Abstract
The present study aimed to investigate the protective effect of aqueous extracts of ginger (GE) and rosemary (RE), both individually and in combination, on carbon tetrachloride (CCl4)-induced liver injury in adult male rats. CCl4 induced significant increase in liver enzymes, bilirubin, triglycerides, and total cholesterol while total protein, albumin, and globulin were significantly decreased. Also, the activity of cytochrome P450 (CYP) and oxidative stress markers were found to be elevated with a concomitant decrease in the activity of antioxidant enzymes in hepatic tissue. Supplementation with extracts of ginger or rosemary effectively relieved most of the CCl4-induced alterations when administered singly. The joint therapy of the two extracts was more effective. The histological investigation strongly confirmed the highly protective effect of the two plant extracts in the hepatocytes. These findings suggest that rosemary and ginger extracts are effective in improving both the function and structure of the hepatocytes through their potent antioxidant effect and point out to the possibility of using a combination of both as an adjunct therapy in liver diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon tetrachloride (CCl4) is a well-recognized hepatotoxin that is routinely used to induce rapid liver injury (Lee et al. 2008). The development of CCl4 toxicity involves cytochrome P450 (CYP)-mediated metabolic biotransformation and formation of highly reactive metabolites which initiate oxidation of cellular structures and macromolecules (Abdelaziz and Ali 2014). Despite the great advances in understanding the molecular mechanisms implicated in the pathogenesis of liver injury, there are only restricted hepatoprotective remedies. Complementary and alternative medicine is one of the promising treatments for liver ailments to replace the currently used drugs. The use of medicinal plants may be an approach in this area because of their high content of valuable therapeutic phytochemicals. Many plants and their extracts have been reported to protect the liver against damage induced by various toxicants (Zhang et al. 2013; Shahbazi et al. 2015).
Ginger (Zingiber officinale) has been used effectively as a herbal medicine for treatment of a variety of disorders and has reported to possess several beneficial pharmacological effects, including gastroprotective, antimicrobial, antiviral, antidiabetic, cardioprotective, anticancer, and chemopreventive (Ali et al. 2008). It contains high level of flavonoids and phenolic compounds which are responsible for its high antioxidant activity (Ghasemzadeh et al. 2010). Several studies pointed out to the protective effect of ginger against the hepatotoxicity induced by various xenobiotics including alcohol (Shati and Elsaid 2009), heavy metals (Khaki and Khaki 2010), and bromobenzene (El-Sharaky et al. 2009).
Similar to ginger, rosemary has been recognized to have several notable pharmacological activities including antidiabetic, antimicrobial, antioxidant, antiproliferative, anti-inflammatory, and antidepressant (Ribeiro-Santos et al. 2015). Furthermore, the protective effect of rosemary has been reported in several experimental models of liver injury (Rašković et al. 2014; Hasanein and Sharifi 2017). Most of the pharmacological effects of rosemary are attributed to its vast arrays of chemical constituents with powerful antioxidant activity such as carnosol, carnosic acid, rosmarinic acid, caffeic acid, and diterpenes, in addition to the essential oil components (Rašković et al. 2014).
Given these beneficial biological effects of ginger and rosemary, this study aimed to evaluate the protective effect of GE and RE when administered singly against liver injury induced by CCl4 in male rats. In addition, we aimed to examine the effect of the joint therapy of both extracts in order to assess the possible synergism between them and compare their effect together with respect to each monotherapy.
Materials and methods
Chemicals
Carbon tetrachloride (99% purity) and olive oil (laboratory grade) were obtained from Sigma chemicals (St Louis, Mo, USA). All other chemicals and reagents used in this study were of analytical grade and were purchased from Biodiagnostic Company (Egypt). The aqueous extracts of ginger and rosemary were prepared based on the methods of Kumari et al. (2014) and Dorman et al. (2003), respectively.
