Abstract
Hexavalent chromium (Cr VI) is widely known as a potential hepatotoxic in humans and animals and its toxicity is associated with oxidative stress. So, an in vivo study was outlined to assess the protective and therapeutic role of Rosmarinus officinalis essential oil (rosemary; REO) against Cr VI-induced hepatotoxicity. Male Wistar rats were assigned into five equal groups (1st group served as control; 2nd and 3rd groups received 0.5 ml/kg BW REO and 2 mg/kg BW Cr VI, respectively; 4th group pretreated with REO then injected with K2Cr2O7; and 5th group received Cr VI then treated with REO for 3 weeks). Results revealed that rats exposed to Cr VI showed a valuable changes in hematological parameters and an increase in oxidative stress markers (Protein carbonyl, TBARS, and H2O2) and a noteworthy decline in glutathione (GSH) content. Furthermore, a considerable decrease in enzymatic antioxidants (SOD, CAT, GPx, and GST), transaminases (AST and ALT), and alkaline phosphatase (ALP) activities, as well as total protein and albumin levels, was detected, while serum liver function biomarkers were increased significantly. In addition, the evaluation of histopathological and immunohistochemical PCNA expression showed significant variations in the liver that confirm the biochemical results. Administration of REO pre- or post-chromium treatment restored the parameters cited above near to the normal values. Otherwise, individual intake with REO slumped lipid peroxidation and gotten better antioxidant status significantly. Conclusively, REO proved to be an effective antioxidant in modulating Cr VI-induced hepatotoxicity, especially in the pretreated rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hexavalent chromium (Cr (VI)) is a major contaminant causing serious environmental problems in many countries due to its wide industrialization. It is utilized in painting, electroplating, leather industry, dyes, and several industries leading to its widespread distribution in the environment and the discharge of chromium-containing solutions may lead to water and soil pollution (Mukesh et al. 2016; Kader and Kalapuram 2017). Also, Cr (VI) presents a potential threat to human health by affecting the food chain. Several health risks may occur due to exposure to Cr (VI) including carcinogenic, cytotoxic, immunotoxic, neurotoxic, hepatotoxic, nephrotoxic, and genotoxic effects as well as general environmental toxicity (Marouani et al. 2017; Mishra and Bharagava 2016). In addition, extensive tissue damage occurs to the animals exposed to Cr (VI), as lesions of the testis, spleen, kidney, and liver. Its mechanism of action is proposed to involve reactive oxygen species (ROS) generation and DNA damage increasing formation of DNA adducts and DNA-protein cross-links, DNA strand breaks, disruption of mitotic cell division, chromosomal aberration, and premature cell division (Khalil et al. 2013) in addition to carcinogenesis (Kim et al. 2018). Moreover, hepatic affection is of great importance due to the essential role of the liver in environmental xenobiotics transformation into their metabolites (Elshazly et al. 2016).
Herbal remedies are being used for the treatment of different diseases and dysfunctions since ancient times. Rosmarinus officinalis L. (Lamiaceae), commonly known as rosemary, is a household plant used worldwide as a food flavoring agent. Rosemary is normally grown in the dry, rocky areas of the Mediterranean, particularly throughout the length of the coast. Previous studies showed that rosemary has powerful anti-inflammatory (Beninca et al. 2011), antibacterial (Hussain et al. 2010), antidiabetic (Naimi et al. 2017), antitumor (Cheung and Tai 2007), antioxidant (El-Demerdash et al. 2016), cytoprotective (Rajgopal et al. 2019), and hepatoprotective (Hegaz et al. 2018; El-Demerdash et al. 2016) properties. Rosemary contains high phenolic content and has high antioxidant activity (Ngo et al. 2011). The rosemary essential oil contains various compounds at a particular concentration. It is distinguished by two or three major components at relatively high concentrations (1,8-cineole, a-pinene, camphor, and p-cymene) compared to others present in trace quantities (Bakirel et al. 2008). Therefore, the present research was conducted to assess the capability of Rosmarinus officinalis essential oil to ameliorate hepatotoxicity, hematotoxicity, and lipid peroxidation as well as histological and immunohistochemical alterations induced by potassium dichromate in male rats.
Materials and methods
Materials
Potassium dichromate (K2Cr2O7, purity 99%) was obtained from Merck (Darmstadt, Germany). Rosemary (Rosmarinus Officinalis L.) dried plant leaves were purchased from a herbal store in Alexandria, Egypt. All other chemicals were of analytical grade.
