroplast thylakoid structure was changed, and thus reduced the energy conversion in the Calvin cycle from C5 to C3. Our work suggests that CeO2 NPs will cause growth changes as well as irreversible damage to soybean plants. Our findings will provide evidence for estimation of plant toxicity induced by CeO2 NPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cerium oxide nanoparticles (CeO2 NPs) are one of the rare earth metal oxide nanoparticles. The use of CeO2 NPs in commercial factories has increased dramatically during the last two decades due to its unique physical–chemical properties (Zhang et al. 2019). The vast use of NPs has brought problems in the environment because of the unregulated release from industrial products (Sendra et al. 2017). The estimated environmental concentrations of CeO2 were 0.001–1000 mg kg−1 (Holden et al. 2014; Roche et al. 2018). CeO2 NPs have strong stability and low solubility in the environment, which enables them to maintain the nanoparticle character and produce toxic effect on plants and human (Tanino et al. 2019). They can be easily transported from the environment to plants (Hawthorne et al. 2014), and then ultimately be transferred to human (Dekani et al. 2019). Toxicology study of NPs on plants has thus become important in recent years.
Most of the research are only focused on the crop yield and growth effect induced by nanoparticles (Hernandez-Viezcas et al. 2013). For example, CuO NPs are found to inhibit the metabolic activity and plant growth under respiring conditions (Kim et al. 2017); TiO2 and CeO2 NPs show different effects on early plant growth of agronomic species (Andersen et al. 2016). Research shows that plant growth and fruit yield of tomato plants were enhanced under low concentrations of CeO2 NPs (≤ 10 mg L−1), while soybean shows a decreased result (Priester et al. 2012). The growth promotion/inhibition induced by NPs exposure are species specific and dose dependent (Yang et al. 2017).
Further research has been working on revealing the mechanism of phototoxicy induced by NPs. However, limited research has been focused on the photosynthetic system of the plants. A relatively poor data on the photosynthesis are mainly descriptive and are based on routine procedures of photosynthesis researches; about photosynthetic pigments, pulse-amplitude modulation (PAM) and gas-exchange studies (Rajput et al. 2019). Some studies show that chlorophyll content was not affected by the addition of CeO2 NPs and ZnO NPs (Wang et al. 2016), while some other studies suggest that the chlorophyll content could be promoted or decreased when being exposed to metal based NPs (Ma et al. 2015). Moreover, studies find that reactive oxygen species (ROS) in chloroplast is one of the reasons that inducing the reduction of chlorophyll content in the plants (Wang et al. 2016) and even cause the upregulated expression of ROS-associated genes (Kohan-Baghkheirati and Geisler-Lee 2015). To date, however, the mechanism of NPs induced photosynthetic problem still needs to be systematically studied. More information needs to be documented in NPs induced plant phototoxicity.
In this study, soybean seeds was cultured and grown in hydroponic solution containing different concentrations of CeO2 NP. Experiments were designed and performed to gain a comprehensive understanding of the effects of CeO2 NPs on plants. The germination rate and plant growth were evaluated. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to observe the structure and subcellular structure of plants. CF Imager were applied in this study to gain the photosynthetic data, including electron transport, opening degree of light system, as well as dark reaction rate. Our study aims to (1) confirm CeO2NPs can be taken up and translocated by soybean plants; (2) analyze the photosynthetic data with the chlorophyll fluorescence image to reveal the electron transport and the damage of dark reaction. This study aims to know the mechanism of photosynthetic damage induced by CeO2 NPs. The findings in this work will help to gain insight into the negative effects induced by CeO2 NPs and provide evidence to assess the environmental risk of NPs.
Materials and Methods
Soybean seeds were prepared and cultured as shown in supplemental document 1. The germination rate was calculated. Soybean plants were prepared and hydroponic cultured as shown in supplemental document 2.