Experimental design
Fifty-six healthy adult male albino rats of 160–180 g were used in the current study. They were kept under observation for 1 week before the beginning of the experiment to be acclimatized. The animals were housed in wire bottomed cages under standard condition of illumination (12:12 h light:dark cycle) and temperature (22–24 °C) and had free access to balanced rat diet and tap water. The animal care and the handling methods of the study were in accordance with the guide for care and use of laboratory animal approved by The Institutional Animal Care and Use Committee (IACUC) of Menoufia University, Egypt, Approval No MNSP155. The animals were randomly divided into eight groups of seven rats each. Animals of the first group received 0.5 ml olive oil intraperitoneally (ip) and served as untreated controls. The second group of animals (GE group) administered orally with 120 mg/kg of GE. The third group (RE group) received 440 mg/kg of RE orally. The fourth group (GE-RE group) received the same doses of both GE and RE. The fifth group (CCl4 group) was given CCl4 (0.5 mL/kg diluted 1:1 (v/v) in olive oil, ip). The sixth group (CCl4-GE group) received 0.5 mL/kg CCl4 and 120 mg/kg GE. The seventh group (CCl4-RE group) received 0.5 mL/kg CCl4 and 440 mg/kg RE. The eighth group (CCl4-GE-RE group) received 0.5 mL/kg CCl4, 120 mg/kg GE, and 440 mg/kg RE. Doses of CCl4, GE, and RE were selected based on previous reports (Eidi et al. 2012; Kamtchouing et al. 2002; Dorman et al. 2003, respectively). These doses were administered twice a week for 6 consecutive weeks.
Biochemical analysis
After 6 weeks of treatment, rats from control and treated groups were fasted overnight and sacrificed under anesthesia. Blood samples were collected from dorsal aorta into dry glass centrifuge tubes and left to clot, then centrifuged at 3500 rpm for 15 min in a Beckman Model T-6 refrigerated centrifuge. Serum was separated and used for biochemical analysis. The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and the level of total bilirubin were determined in serum using the available assay kits according to the manufacturer’s protocol. The levels of total protein, albumin, globulin, triglycerides, and total cholesterol were also determined in serum using the assay kits according to the manufacturer’s protocol.
Assessment of lipid peroxidation, enzymatic antioxidants, and CYP
Liver homogenate was prepared from animals of different groups and used for determination of the level of lipid peroxidation (measured as malondialdehyde, MDA) according to the method of Ohkawa et al. (1979) and the activity of the antioxidant enzymes glutathione peroxidase (GPx) (Paglia and Valentine 1967), glutathione-S-transferase (GST) (Habig et al. 1974), superoxide dismutase (SOD) (Kakkar et al. 1984), and catalase (CAT) (Aebi 1984). Also, the activity of CYP was determined according to the method of Estabrook and Werringloer (1978).
Histological study
For histological study, small pieces of liver were quickly excised from rats in different groups, fixed in 10% buffered formalin solution, and processed routinely for paraffin embedding. Sections at a thickness of 4 μm were stained with hematoxylin and eosin (H&E) (Bancroft and Gamble 2002) and examined under light microscope (Nikon 80i, Japan).
Statistical analysis
Results are expressed as mean ± standard error for each group (n = 7). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by least significance difference (LSD) post-hoc test using Statistical Package for Social Sciences (SPSS) software version 15.0 for Windows. P ≤ 0.05 was considered statistically significant.
Results
Biochemical results
The effect of GE, RE, and their combination on indices related to liver injury in CCl4 intoxicated rats are illustrated in Table 1. Rats administered CCl4 had significantly higher levels of serum ALT (158.2%), AST (148.2%), ALP (164.5%), and total bilirubin (77.8%) compared to the normal control group. The levels of these parameters were significantly (P ≤ 0.05) improved upon treatment with extracts of GE and/or RE.