Essential oil extraction
Authentication of plant was carried out by the Herbarium of the Department of Botany, Faculty of Science, Alexandria University. Dried rosemary leaves were used for the extraction process. Essential oils were extracted by hydrodistillation using a Clevenger type apparatus (Guenther 1948). A flask containing 100 gm of the plant material and 1000 ml of distilled water was heated for 3 h and the condensed vapor laden with essential oil was separated throughout an auto oil/water separator; hydrodistillation was continued until successive readings of the oil volume did not change. Oil recovered directly, from above the distillate without any solvent addition. The essential oil was gathered in dark glass vials wrapped with aluminum foil to avoid the negative effects of light and stored at 4 °C. The oil yield was calculated in terms of w/v%.
GC/MS analysis of essential oils
The chemical composition of the extracted essential oil was identified using a Thermo Scientific gas chromatography-mass spectrometer (GC/MS) version (5) 2009 system with TG-5MS column (30 m × 0.32 mmID). Helium was used as a carrier gas at a flow rate of 1 ml/min. Five-microliter essential oil was diluted to 1 ml with dichloromethane, and then 2 μl was injected on splitless mode for 1 min followed by a split flow with a ratio of 1:10. GC oven temperature was held at 45 °C for 2 min then was programmed from 45 to 165 °C at 4 °C/min; from 165 to 280 °C at 15 °C/min after which was kept constant at 280 °C for 10 min. Both the interface and injection temperatures were adjusted at 250 °C. The ionization voltage was 70 eV with a mass range between 40 and 800 m/z (Adams 2006). The components of the essential oil were identified by mass fragmentation patterns, which were compared with the National Institute of Standards and Technology (NIST) mass spectral database (version 2), 13,531 components scanned for 46.01 min, and their relative percentages were calculated based on GC peak areas (Adams 2006).
Experimental design
Thirty-five male Wistar rats (weighing 150–170 g) were bought from the animal house of the Faculty of Medicine, Alexandria University. Animal handling and experimental design procedures were approved by the Research Ethical Committee of the Faculty of Medicine, Alexandria University, Alexandria, Egypt, and the protocol conforms to the National Institutes of Health guidelines. Rats were housed in stainless steel-bottomed wire cages and kept at a temperature of 22 ± 2 °C, relative humidity of 40–60%, with a 12-h/12-h light/dark cycle and free access to commercial diet and water ad libitum. After 2 weeks of acclimatization, rats were assigned into five groups randomly with seven animals each. Group I used as a control, and group II administered orally with rosemary essential oil (REO; 1/10 LD50, 0.5 ml/kg BW, LD50 = 5 ml/kg). Group III was injected intraperitoneally with potassium dichromate (CrVI; 2 mg/kg BW). Group IV administered orally with REO (0.5 ml/kg BW) and after 30 min, injected with CrVI (2 mg/kg BW). Group V was injected with CrVI (2 mg/kg BW) and after 30 min was administered with REO. Rosemary essential oil and potassium dichromate doses were given daily for 3 weeks according to the previous studies of Opdyke (1974), Fahim et al. (1999), and Murthy et al. (1991), respectively. There are no adverse effects observed in the group treated with REO (1/10 LD50) alone in addition; several studies confirmed the safety of high doses of REO (Machado et al. 2013; Takaki et al. 2008; Fahim et al. 1999). At the experiment termination, rats were anesthetized by isoflurane and then killed via cervical dislocation, and the livers were immediately removed. The liver was chopped into two portions: one was fixed in 10% formalin for histopathology and immunohistochemistry, and the other was stored at − 80 °C for biochemical analyses.
Body and organ weights
The body weight was recorded on the first day of treatment (initial), and on the day of sacrifice (final). The livers were dissected out, trimmed off the attached tissues, and weighed. The relative weight of organs was expressed as g/100 g of the body weight.
Blood and serum samples
Blood samples were taken by cardiac puncture and allowed to stand for 30 min at 25 °C to clot before being centrifuged at 3000g for 15 min. Serum was obtained and stored at − 80 °C until used for liver function biomarkers’ determination. Other blood samples put in EDTA tubes were taken for the identification of complete blood count (CBC) including hemoglobin (Hb), white blood cells (WBCs), red blood cells (RBCs), platelets, and hematocrit using an automatic analyzer (Sysmex kx-21n Automated Hematology Analyzer; JAPAN CARE CO., LTD).