CeO2 NPs (< 25 nm) were purchased from Sigma-Aldrich, USA. According to Yang et al. (2015) and our previous research (Li et al. 2020), CeO2 NPs in the nutrient solution were negligible. Transmission electron microscopy (TEM) (H-7650, Hitachi, Japan) showed the morphology of CeO2 NPs, operated at 200 kV. The image is shown in Fig. S1. Hydroponic nutrient solution with different CeO2 NPs concentrations (0.01, 0.05, 0.1, 0.5, g L−1, respectively) was agitated by ultrasonic vibration (output frequency 53 kHz, power 500 W, SK20GT, Ishine, China) for 30 min to increase dispersion. Metal concentrations in the nutrient solution were measured after mixed with the triacid mix (HNO3:HCIO3:HF = 5:1:1). Zeta potential was used to determine the stability of NPs. The results are shown in Table S1.
Fresh biomass of shoots and roots of tissues from all treatments were measured and digested by HNO3–H2O2 (v/v = 5:1) (Roche et al. 2018). Metal content was determined by ICP-MS (7500a, Agilent, USA).
Scanning electron microscope (SEM) (SU8010, Hitachi, Japan) with energy dispersive spectroscopy (INCA100, Oxfordshire, U.K.) was performed to localize the transport of nanoparticles in plants and their effects on plant morphology. TEM was used to analyze the structure change of chloroplast, thus revealing the reasons for inhibition of photosynthetic system. The sample preparations were shown in supplemental document 3.
After the chlorophyll content was recorded with a chlorophyll meter (SPAD-502; Minolta, Japan), the leaves were kept in dark for CF Imager measurement.
Samples were harvested. Well-shaped blades were chosen from the second batch and ready to test the effect of CeO2 NPs on PSII electron transport. Soybean leaves were placed in the dark for 30 min for dark adaptation to evaluate the dark-adapted minimum fluorescence (Fo), dark-adapted maximum fluorescence (Fm), variable fluorescence Fv (Fv = Fm–Fo). Fo′ and Fm′ were measured after 30 min of light intensity1000 μmol/(m2 s) for the potential capture efficiencies of Fv/Fm (XE), non-photochemical quenching Fm/Fm′−1 (NPQ) and the capture rate of excitation energy of PSII reaction center Fv′/Fm (XE′), photochemical quenching coefficients Fq′/Fv′ (qP) and φPSII (Fq′/Fm′). The leaves were ready to be monitored in vivo with CF Imager (CF0056, TNC, America). All the leaves were measured under the same condition.
Data were analyzed statistically using the SPSS package (version 11.0; SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was performed on the datasets. Normality (Shapiro–Wilk test) and homogeneity (Leven’s test) were applied. In this dataset, ANOVA can be used as evident from the test of homogeneity and normality (Naz et al. 2018). We used the Tukey HSD pot-doc test to perform multiple comparisons to compare whether there were significant differences between different concentrations.
Results and Discussion
The measured metal content in the nutrient solution were (0.009, 0.048, 0.097, 0.491 g L−1, respectively). According to Ebbs et al. (2016) and our previous research (Li et al. 2020), the dissolution of CeO2 NPs is negligible. After the seeds were cultured in the dark environment for three days, we found that the germination rate of the experimental soybean seeds was the same as that of the control group (Fig. S2.), indicating that the nanoparticles did not affect the germination of the soybean seeds. Then soybean seed were cultured in nutrient solution with different concentrations of CeO2 NPs. After a 14d exposure, the plant biomass decreased with the increasing content of CeO2 NPs in the solution. Significant decrease in shoot weight was found with the addition of CeO2 NPs (Figs. 1a, S3). Compared with control group (CK) (0 g L−1 CeO2 NPs), shoot weight of the NPs exposed groups (0.01, 0.05,0.1 and 0.5, g L−1) decreased by 11.84%, 20.58%, 29.12% and 55.29% respectively, but interestingly, CeO2 NPs were observed to promote root elongation of the soybean plants. Root weight was promoted and the corresponding values for root weight remarkably increased by 9.56%, 20.99%, 33.34%, 40.12%, respectively.