According to Table 2, CCl4 elicited a significant (P ≤ 0.05) decrease in the level of total protein (34.3%), albumin (30.1%), and globulin (41.7%), while triglycerides and total cholesterol levels were significantly increased (65.1 and 42.3%, respectively) when compared to the control group. Treatment with GE or RE singly after CCl4 intoxication partially improved the protein synthetic capacity of the liver, while a combination of both extracts completely restored the normal value of the protein content. Moreover, supplementation with GE and/or RE completely improved the measured lipid parameters and recovered the normal value of triglycerides and total cholesterol. Notably, the intake of GE and RE, both individually and in combination, did not significantly affect the metabolic function of the liver in normal rats.
Changes in the activity of CYP in hepatic tissue
Administration of CCl4 resulted in a significant (P ≤ 0.05) increase in the activity of CYP by 291.8% compared to the control group. Supplementation with GE or RE or both collectively to CCl4 intoxicated rats decreased the high activity of CYP (by 67.1, 71.3, and 72.7%, respectively) compared to the CCl4 group. Normal rats treated with GE or RE or both showed no marked changes in the activity of CYP (Table 3).
Changes in oxidant/antioxidant status in liver tissue
Administration of CCl4 induced significant (P ≤ 0.05) increase of 242.3% in the level of hepatic LPO (measured as MDA) compared to the control group (Table 3). Treatment with GE, RE, and a combination of both significantly (P ≤ 0.05) reduced the elevation in MDA level (72.4, 72.8, and 74.5%, respectively). The enzymatic antioxidant activity of the liver was significantly suppressed upon administration of CCl4 (Table 3). It significantly reduced the activity of GPx (43.3%), GST (48.4%), SOD (57%), and CAT (56.4%) compared to the normal control group. The suppressed activities of GST, SOD, and CAT were partially recovered upon treatment with GE and RE individually while the activity of GPx was recovered to almost normal level of the control. Notably, there was no statistically significant difference in MDA level or in the activity of antioxidants in rats treated only with GE and/or RE compared to the control group.
Histopathological results
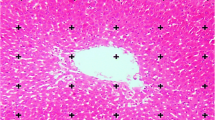
Liver sections from untreated rats showed normal lobular architecture with normal hepatocytes forming hepatic strands arranged around the central vein and separated by clear blood sinusoids (Fig. 1a). Treatment with CCl4 results in the loss of normal liver architecture and induced obvious histopathological changes including deformed hepatocytes with atrophied densely stained nuclei, intense infiltration of inflammatory cells, dilatation and congestion of blood vessels, and collapsed blood sinusoids with more dense Kupffer cells (Fig. 1b, c). Swelling hepatocytes and hepatocytes with cytoplasmic vacuolization and karyolytic nuclei were also recorded. Administration of GE to CCl4-intoxicated rats conspicuously weakened most of the destruction in the hepatic lobular architecture (Fig. 1d). Rats treated with both CCl4 and RE showed preservation of hepatic lobular structure with some cellular infiltrations (Fig. 1e). Animals intoxicated with CCl4 and treated with both GE and RE showed a marked degree of tissue recovery (Fig. 1f). Hepatocytes appeared with improved nuclei, less vacuolated cytoplasm, and defined outline. Normal central vein and blood sinusoids were also recorded.
Photomicrographs of rat liver sections (H&E stain). a Control liver showing normal and binucleated hepatocytes (dashed-circle) arranged in hepatic strands radiating from a central vein (CV) and separated by blood sinusoids (BS) with their kupffer cells (arrow). b, c Sections of liver from rats treated with CCl4. In b, loss of the normal liver architecture, dilatation and congestion of portal vein (PV) with detached endothelial lining (arrow), hepatocytes with cytoplasmic vacuolization (dashed-circle), and infiltration of leucocytes (white star). In c, hepatic necrosis associated with degenerated nuclei (black star), swelling of hepatocytes (dashed arrow), infiltration of leucocyte (long arrow), and lipid droplets accumulations (short arrow). d, e Photomicrographs of liver sections from rats treated with CCl4-GE and CCl4-RE, respectively, showing less hepatic injury compared to b and c. f Liver section from rat treated with CCl4, GE, and RE, showing more or less normal hepatic architecture
Discussion
Oxidative stress is identified as a key contributor in pathophysiological changes associated with many liver diseases (Rolo et al. 2012). Natural antioxidant products, especially phytochemicals, have gained interest due to their safety and ability to strengthen the endogenous antioxidants defenses. In the current study, we aimed to evaluate the antioxidant and the protective effect of GE and RE, both individually and collectively, using CCl4-induced liver injury in rats as an experimental model.