Tissue preparation
Liver tissues were weighed, minced, and homogenized (10% w/v) in ice-cold 1.15% KCl–0.01 mol/l sodium-potassium phosphate buffer (pH 7.4) using a Potter-Elvehjem type homogenizer. This is followed by the centrifugation process (10,000g; 20 min; 4 °C), and the obtained supernatants were stored for various assays.
Biochemical analysis
Thiobarbituric acid-reactive substances (TBARS), hydrogen peroxide (H2O2), reduced glutathione (GSH), and antioxidant enzymes as superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), glutathione peroxidase (GPx, EC 1.11.1.9), and glutathione S-transferase (GST; EC 2.5.1.18) activities were measured in liver homogenate using commercially available kits (Biodiagnostic, Egypt). Protein carbonyl content was assessed according to the method of Reznick and Packer (1994). Aspartate aminotransferase (AST; EC 2.6.1.1) and alanine aminotransferase (ALT; EC 2.6.1.2) activities were determined using kits from SENTINEL CH. (via principle Eugenio 5-20155, Milan, Italy). Alkaline phosphatase (ALP; EC 3.1.3.1) activity was assayed according to Principato et al. (1985). Protein content and albumin concentration were evaluated according to the methods of Lowry et al. (1951) and Doumas et al. (1977), respectively. Globulin concentrations were detected by the difference between total protein and albumin.
Histopathological examination
The livers were fixed in 10% formalin solution and serial paraffin sections were obtained to examine the histological changes using hematoxylin and eosin stain (Bancroft and Steven 1990), then slides were photographed by a light microscope (Olympus BX 41, Japan).
Immunohistochemical examinations
Distribution of proliferating cell nuclear antigen immunoreactivity (PCNA-ir)-stained nuclei was investigated in deparaffinized sections (5 μm) using an avidin-biotin-peroxidase complex (ABC) immunohistochemical method (Elite–ABC, Vector Laboratories, CA, USA) with PCNA monoclonal antibody (dilution 1:100; DAKO Japan Co, Tokyo, Japan) and mounted on poly-L-lysine-coated glass slides, then they have been evaluated and photographed using light microscopy (Olympus BX 41, Japan).
Statistical analysis
Data from different groups were represented as means ± standard errors (SEM) then analyzed by SPSS software (version 22, IBM Co., Armonk, NY). Comparison between groups was done through one-way ANOVA followed by Tukey’s post hoc test. P value ≤ 0.05 was approved to be significant.
Results
GC/MS analysis of rosemary essential oil
In the current research, dried rosemary leaves yielded a high amount of essential oil (3.2 ml/100 g DW). Rosemary essential oil (REO) is yellowish in color with a slightly camphoraceous odor. Twenty-nine compounds were identified in the essential oils representing 99.87% of the total oil content. As a consequence of GC–MS analyses, REO contained 1,8-cineole (41.75%), camphor (17.66%), and α-pinene (13.64%) as the major compounds. Also, REO contains hydrocarbons of monoterpenes (like α-thujene, α-pinene, camphene, β-myrcene, α-terpinene, p-cymene, Ɣ-terpinene, and α-terpinolene), oxygenated monoterpens (such as 1,8-cineole, linalool, camphor, borneol, terpinen-4-ol, α-terpineol, bornyl acetate, and trans-3-caren-2-ol), and sesquiterpene hydrocarbon compounds such as (T-cadinol, α-copaene, β-caryophyllene and γ-cadinene) (Table 1 and Fig. 1).
General health
None of the CrVI-intoxicated rats showed signs of morbidity or mortality during the study. However, Table 2 shows a significant decrease in body weight accompanied by an increase in liver weights in chromium-treated rats in comparison with the control group. On the contrary, the REO supplementation in protective and therapeutic groups ameliorated these settings compared to CrVI group while REO alone did not cause any significant change.
Hematological parameters
Rats administered with potassium dichromate showed a significant drop in red blood cells (RBCs), hemoglobin (Hb) concentration, and hematocrit (HCT) while white blood cells (WBCs) and platelets are significantly increased with respect to control. Administration of REO alone showed insignificant changes in most of the hematological parameters except WBCs which is relatively increased as compared to the control group. On the other hand, protection or treatment with REO to CrVI treated rats showed significant restoration near the normal level as compared to CrVI-treated ones (Table 3).