Effect of CeO2 NPs on biomass (a). Ce metal contents in shoot and root (b). Bioaccumulation factor (BAFs) and translocation factor (TFs) for Ce of soybean plants (c). Chlorophyll content of soybean leaves after 14 days exposure of CeO2 NPs (d). All plants were treated with different CeO2 NPs levels. The means were averaged from 3 replicates of every experimental group. There are significant difference among different treatments (p < 0.05).The image was taken on the 14th day of treatment, and biomass and Ce concentration were analyzed
The Ce contents in shoots and roots of soybean raised with the increasing concentration of CeO2 NPs level (Fig. 1b). Much more CeO2NPs were found in roots than in shoots, indicating that the metal absorbance of soybean plant roots is larger than that of shoots. As for the roots, the content of Ce increased with the growing concentrations of NPs from 389.63 to 1302.27 mg kg−1. As for the shoots, the content of Ce increased from 79.78 to 404.83 mg kg−1. Notably, when the concentration reached 0.1 g L−1, the absorption of the roots is stabilized at about 410 mg kg−1, illustrating that the root absorption of CeO2 NPs may reach saturation after 0.1 g L−1. Bioaccumulation factors (BAFs = root metal content/metal content in hydroponic nutrient solution) and translocation factors (TFs = aerial tissue metal content/root metal content) for Ce were calculated to quantify the translocation via roots and shoots (Fig. 1c) (Chua, 1998; Chowdhury and Maiti 2016). Evidence showed that CeO2 NPs can be accumulated and translocated from roots to shoots. TFs values in shoots consistently trended downward for all exposure treatments (TF = 0.57–0.27).
The bioaccumulation and translocation were further observed by SEM. The internal structure of the soybean stem can be clearly seen from the cross-sectional and vertical-sectional view of the stem, and large number of nanoparticles are distributed in the ducts of the stem. The volume of the attached nanoparticles is about thousand times the volume of the original nanoparticles. The stem section diagram was shown in Fig. 2a, in which the xylem catheters were attached by a large number of particles (Fig. 2b). The mass particles adhering to the xylem catheter and the vascular bundles had attached clusters of particles as shown in the report of EDS. Results above indicates that CeO2 NPs can be transported and accumulated in plants.
According to the biomass shown in Fig. 1a, soybean growth in this research has a tendency to decrease with increasing CeO2 NPs concentration, indicating that the concentration of nanoparticles is closely related to the growth of plants, and the ability of the aboveground and underground parts in transferring nanoparticles were different. Furthermore, it is ensured by the patterns observed (Fig. 2) and the data of metal accumulation (Fig. 2b) that CeO2 NPs can be taken up and transported by soybean plants in the form of nanoparticles. The accumulation of CeO2 NPs in the aboveground rose slowly after 0.05 g L−1, while the underground quarter maintained a steady growth, and this might be due to weakened absorption efficiency of metals by roots. Some reports have shown that NPs or metal ions separated from NPs penetrated into the roots and might then precipitate or accumulate in the root cell wall network, limiting further transport and accumulation (López-Moreno et al. 2010). Some studies also have reported that NPs can only be found immobilized in the surface of the root epidermal cell wall, instead of entering the root axis, thus translocation of the aboveground of the plants is limited (Wild and Jones 2009).