According to the data of our study, administration of CCl4 significantly enhanced the level of ALT, AST, ALP, and total bilirubin in the serum which agrees with the context of liver injury. These results correlate with those of Eidi et al. (2012), Wu et al. (2017), and Fu et al. (2018). Elevation in the serum level of ALT and AST indicates hepatocellular necrosis (Chen et al. 2015). Also, elevation in the level of ALP and bilirubin provides an evidence of liver injury and indicates an interruption of bile flow from the liver (Ranawat et al. 2010). The development of CCl4-induced liver injury may be a secondary event to the generation of free radicals which initiate LPO reactions and destroy the membranes of hepatocytes finally leading to the release of their cytosolic enzymes into the blood (Kyung et al. 2007).
Hepatotoxicity is always associated with perturbations in the synthesis and metabolism of proteins and lipids. In our study, a significant increase was observed in the serum level of triglycerides and total cholesterol after administration of CCl4. These results coincide with those of Essawy et al. (2012) and Abdel-Moneim et al. (2015). CCl4 can stimulate lipid synthesis from fatty acid, triglyceride, and cholesterol through enhancing the transport of acetate into the liver cells, resulting in increased substrate availability (Soni et al. 2008). CCl4-induced oxidative stress may enhance the non-essential fatty acids which in turn, elevate the level of cholesterol and triglyceride (Andritoiu et al. 2014). Diminution in protein content is a feature of liver damage and its subsequent fall in the capacity of the liver to synthesize proteins. In the present work, CCl4 induced a significant decline in the serum level of total protein, albumin, and globulin which affirms liver damage. These results go in parallel with Ali et al. (2010) and Huo et al. (2011). Also, CCl4-induced cellular damage may be responsible for a decrease in the number of hepatocytes which in turn affect the hepatic protein synthesis (El-Shenawy and Abdel-Nabi 2006).
Cytochromes P450 is a superfamily of enzymes localized mainly in the liver. They are involved in oxidative biotransformation of xenobiotic including medications, environmental toxins, and carcinogens into their metabolites (Zanger and Schwab 2013). It is well established that the metabolism of CCl4 into reactive hepatotoxic metabolites is mediated through CYP (Zangar et al. 2000). Our results revealed a significant increase in the activity of CYP which further provides an evidence for the correlation between CCl4 and over-expression of CYP. These results agree with Weber et al. (2003) and Xie et al. (2012). The catalytic activity of CYP isozyme has been reported to be correlated with enhanced generation of reactive oxygen species (ROS) and deterioration of antioxidant defense system (Dadkhah et al. 2015).