Liver function biomarkers in rat serum and liver homogenate
In comparison to the control group, rats treated with Cr (VI) showed considerable changes (P < 0.05) in the activities of ALT, AST, and ALP in rat serum and liver homogenate indicating hepatic damage. A significant decrease in serum total protein, albumin, and globulin in addition to liver protein content was observed due to Cr (VI) treatment as compared to control. REO administration alone had a significant influence on some of the studied parameters. Moreover, rats treated with both REO and Cr (VI) showed significant improvement especially in the protection group (Table 4).
Lipid peroxidation, protein oxidation, and reduced glutathione content
As shown in Table 5, rats treated with Cr (VI) exhibited a significant increase in protein carbonyl, TBARS, and H2O2 levels accompanied by depletion in non-enzymatic antioxidant (GSH) content in liver homogenate. Rats supplemented with rosemary essential oils alone showed a significant (P < 0.05) decrease in protein carbonyl content, TBARS level, and H2O2 concentration and increased the GSH content as related to the control group. Rats protected or treated with REO attenuated the observed disturbance in TBARS, H2O2, protein carbonyl, and GSH as compared to Cr (VI) treated rats.
Antioxidant enzyme activities
Data in Table 5 showed the changes in liver GPx, SOD, CAT, and GST of rats intoxicated with Cr (VI), REO, and their combination. Treatment with Cr (VI) decreased the activities of GST, SOD, CAT, and GPx in liver homogenates. On the other hand, administration of REO alone showed a significant (P < 0.05) increase in GST, SOD, and CAT activities and insignificant changes in GPx activity compared with the control group. Rats protected or treated with REO maintained antioxidant enzyme activities near to the normal values as compared to Cr (VI)-treated rats.
Liver histopathology
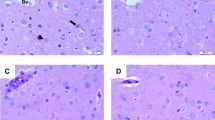
Histopathological examination of control and REO liver sections exhibited normal architecture (Fig. 2a–b). In contrast, liver sections in Cr (VI)-treated rats (G3) showed severe hepatic damage including hepatocytes degeneration, necrosis, inflammation, and vacuolization (Fig. 2c and d) when compared with control rats. However, rats pretreated with REO then intoxicated with Cr (VI) (G4) showed detectable recovery in the liver architecture with normal sinusoidal spaces (Fig. 2e). On the other hand, liver sections of rats treated with CrVI + REO (G5) revealed a mild degree of improvement in the structure of the hepatocytes (Fig. 2f; Table 6).
Photomicrographs of rat liver sections in the different experimental groups stained with haematoxylin & eosin. (a) and (b) Liver sections in control and REO rat groups revealed normal structure of hepatocytes with normal central veins (CV). (c) and (d) Liver sections of CrVI group showed severe hepatic damage including massive vacuolar degeneration of hepatocytes with necrosis, inflammation (black arrows), slight areas of hemorrhage, presence of cellular debris within a central vein (CV), and cytological vacuolization of hepatocytes (white arrows). (e) Liver sections in the treatment group (REO+CrVI) (G4) showed prominent recovery in the form of the liver histo-architecture such as a minimal vacuolization in hepatocytes and mild cellular infiltrations (black arrows). (f) Liver sections in the therapeutic rat group (CrVI+REO) (G5) showed moderate to mild congestion in central veins, mild cytoplasmic vacuolization of hepatocytes (white arrows), and mild cellular infiltrations (black arrows)
PCNA immunohistochemistry
The identification and distribution of PCNA immunoreactivity (PCNA-ir) in liver sections in control and REO groups showed negative or very low reactions for PCNA-ir in hepatocytes (Fig. 3a–b), while high reactions were observed for PCNA-ir in the liver of rats treated with chromium (Fig. 4a). On the other hand, intermediate positive reactions for PCNA-ir were observed in liver sections of REO and Cr (VI) group (G4) (Fig. 4b). On the contrary, liver sections of Cr (VI) + REO group (G5) revealed high reactions for PCNA-ir in the hepatocytes (Fig. 4c).