In addition to the metal content, the effect on the morphological change of plants were investigated. Deng et al. (2016) showed that increasing NPs concentrations affect onion roots on the length, morphology, structure and metabolic activity. Priester et al. (2012) suggested that CeO2 NPs can lead to the decrease of the nitrogen fixation of soybean thus inhibit the growth and pod biomass. Similarly, the growth of the soybean in this study decreased either. Besides, it is determined that the transport of CeO2 NPs was accompanied by the change of valence state (Née Röhder et al. 2018). The redox state of Ce NPs are found partially reduced from Ce(IV) to Ce(III) in soybean (Hernandez-Viezcas et al. 2013). Plants are oxidatively stressed as the valence of the Ce changes, thus causing damage to plant growth. In addition, the shape of the leaf under higher concentration has shown greater change than the lower treatment group, the leaf of which was narrower and showed a weakened growth trend according to the biomass. The estimate of total leaf area indicates the plant health is affected by water stress (Gutiérrez-Boem and Thomas 2001) and metal exposure (Weryszko-Chmielewska and Chwil 2005). We assume that CeO2 NPs can block plant water transport channels, raise oxidative stress and decrease the nitrogen fixation of soybean plant, and thus cause negative effect on plant, but more studies are needed to support this statement.
Interestingly, unlike other nanoparticles, CeO2 NPs can inhibit the growth of the aboveground while promote the root elongation. In consistence with López-Moreno et al. (2010), the root growth of soybean, like cucumber and corn, was promoted. Previous results have shown that dissolution of the NPs resulted in raising the levels of metal ion that modified IAA distribution, causing root morphology changes and raising NO cell signaling to regulate root proliferation (Adams et al. 2017). In order to determine the ultrastructure of the root cells, TEM was conducted. As shown in the figure, NPs cluster were found in the vacuoles. Importantly, the cell walls were found thicker than that of CK. In consistency, a previous study showed that NPs can promote OH radicals in root cells, causing cell wall loosening, promoting the root elongation (Kim et al. 2014).
After 14 days growth, the new batch of soybean leaves were changed with a SPAD reading from 31.5 to 17.4. By comparison, leaves of soybean plants grown in the control group had SPAD reading at 35 (Fig. 1d), indicating that CeO2 NPs treatment significantly reduced the chlorophyll synthesis of soybean plants. Moreover, at low concentrations, the content of chlorophyll in the leaves began to change, and as the concentration increased, the content of chlorophyll in the leaves decreased significantly, suggesting the inhibition of chlorophyll was more pronounced in plants exposed to higher concentrations of nanoparticles.
Chlorophyll fluorescence analysis is one of the reliable techniques in photochemical activities estimation and electron transport through PSII (Baker and Rosenqvist 2004), providing insight into intra-cellular photosynthetic responses to abiotic stress (Redondo-Gómez et al. 2010). The reduction in chlorophyll may affect the efficiency of the photosynthetic system, thereby reducing the photosynthetic rate. Therefore, Chlorophyll Fluorescence Imager is used to characterize soybean leaves. The chlorophyll fluorescence parameters of soybean leaves were collected after 14 days of growth. According to the results, the leaves of the treated group with the highest CeO2 NPs (0.5 g L−1) showed a narrower shape. When illuminated, φPSII showed an inhibition of both photochemical and non-photochemical PSII processes, the image of which reflected a concentration free downward trend. The maximum quantum yield of the control group of PSII (Fv/Fm) was measured after 30 min dark recovery (Fig. 3a). The maximum quantum yield of the control group of PSII (Fv/Fm) was 0.854 ± 0.011, while the test group results were decreased by 8.7–19.6%, which indicated that with the growing concentration of CeO2 NPs, the non-photochemical quenching had reversed and decreased during the dark period. Besides, φPSII was decreased by 64.8–71.9%, and the photochemical quenching (qP) declined significantly with the addition of CeO2 NPs, those were 24.24%, 25.76%, 27.28%, 31.82%, respectively. The non-photochemical quenching (NPQ), indicating the strength of heat dissipation of the leaves, were enhanced by 28% and 44.52% when added with CeO2 NPs, and results grew slower when the concentration reached 0.1 g L−1, which are 66.11% and 69.92%. Chlorophyll content of soybean leaves was as measured by a hand-held chlorophyll meter (Fig. 1d.), the chlorophyll content was linearly dropped by 50.33% from CK to group 0.5 g L−1.