It is well established that generation of ROS and induction of oxidative stress are major steps in many chemical-induced liver injuries (Banerjee et al. 2016). These mechanisms are strongly involved in the development of CCl4 hepatotoxicity which seems to be an example of free radical-mediated injury (Williams and Burk 1990). In our study, administration of CCl4 significantly enhanced the level of MDA which indicates enhanced LPO. These results are in concert with to the findings of Abdel-Moneim et al. (2015) and Wu et al. (2017). CCl4 undergoes reductive metabolism by CYP and is bio-transformed into highly reactive metabolites such as trichloromethyl (CCl3•) and trichloromethylperoxy (CCl3OO•). These radicals can attack polyunsaturated fatty acids and initiate a cascade of reactions leading to peroxidation of membrane lipids and disruption of membrane integrity, ultimately leading to necrosis of hepatocytes (Weber et al. 2003; Khan et al. 2012). Cells have their own antioxidant defense system to scavenge or inhibit the formation of the reactive species. In our study, a remarkable decline in the activity of GPx, GST, SOD, and CAT was reported in the hepatic tissue upon administration of CCl4. These results are in agreement with Sreelatha et al. (2009), Abdel-Moneim et al. (2015), and Lee et al. (2017). The depletion in antioxidant enzymes with concomitant enhancement in LPO indicates that these enzymes are being used in detoxification of the reactive metabolites of CCl4. The observed change in the hepatic oxidant/antioxidant status clearly indicates the development of oxidative stress following CCl4 administration which may be the main reason behind the observed disturbance in the liver function.
Ginger is a commonly used flavoring agent and has been recognized to possess various pharmacological effects. In the current work, administration of GE modulated the CCl4-induced hepatotoxicity and recovered the proper function of the liver. This was indicated by the significant decrease in liver function markers ALT, AST, ALP, and total bilirubin. The disturbance in lipid profile, protein content, and CYP induced by CCl4 was also significantly ameliorated. Furthermore, the redox status of the liver was greatly improved upon treatment of CCl4-intoxicated rats with GE as indicated by a significant reduction in the hepatic MDA level and the significant increase in the activity of the major hepatic enzymatic antioxidants. These results are in concert with previous studies that revealed a similar protective effect for GE against different hepatotoxicants (El-Sharaky et al. 2009; Khaki and Khaki 2010). The observed protective effect of GE is mediated, at least in part, through modulating the hepatic redox status. Several studies have shown that ginger reduces oxidative stress through inhibiting LPO and enhancing the activity of the antioxidant enzymes (Mallikarjuna et al. 2008). All of the essential phenolic compounds of ginger including zingerone, zingibrene, gingerols, and shogaols are reported to possess potent antioxidant activities and subsequently contribute to the hepatoprotective effects (Ghasemzadeh et al. 2010). Ginger may also exert its protective effect through inhibition of CYP. The major components of ginger were found to suppress the major CYP isozymes which are responsible for the metabolism of most xenobiotics (Li et al. 2013). This was confirmed in our study by the marked reduction in the activity of CYP when compared with the hepatotoxic group which subsequently inhibits the initiation and propagation of a cascade of reactions leading finally to CCl4 hepatotoxicity.
Rosemary is one of the plants rich in different phytochemical derivatives including flavonoids, polyphenols, and triterpenes. In our study, oral supplementation with RE mitigated the CCl4-induced hepatotoxicity and restored the proper function of the liver as indicated by the improvement in ALT, AST, ALP, and total bilirubin. It also restored the normal capacity of the liver to synthesize lipids and proteins and improved the level of CYP. Furthermore, RE improved the redox status of the liver as indicated by suppression of LPO and restoration of the enzymatic antioxidant capacity. Dietary supplementation with RE has been reported to stabilize membrane and enhance the structural integrity of hepatocytes and to prevent liver damage induced by CCl4 (Botsoglou et al. 2009; Rašković et al. 2014). The protective effect of rosemary is largely attributed to its antioxidant, radical scavenging, and electron donation properties against different reactive oxygen and nitrogen species (Ban et al. 2016; Hasanein and Sharifi 2017). Phytochemical screening of RE revealed the presence of active ingredients such as polyphenols, diterpenes, and flavonoids which have been reported to possess strong antioxidant activities (Moreno et al. 2006). These phytochemicals have the ability to donate electrons to reactive radicals, making them non-reactive and more stable species, therefore preventing them from interacting with biomolecules, such as polyunsaturated fatty acids, lipoproteins, and DNA (Rašković et al. 2014). The protective action of rosemary can alternatively be mediated through the induction of detoxification enzymes. Rosemary or one of its constituents may act as a co-factor in the synthesis of endogenous antioxidants such as GST and quinine reductase (Singletary 1996). Furthermore, rosemary may affect the activity of CYP (Letelier et al. 2009) and therefore interferes with the biotransformation of CCl4 into its reactive metabolites. It is worth to mention that a combined therapy of both extracts maximizes the protection against CCl4-induced liver injury which can be a flawless combination for therapeutic approaches when compared to each individual therapy. These results suggest a synergistic and/or additive protective effect of phytochemicals present in the extracts of both ginger and rosemary.