Photomicrographs of rat liver sections in the chromium (G3), protection group (REO+CrVI) (G4), and therapeutic group (CrVI+REO) (G5), respectively. a (+++) High PCNA-ir reaction in hepatocytes in chromium group. b (++) Mild positive reactions for PCNA-ir in the protection group was observed. c (+++) Liver sections in rat liver of the therapeutic group showed high PCNA-ir reaction in hepatocytes
Discussion
In the current investigation, the potential benefit of Rosmarinus officinalis essential oil against Cr (VI)-induced hazardous effects was evaluated in the blood and liver of male rats. Cr (VI) administration induced a critical decrease in the body weight of rats with an increase in liver weight and this agreed with the finding of Saidi et al. (2019). This might be due to the loss of appetite following the alteration of the food organoleptic proprieties caused by the chromium (Saka and Aouacheri 2017). Also, the observed hematotoxicity induced by Cr (VI) is related to impairment of hematogenesis through the inhibition of RBC production from pro-erythroblast and/or the destruction of erythrocyte in hemopoietic organs (De Vizcaya-Ruiz et al. 2003). Moreover, Cr (VI) was bound to the beta-chain of hemoglobin in erythrocytes (Barceloux 1999) which explains the depletion of hemoglobin concentrations. Furthermore, leukocytosis observed indicates the improved defense mechanism and immune system against xenobiotics infection (Srivastava and Narain 1985).
K2Cr2O7 induced oxidative stress as assessed by increased levels of lipid peroxidation markers, oxidized proteins, and decreased GSH content (Halliwell and Gutteridge 2007). Carbonyl groups are mainly generated by the oxidation of amino acid side chains of proteins or caused by ROS-mediated protein damage (Dalle-Donne et al. 2003). Otherwise, protein sulfhydryl groups from cysteine residues can be oxidized to form disulfide, possibly altering the redox state of proteins and driving to their inactivation (Kuhn et al. 1999). Moreover, TBARS and H2O2 are important oxidation products, and their increase is deemed as lipid peroxidation predictors (Celik and Suzek 2009). Similarly, several studies reported that chromium acts as an oxidant and affects many organs causing oxidative stress (Marouani et al. 2017; Navya et al. 2018).
Reduced glutathione is responsible for protection against ROS and detoxification of several toxins. It is known that oxidants may react with GSH directly and generate free radicals by altering the redox status or may be through their metabolism that leads to the release of excess free radicals (Maran et al. 2009). The observed reduction in hepatic GSH content may be related to its consumption in scavenging free radicals induced by chromium (Chandra et al. 2007) as its sulfhydryl group is converted into its oxidized form (GSSG) during its metabolism (Meister and Anderson 1983). The redox cycling of oxidized glutathione is catalyzed by glutathione reductase, whereas the supply of the major reducing agent, NADPH, is provided by the glucose-6-phosphate dehydrogenase enzyme (Halliwell and Gutteridge 2007). The decline in antioxidant enzyme activities in chromium-exposed animals might be attributed to the loss of cells expressing these enzymes and/or due to direct inhibitory actions of ROS on these enzymes. Catalase and superoxide dismutase enzymes are considered the first line of defense system against oxygen toxicity (Tan et al. 2018). Superoxide dismutase catalyzes superoxide anion (O2-) to O2 and H2O2, which then is reduced to H2O by the H2O2-scavenging enzyme, catalase, and the decline in their activities may lead to a massive generation of free radicals (Choudhuri et al. 2020). Also, GPx and GSH are also important sensitive indicators of oxidative stress. GPx modifies peroxide to a nontoxic hydroxyl compound to protect the cell membrane structure, while GST plays a critical role in the detoxification process of xenobiotics to non-toxic products, protecting against electrophiles and oxidative stress (Ghosh et al. 2012; Choudhuri et al. 2020).
In the current study, rats treated with CrVI showed significant variations in serum and liver ALP, ALT, and AST activities and their alterations pointed out hepatocyte damage that altered the transport function and membrane permeability as well as leakage of enzymes from the cells to the bloodstream indicating hepatotoxicity (Chen et al. 2019; Albasher et al. 2020). Also, lipid peroxidation has a fundamental role in the disruption of hepatocellular membrane integrity, leading to the leakage of cytoplasmic enzymes and this confirmed the possible mechanism of oxidative stress in liver damage stimulated by Cr (Soudani et al. 2011; Farag and El-Shetry 2020). Alkaline phosphatase is a polyfunctional critical membrane-bound enzyme used as a biomarker for heavy metal toxicity (Yarbrough et al. 1982). So, alterations in this enzyme activity could be expected due to cellular necrosis of the liver, kidney, and lung (Bashandy et al. 2020). Protein is an essential cellular component susceptible to damage by free radicals and its reduction might be linked to exaggerated leakage via nephrosis (Chatterjea and Shinde 2002) and/or related to a disturbance in protein anabolic and catabolic processes (Balakrishnan et al. 2013). Liver histopathological examination of chromium-intoxicated rats revealed severe hepatic damage and this could be attributed to the oxidative toxicity induced by Cr (VI) which may have apparently led to severe alterations in liver architecture. Similar observations exhibited cellular damage, morphological changes, chromatin condensation, and DNA fragmentation (Bashandy et al. 2020; Lukas et al. 2011). Also, an intense expression of PCNA in the nuclei of liver tissue of rats treated with Cr (VI) and the accelerated proliferation might indicate an increased mutagenic risk on cells (Cardoso et al. 2000).