In consistence with Perreault et al. (2010), NPs induced photosynthetic decrease is remarkable based on the measurement of photosynthetic parameters. Light harvesting pigment systems of PSII from PSII core is reflected by the maximum quantum yield of PSII (Fv/Fm). The dark response and light system of the soybean plants were damaged with the addition of CeO2 NPs and also showed a concentration-dependent reduction level. The level of non-radiative energy dissipation in the light-harvesting antenna of photosystem II and plant respiration can be assessed by NPQ and qP. Data shows the degree of openness of the plant was linearly increased, while the degree of openness of the light system decreased linearly. This suggests that plant energy consumption is greater than production in the process of photosynthesis, thereby inhibiting the trend of plant leaf growth.
Apart from the energy consumption, plant capacity for photochemistry and electron transportation is also essential to the photosynthetic system. We use PS II quantum efficiency φPSII (Fq′/Fm′) to measure the proportion of the light absorbed by photosystem II in photochemistry, and it states the level of electron acceptors (NADP+) at the acceptor side of PS I (Murchie and Lawson 2013). According to chlorophyll fluorescence mapping, electron transport is inhibited in photosynthesis, which was related to the addition of CeO2 NPs, and showed a concentration-dependent relation. As the electron transfer efficiency was affected, we speculate that the structure of the chloroplast will change or even be damaged (Redondo-Gómez et al. 2010). Besides, previous research pointed out that NPs are potentially responsible for conformational changes in polysaccharides, lipids, proteins, pectin, suberin and lignin molecules (Missaoui et al. 2018), thus the substructure of the soybean plant were observed.
Transmission electron microscopy was used to analyze the subcellular structures of the high concentration group (CeO2 NPs 0.5 g L−1). As for CK (Fig. 4a), the structure of the cells observed under transmission electron microscope were intact. The grana stacking in the chloroplasts were clearly shown and starch granules were evenly distributed between the granas. As for the high CeO2NPs concentration exposure group (Fig. 4c), the vacuole in the cell was relatively larger than the control group. The stacking of grana was broken and relatively more starch granules can be observed in the view. The ultrastructure of the roots from the treated group (Fig. 4f) was subsequentially different from the control group (Fig. 4e). The cell wall of the control group was clear and the cell structure was intact. Compared with the control group, the treatment group of the cell wall edge was thinner, the vacuoles were suspected to contain agglomerated Ce nanoparticles. Starch granules are the intermediate products of effective energy conversion in chloroplast. Starch grains are produced during the photoreaction stage to keep the energy in store and will be delivered to dark reaction for the Calvin cycle (Slack et al. 1969), thus the amount of starch granule is a key that can assess the circulation of the cycle system. As it is shown in Fig. 4. each cell of the exposed group contained more chloroplasts with more starch grains and less grana. It is possible that the conversion process from C5 to C3 of Calvin cycle is affected by the inhibition of electron transport, leading to the stagnation of starch grains and energy transmission interruption. Moreover, CeO2 NPs negatively impacts the production of chlorophyll pigments with the increasing concentrations of CeO2 NPs and thus reducing the raw materials for photosynthesis. Combined with the information mentioned above, results illustrate that CeO2 NPs could inhibit the electron transport during the optoelectronic circulation of PS II, causing damage to the photosynthetic system, thus reducing the photosynthetic rate of soybean plants.
The addition of CeO2 NPs decreased the quantum yield of PSII electron transport thus caused damage to the chloroplasts of soybean leaves and reduced the photosynthetic process. This process is accompanied by promoted respiration process and the reduced energy conversion of soybean plants. Furthermore, results indicate that the addition of CeO2 NPs could result in the decrease of photosynthesis of soybean plants due to lack of raw material, inhibition of electron transport and structural damage of chloroplast.