The histopathological results recorded in the present study supported the obtained biochemical results and indicated that CCl4 induced severe histological alterations in the hepatic tissue as mentioned in the result section. Similar changes were recorded by other investigators (Eidi et al. 2012; Abdel-Moneim et al. 2015; Wu et al. 2017). The massive degenerative changes induced by CCl4 are primarily attributed to the generation of free radicals. Furthermore, the histopathological examination confirmed the protective effect of GE and RE. Most of the observed CCl4-induced damage in liver tissue was greatly improved upon treatment with GE and RE which points out to their ability to scavenge free radicals. The combination of both extracts maximizes the protective effect against hepatotoxicity of CCl4 possibly due to their synergistic or additive effect.
Conclusion
Aqueous extracts of ginger and/or rosemary alleviated the CCl4-induced hepatotoxicity in rats especially when administered in combination. The protective effect could be attributed, at least in part, to their bioactive ingredients which possess the ability to scavenge and/or inhibit the formation of free radicals during the metabolism of CCl4. They may also enhance the endogenous detoxifying enzymes and/or inhibit CYP. The potency of the plant extracts under investigation may open new fields for developing safe and cheap protective remedies for treatment of liver diseases.
References
Abdelaziz DH, Ali SA (2014) The protective effect of Phoenix dactylifera L. seeds against CCl4-induced hepatotoxicity in rats. J Ethnopharmacol 155(1):736–743
Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA (2015) Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One 10(12):e0144509
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ali BH, Blunden G, Tanira MO, Nemmar A (2008) Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale). Food Chem Toxicol 46:409–420
Ali SA, Rizk MZ, Ibrahim NA, Abdallah MS, Sharara HM, Moustafa MM (2010) Protective role of Juniperus phoenicea and Cupressus sempervirens against CCl4. World J Gastrointest Pharmacol Ther 1(6):123–131
Andritoiu CV, Ochiuz L, Andritoiu V, Popa M (2014) Effect of apitherapy formulations against carbon tetrachloride-induced toxicity in Wistar rats after three weeks of treatment. Molecules 19(9):13374–13391
Ban L, Narasimhamoorthy B, Zhao L, Greaves JA, Schroeder WD (2016) Antioxidant activities from different rosemary clonal lines. Food Chem 201:259–263
Bancroft JD, Gamble M (2002) Theory and practice of histological techniques, 5th edn. Churchill Livingstone, Edinburgh, p 796
Banerjee S, Ghosh J, Sil PC (2016) Drug metabolism and oxidative stress: cellular mechanism and new therapeutic insights. Biochem Anal Biochem 5(1):255
Botsoglou AN, Taitzoglou AI, Botsoglou E, Zervos I, Kokoli A, Christakia E, Nikolaidisc E (2009) Effect of long-term dietary administration of oregano and rosemary on the antioxidant status of rat serum, liver, kidney and heart after carbon tetrachloride-induced oxidative stress. J Sci Food Agric 89:1397–1406
Chen J, Zhao Y, Tao X, Zhang M, Sun A (2015) Protective effect of blueberry anthocyanins in a CCl4-induced liver cell model. LWT Food Sci Technol 60:1105–1112
Dadkhah A, Fatemi F, Alipour M, Ghaderi Z, Zolfaghari F, Razdan F (2015) Protective effects of Iranian Achillea wilhelmsii essential oil on acetaminophen-induced oxidative stress in rat liver. Pharm Biol 53:220–227
Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ (2003) Characterization of the antioxidant properties of deodourised aqueous extracts from selected Lamiaceae herbs. Food Chem 83:255–262
Eidi A, Mortazavi P, Bazargan M, Zaringhalam J (2012) Hepatoprotective activity of cinnamon ethanolic extract against CCl4-induced liver injury in rats. EXCLI J 11:495–507
El-Sharaky AS, Newairy AA, Kamel MA, Eweda SM (2009) Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats. Food Chem Toxicol 47:1584–1590
El-Shenawy NS, Abdel-Nabi IM (2006) Hypoglycemic effect of Cleome droserifolia ethanolic leaf extract in experimental diabetes, and on non-enzymatic antioxidant, glycogen, thyroid hormone and insulin levels. Diabetol Croat 35:15–22
Essawy AE, Abdel-Moneim AM, Khayyat LI, Elzergy AA (2012) Nigella sativa seeds protect against hepatotoxicity and dyslipidemia induced by carbon tetrachloride in mice. J Appl Pharma Sci 2(10):21–25
Estabrook RW, Werringloer J (1978) The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol 52:212–220
Fu X, Jiang B, Zheng B, Yan Y, Wang J, Duan Y, Li S, Yan L, Wang H, Chen B, Sang X, Ji W, Xu R, Si W (2018) Heterogenic transplantation of bone marrow-derived rhesus macaque mesenchymal stem cells ameliorates liver fibrosis induced by carbon tetrachloride in mouse. PeerJ 6:e4336. https://doi.org/10.7717/peerj.4336
Ghasemzadeh A, Jaafar HZ, Rahmat A (2010) Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale R.). Molecules 15(6):4324–4333
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Hasanein P, Sharifi M (2017) Effect of rosmarinic acid on acetaminophen-induced hepatotoxicity in male Wistar rats. Pharm Biol 55:1809–1816
Huo HZ, Wang B, Liang YK, Bao YY, Gu Y (2011) Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int J Mol Sci 12(10):6529–6543
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21(2):130–132
Kamtchouing P, Mbongue-Fandio GY, Dimo T, Jatsa HB (2002) Evaluation of androgenic activity of Zingiber officinale and Pentadiplandra brazzeana in male rats. Asian J Androl 4(4):299–301
Khaki AA, Khaki A (2010) Antioxidant effect of ginger to prevents lead-induced liver tissue apoptosis in rat. J Med Plants Res 4:1492–1495
Khan RA, Khan MR, Sahreen S, Shah NA (2012) Hepatoprotective activity of Sonchus asper against carbon tetrachloride-induced injuries in male rats: a randomized controlled trial. BMC Complement Altern Med 12:90
Kumari JA, Venkateshwarlu G, Choukse MK, Anandan R (2014) Effect of essential oil and aqueous extract of ginger (Zingiber officinale) on oxidative stability of fish oil-in-water emulsion. J Food Process Technol 6:412. https://doi.org/10.4172/2157-7110.1000412
Kyung JL, Jea HC, Hye GJ (2007) Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol 45:2118–2125
Lee KJ, Choi JH, Khanal T, Hwang YP, Chung YC, Jeong HG (2008) Protective effect of caffeic acid phenethyl ester against carbon tetrachloride-induced hepatotoxicity in mice. Toxicology 248:18–24
Lee GH, Lee HY, Choi MK, Chung HW, Kim SW, Chae HJ (2017) Protective effect of Curcuma longa L. extract on CCl4-induced acute hepatic stress. BMC Res Notes 10:77–85
Letelier ME, Terán A, Barra MA, Aracena-Parks P (2009) Antioxidant properties of Rosmarinus officinalis and its effects on xenobiotic biotransformation. Bol Latinoam Caribe Plant Med Aromat 8(6):487–497
Li M, Chen PZ, Yue QX, Li JQ, Chu RA, Zhang W, Wang H (2013) Pungent ginger components modulates human cytochrome P450 enzymes in vitro. Acta Pharmacol Sin 34(9):1237–1242