Rosmarinus officinalis (L.) is well known for its antioxidant properties as well as a hepatoprotector (Hamed et al. 2019). The antioxidant activity of REO assigned to both carnosic acid and carnosol, the main diterpene contents (Hussain et al. 2010), and its essential oil constituents (Hcini et al. 2013). Administration of REO markedly ameliorated the toxic action produced by Cr (VI) on hematological parameters. It has been observed that rosemary is effective in relation to blood circulation improving hemodynamics in occlusive arterial diseases (de Oliveira et al. 2019). The activity of REO might be related to the stimulation or protection of bone marrow hematopoiesis and protecting stem cells from hematopoietic responsible for the regeneration and recovery of the system (Abd El-Aziz et al. 2014). Rosemary has shown significant antithrombotic activity in mice that may encompass a direct inhibitory effect on platelets, so protection or treatment with rosemary showed a considerable decline in platelets (Naemura et al. 2008). Also, rosemary extract has been found to exert hepatoprotective effect against Cr (VI) via attenuating the alterations in AST, ALT, and ALP activities in acute liver damage and liver cirrhosis of the animals due to its antioxidant properties (Gutiérrez et al. 2009; Botsoglou et al. 2010). Additionally, the administration of rosemary extract recovers the alterations in serum ALT, AST, and ALP activities, improves the oxidant-antioxidant status, and inhibits oxidative stress and inflammation (Samaha 2017; Gao et al. 2016).
The present results showed that administration of REO alone decreased oxidative stress markers, and activated the antioxidant status in rat liver with respect to control. However, a significant modulation in protein carbonyl, TBARS, and H2O2 concentrations was observed in rats administered with REO plus Cr (VI). According to Jongberg et al. (2013), the high phenolic content of rosemary extracts protected against the formation of TBARS and protein carbonyls. Furthermore, the increase demonstrated in antioxidant enzymes is related to the decrease in the level of free radicals that are prohibited by REO. Similarly, Parmar et al. (2011) reported that rosemary maintained the levels of antioxidants, membrane-bound enzymes, and the antioxidant enzyme activities near-normal levels, thus emphasizing its effect as an antioxidant. Besides, rosemary protects the liver against toxins through the prohibition of lipid peroxidation, activation of antioxidants status (Malo et al. 2010), stabilization of the cell membrane permeability, enhancement of protein biosynthesis (Habtemariam 2016), and reduction of enzyme leakage into the bloodstream (Gutiérrez et al. 2009).
Furthermore, administration of rosemary elevated the level of GSH due to its antioxidant property and this is in agreement with El-Demerdash et al. (2016). The observed increase in the GSH level could protect cellular proteins against oxidation via the glutathione redox cycle in addition to ROS elimination. SOD and CAT act as mutually supportive antioxidant enzymes, which protect against ROS (Cerutti et al. 1994). Supporting this finding, Ciftci et al. (2011) found that 1.8-cineole enforced the antioxidant defense system in rats treated with 2,3,7,8-tetracholorodibenzo-p-dioxin, while reducing malondialdehyde concentration near the normal level. Besides, α-pinene, a bicyclic monoterpene observed in REO, in a relatively high amount (13.64%), could have a hepatoprotective activity as well (Türkez and Aydin 2013). In addition, rosemary extract has the potential effect to prevent hepatic fibrosis due to chronic liver damage and thus delay cirrhosis development (Li et al. 2010). In agreement with the current study, Wang et al. (2017) reported that rosemary extract supply increases SOD and CAT activities, and decreases the level of malondialdehyde, significantly. Finally, REO can effectively inhibit hepatotoxicity induced by Cr (VI) and this is may be attributed to its antioxidant properties.