The result shows that soybean plants could take up CeO2 NPs through xylem, causing negative effect on plant growth by (1) inhibiting conversion efficiency of C5 to C3 in the Calvin cycle, and the photosynthesis is inhibited. (2) increasing starch granules, alter thylakoid membrane, and destroy the structure of chloroplast of soybean plants; (3) blocking the level of electron acceptor during photosynthesis and reducing chlorophyll formation and activity, and irreversibly damaging to the plant. CeO2 NPs can promote root elongation by triggering cell wall loosening. However, the direct cause of short root elongation and leaf deformation caused by CeO2 NPs has not yet been clarified. Further study is needed to reveal how CeO2 NPs decrease the nitrification process and even cause gene damage of plants. The information obtained from the continuation will help to understand the environmental behavior and the fate of CeO2 NPs, in order to provide insights into the assessment of the potential food safety associated with other edible plants grown in the presence of CeO2 NPs contaminated environment.
References
Adams J, Wright M, Wagner H, Valiente J, Britt D, Anderson A (2017) Cu from dissolution of CuO nanoparticles signals changes in root morphology. Plant Physiol Biochem 110:108–117
Andersen CP, King G, Plocher M, Storm M, Pokhrel LR, Johnson MG et al (2016) Germination and early plant development of ten plant species exposed to TiO2 and CeO2 nanoparticles. Environ Toxicol Chem 35(9):2223–2229
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55(403):1607–1621
Chowdhury A, Maiti SK (2016) Identification of metal tolerant plant species in mangrove ecosystem by using community study and multivariate analysis: a case study from Indian Sunderban. Environ Earth Sci 75:744. https://doi.org/10.1007/s12665-016-5391-1
Chua H (1998) Bio-accumulation of environmental residues of rare earth elements in aquatic flora Eichhornia crassipes (Mart.) Solms in Guangdong Province of China. Sci Total Environ 214(1–3):79–85
Dekani L, Johari SA, Joo HS (2019) Comparative toxicity of organic, inorganic and nanoparticulate zinc following dietary exposure to common carp (Cyprinus carpio). Sci Total Environ 656:1191–1198
Deng F, Wang S, Xin H (2016) Toxicity of CuO nanoparticles to structure and metabolic activity of Allium cepa root tips. Bull Environ Contam Toxicol 97(5):702–708
Ebbs SD, Bradfield SJ, Kumar P, White JC, Musante C, Ma X (2016) Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ Sci 3(1):114–126
Gutiérrez-Boem FH, Thomas GW (2001) Leaf area development in soybean as affected by phosphorus nutrition and water deficit. J Plant Nutr 24(11):1711–1729
Hawthorne J, De la Torre Roche R, Xing B, Newman LA, Ma X, Majumdar S, White JC (2014) Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain. Environ Sci Technol 48(22):13102–13109
Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Gardea-Torresdey JL (2013) In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 7(2):1415–1423
Holden PA, Klaessig F, Turco RF, Priester JH, Rico CM, Avila-Arias H, Gardea-Torresdey JL (2014) Evaluation of exposure concentrations used in assessing manufactured nanomaterial environmental hazards: are they relevant? Environ Sci Technol 48(18):10541–10551
Kim JH, Lee Y, Kim EJ, Gu S, Sohn EJ, Seo YS, Chang YS (2014) Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environ Sci Technol 48(6):3477–3485
Kim S, Samanta P, Yoo J, Kim WK, Jung J (2017) Time-dependent toxicity responses in Daphnia magna exposed to CuO and ZnO nanoparticles. Bull Environ Contam Toxicol 98(4):502–507
Kohan-Baghkheirati E, Geisler-Lee J (2015) Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 5(2):436–467
Li J, Song Y, Vogt RD, Liu Y, Luo J, Li T (2020) Bioavailability and cytotoxicity of Cerium-(IV), Copper-(II), and Zinc oxide nanoparticles to human intestinal and liver cells through food. Sci Total Environ 702:134700