Mallikarjuna K, Sahitya Chetan P, Sathyavelu Reddy K, Rajendra W (2008) Ethanol toxicity: rehabilitation of hepatic antioxidant defence system with dietary ginger. Fitoterapia 79:174–178
Moreno S, Scheyer T, Romano CS, Vojnov AA (2006) Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res 40(2):223–231
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Ann Biochemist 95(2):351–358
Paglia DE, Valentine WN (1967) Studies on quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Ranawat L, Bhatt J, Patel J (2010) Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum in CCl4 induced hepatic damage in rats. J Ethnopharmacol 127(3):777–780
Rašković A, Milanović I, Pavlović N, Ćebović T, Vukmirović S, Mikov M (2014) Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement Altern Med 14:225
Ribeiro-Santos R, Carvalho-Costa D, Cavaleiro C, Costa HS, Albuquerque TG, Castilho MC (2015) A novel insight on an ancient aromatic plant: the rosemary (Rosmarinus officinalis L.). Trends Food Sci Technol 45(2):355–368
Rolo AP, Teodoro JS, Palmeira CM (2012) Role of oxidative stress in the pathogenesis of non-alcoholic steatohepatitis. Free Radic Biol Med 52(1):59–69
Shahbazi F, Sadighi S, Dashti-Khavidaki S, Shahi F, Mirzania M, Abdollahi A, Ghahremani MH (2015) Effect of silymarin administration on cisplatin nephrotoxicity: report from a pilot, randomized, double-blinded, placebo controlled clinical trial. Phytother Res 29(7):1046–1053
Shati AA, Elsaid FG (2009) Effects of water extracts of thyme (Thymus vulgaris) and ginger (Zingiber officinale R.) on alcohol abuse. Food Chem Toxicol 47:1945–1949
Singletary KW (1996) Rosemary extract and carnosol stimulate rat liver glutathione-S-transferase and quinone reductase activities. Cancer Lett 100:139–144
Soni B, Visavadiya NP, Madamwar D (2008) Ameliorative action of cyanobacterial phycoerythrin on CCl4-induced toxicity in rats. Toxicology 248:59–65
Sreelatha S, Padma PR, Umadevi M (2009) Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem Toxicol 47(4):702–708
Weber LW, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33(2):105–136
Williams AT, Burk RF (1990) Carbon tetrachloride hepatotoxicity: an example of free radical mediated injury. Semin Liver Dis 10(4):279–284
Wu T, Li J, Li Y, Song H (2017) Antioxidant and hepatoprotective effect of swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cell Physiol Biochem 41(6):2242–2254
Xie Q, Guo FF, Zhou W (2012) Protective effects of cassia seed ethanol extract against carbon tetrachloride-induced liver injury in mice. Acta Biochim Pol 59(2):265–270
Zangar RC, Benson JM, Burnett VL, Springer DL (2000) Cytochrome P450 2E1 is the primary enzyme responsible for low dose carbon tetrachloride metabolism in human liver microsomes. Chem Biol Interact 125(3):233–243
Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138(1):103–140
Zhang A, Sun H, Wang X (2013) Recent advances in natural products from plants for treatment of liver diseases. Eur J Med Chem 63:570–577
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experimental design was conducted in accord with the Institutional Animal Care and Use Committee (IACUC) of Menoufia University, Egypt, Approval No: MNSP155.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Essawy, A.E., Abdel-Wahab, W.M., Sadek, I.A. et al. Dual protective effect of ginger and rosemary extracts against CCl4-induced hepatotoxicity in rats. Environ Sci Pollut Res 25, 19510–19517 (2018). https://doi.org/10.1007/s11356-018-2129-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2129-5