Conclusion
In conclusion, GC-MS analysis indicated that REO is rich in phenolic compounds. Otherwise, chromium hexavalent treatment led to LPO and disturbances in the antioxidant defense system, biochemical indices, and hematological parameters as well as liver histological and immunohistochemical changes. Furthermore, REO supplementation to Cr (VI)-treated rats attenuates oxidative damage and restores the disturbances in most of the measured parameters and its effect is more pronounced in the protection group. So, REO had a powerful antioxidant role in mitigating Cr toxicity by potentiating antioxidant defense system status and reducing free radicals’ production; in addition, our data support the use of REO as a hepatoprotective natural product.
Availability of data and materials
The authors declare that all relevant data that support the findings of this study are available within the article. Additional data are available from the corresponding author upon reasonable request
References
Abd El-Aziz AF, Hefni ME, Shalaby AM (2014) Inhibitory effects of rosemary (Rosmarinus officinalis L.) on Ehrlich ascites carcinoma in mice. Int J Curr Res Acad Rev 2:330–335
Adams RP (2006) Identification of essential oil components by gas chromatography and mass spectrometry, 4th edn. Allured Pub. Corp., Carol Stream
Albasher G, Abdel-Daim MM, Almeer R, Ibrahim KA, Hamza RZ, Bungau S, Aleya L (2020) Synergistic antioxidant effects of resveratrol and curcumin against fipronil-triggered oxidative damage in male albino rats. Environ Sci Pollut Res Int 27:6505–6514
Bakirel T, Bakire U, Keles OU, Ulgen SG, Yardibi H (2008) In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J Ethnopharmacol 116:64–73
Balakrishnan B, Praveen C, Seshadri PR, Perumal PT (2013) 5-(3-Meth-oxy-phen-yl)-3-phenyl-1,2-oxazole. Acta Crystallogr Sect E Struct Rep Online 69(Pt 4):o597
Bancroft JD, Steven A (1990) Theory and practice of histological technique, 3rd edn. Churdchill Livingstone, New York, p 726
Barceloux DG (1999) Chromium. Clin Toxicol 37:173–194
Bashandy SAE, Salama A, Fayed AM, Omara EA, El-Toumy SA, Salib JY (2020) Protective effect of Mandrin (Citrus Reticulata) peel extract on potassium dichromate induced hepatotoxicity and nephrotoxicity in rats. Plant Arch 20:2231–2242
Beninca JP, Dalmarco JB, Pizzolatti MG, Frode TS (2011) Analysis of the anti-inflammatory properties of Rosmarinus officinalis L in mice. Food Chem 124:468–475
Botsoglou N, Taitzoglou I, Zervos I, Botsoglou E, Tsantarliotou M (2010) Potential of long-term dietry administration of rosemary in improving the antioxidant status of rat tissues following carbon tetrachloride intoxication. Food Chem Toxicol 48:944–950
Cardoso WP, Denardin OVP, Rapoport A, Araujo CV, Carvalho MB (2000) Proliferating cell nuclear antigen expression in mucoepidermoid carcinoma of salivary glands. Sao Paulo Med J 118:69–74
Celik I, Suzek H (2009) Effects of subacute exposure of dichlorvos at sublethal dosages on erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Ecotoxicol Environ Saf 72:905–908
Cerutti P, Ghosh R, Oya Y, Amstad P (1994) The role of cellular antioxidants defense in oxidant carcinogenesis. Environ Health Perspect 102:123–129
Chandra AK, Chatterjee A, Ghosh R, Sarkar M (2007) Effect of curcumin on chromium-induced oxidative damage in male reproductive system. Environ Toxicol Pharmacol 24:160–166
Chatterjea MN, Shinde R (2002) Text book of medical biochemistry, 5th edn. Jaypee Brothers, New Delhi
Chen C, Lin B, Qi S, He J, Zheng H (2019) Protective effects of salidroside on lead acetate-induced oxidative stress and hepatotoxicity in Sprague-Dawley rats. Biol Trace Elem Res 191:426–434
Cheung S, Tai J (2007) Anti-proliferative and antioxidant properties of rosemary (Rosmarinus officinalis). Oncol Rep 17:1525–1531
Choudhuri S, Saha J, Das S, Choudhuri D (2020) Modulatory role of selenium and vitamin E against oxidative stress induced hepatotoxicity and nephrotoxicity in rats exposed sub-chronically to hexavalent chromium. Asian J Pharm Clin Res 13:113–118