López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL (2010) X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem 58(6):3689–3693
Ma C, White JC, Dhankher OP, Xing B (2015) Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol 49(12):7109–7122
Missaoui T, Smiri M, Chemingui H, Jbira E, Hafiane A (2018) Regulation of mitochondrial and cytosol antioxidant systems of Fenugreek (Trigonella foenum graecum L.) exposed to nanosized titanium dioxide. Bull Environ Contam Toxicol 101(3):326–337
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64(13):3983–3998
Naz A, Chowdhury A, Mishra BK, Karthikeyan K (2018) Distribution of heavy metals and associated human health risk in mine, agricultural and roadside soils at the largest chromite mine of India. Environ Geochem Health 40(5):2155–2175
Née Röhder LAK, Brandt T, Sigg L, Behra R (2018) Uptake and effects of cerium (III) and cerium oxide nanoparticles to Chlamydomonas reinhardtii. Aqua Toxicol 197:41–46
Perreault F, Oukarroum A, Pirastru L, Sirois L, Matias WG, Popovic R (2010) Evaluation of copper oxide nanoparticles toxicity using chlorophyll a fluorescence imaging in lemna gibba. J Bot 2010(4):807–826
Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Schimel JP (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. PNAS 109(37):E2451–E2456
Rajput VD, Minkina T, Sushkova S, Mandzhieva S, Fedorenko A, Lysenko V, Chaplygin V (2019) Structural and ultrastructural changes in nanoparticle exposed plants. In: Nanoscience for sustainable agriculture. Springer, Cham, pp 281–295
Redondo-Gómez S, Mateos-Naranjo E, Moreno FJ (2010) Physiological characterization of photosynthesis, chloroplast ultrastructure, and nutrient content in bracts and rosette leaves from glaucium flavum. Photosynthetica 48(4):488–493
Roche RDLT, Pagano L, Majumdar S, Eitzer BD, Zuverza-Mena N, Ma C, White JC (2018) Co-exposure of imidacloprid and nanoparticle Ag or CeO2 to Cucurbita pepo (zucchini): contaminant bioaccumulation and translocation. NanoImpact 11:136–145
Sendra M, Yeste PM, Moreno-Garrido I, Gatica JM, Blasco J (2017) CeO2 NPs, toxic or protective to phytoplankton? Charge of nanoparticles and cell wall as factors which cause changes in cell complexity. Sci Total Environ 590:304–315
Slack CR, Hatch MD, Goodchild DJ (1969) Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J 114(3):489–498
Tanino R, Amano Y, Tong X, Sun R, Umemoto J, Kobayashi M, Hotta T (2019). Therapeutic efficacy of zinc oxide nanoparticles against small cell lung cancer in an Orthotopic Xenograft Model. In: B68. Oncogenic mutations, MET
Wang Z, Xu L, Zhao J, Wang X, White JC, Xing B (2016) CuO Nanoparticle interaction with Arabidopsis thaliana: toxicity, parent-progeny transfer, and gene expression. Environ Sci Technol 50(11):6008–6016
Weryszko-Chmielewska E, Chwil M (2005) Lead-induced histological and ultrastructural changes in the leaves of soybean (Glycine max (L.) Merr.). Soil Sci Plant Nutr 51(2):203–212
Wild E, Jones KC (2009) Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environ Sci Technol 43(14):5290–5294
Yang Z, Chen J, Dou R, Gao X, Mao C, Wang L (2015) Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int J Environ Res Public Health 12(12):15100–15109
Yang X, Pan H, Wang P, Zhao FJ (2017) Particle-specific toxicity and bioavailability of cerium oxide (CeO2) nanoparticles to Arabidopsis thaliana. J Hazard Mater 322:292–300
Zhang W, Huang Y, Gong H, Dang F, Zhou D (2019) Different uptake of metal dioxide nanoparticles (ceria nanoparticles, zirconia nanoparticles and silica nanoparticles) by Wheat. Bull Environ Contam Toxicol 1–7.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (#41271333, #21477104, #41671315).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Mu, Q., Du, Y. et al. Growth and Photosynthetic Inhibition of Cerium Oxide Nanoparticles on Soybean (Glycine max). Bull Environ Contam Toxicol 105, 119–126 (2020). https://doi.org/10.1007/s00128-020-02892-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02892-z