Ciftci O, Ozdemir I, Tanyildizi S, Yildiz S, Oguzturk H (2011) Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzop-dioxin-induced oxidative stress in rats liver. Toxicol Ind Health 27:447–453
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta: Inter J Clin Chem 329:23–38
de Oliveira JR, Camargo SEA, de Oliveira LD (2019) Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J Biomed Sci 26:5
De Vizcaya-Ruiz A, Rivero-Muller A, Ruiz-Ramirez L, Howarth JA, Dobrota M (2003) Hematotoxicity response in rats by the novel copper-based anticancer agent: casiopeina II. Toxicol 194:103–113
Doumas BT, Watson WA, Biggs HG (1977) Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chem Acta 31:87–96
El-Demerdash FM, Abbady EA, Baghdadi HH (2016) Oxidative stress modulation by Rosmarinus officinalis in creosote –induced hepatotoxicity. Environ Toxicol 31:85–92
Elshazly MO, Morgan AM, Ali ME, Abdel-mawla E, Abd el-Rahman SS (2016) The mitigative effect of Raphanus sativus oil on chromium-induced geno- and hepatotoxicity in male rats. J Adv Res 7:413–421
Fahim FA, Esmat AY, Fadel HM, Hassan KFS (1999) Allied studies on the effect of Rosmarinus officinalis L. on experimental hepatotoxicity and mutagenesis. Int J Food Sci Nutr 50:413–427
Farag AI, El-Shetry ES (2020) Chromium-induced hepatotoxicity and potential protective effect of selenium in adult male albino rat: a histological, immuno-histochemical and molecular study. Med J Cairo Univ 88:187–196
Gao L, Shan W, Zeng W, Hu Y, Wang G, Tian X, Zhang N, Shi X, Zhao Y, Ding C, Zhang F, Liu K, Yao J (2016) Carnosic acid alleviates chronic alcoholic liver injury by regulating the SIRT1/ChREBP and SIRT1/p66shc pathways in rats. Mol Nutr Food Res 60:1902–1911
Ghosh T, Mustafa MD, Kumar V, Datta SK, Bhatia MS, Sircar S, Banerjee B (2012) A preliminary study on the influence of glutathione S transferase T1 (GSTT1) as a risk factor for late onset Alzheimer’s disease in North Indian population. Asian J Psychiatr 5:160–163
Guenther E (1948) The essential oils. D. Van Nostrand Co. priceton, New York, pp 316–318
Gutiérrez R, Alvarado JL, Presno M, Perez-Veyna O, Serrano CJ, Yahuaca P (2009) Oxidative stress modulation by Rosmarinus officinalis in CCl4-induced liver cirrhosis. Phytother Res 24:595–601
Habtemariam S (2016) The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. Evid Based Complement Alternat Med 2016:2680409
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, New York
Hamed H, Bellassoued K, El Feki A, Gargouri A (2019) Evaluation of the hepatoprotective effect of combination between fermented camel milk and Rosmarinus officinalis leaves extract against CCl4 induced liver toxicity in mice. J Food Sci Technol 56:824–834
Hcini K, Sotomayor JA, Jordan MJ, Bouzid S (2013) Identification and quantification of phenolic compounds of Tunisian Rosmarinus officinalis L. Asian J Chem 25:9299–9301
Hegaz AM, Abdel-Azeem AS, Zeidan HM, Ibrahim KS, El Sayed EM (2018) Hypolipidemic and hepatoprotective activities of rosemary and thyme in gentamicin-treated rats. Hum Exp Toxicol 37:420–430
Hussain AI, Anwar F, Chatha SAS, Jabbar A, Mahboob S, Nigam PS (2010) Rosmarinus officinalis essential oil: antiproliferative, antioxidant and antibacterial activities. Braz J Microbiol 41:1070–1078
Jongberg S, Tørngren MA, Gunvig A, Skibsted LH, Lund MN (2013) Effect of green tea or rosemary extract on protein oxidation in Bologna type sausages prepared from oxidatively stressed pork. Meat Sci 93:538–546
Kader AF, Kalapuram SP (2017) Ameliorative effect of Emblica officinalis in potassium dichromate induced toxicity in rats. Eur J Pharm Med Res 4:267–274
Khalil S, Awad A, Elewa Y (2013) Antidotal impact of extra virgin olive oil against genotoxicity, cytotoxicity and immunotoxicity induced by hexavalent chromium in rat. Int J Vet Sci Med 1:65–73
Kim J, Seo S, Kim Y, Kim DH (2018) Review of carcinogenicity of hexavalent chrome and proposal of revising approval standards for an occupational cancer in Korea. Ann Occup Environ Med 30:7
Kuhn DM, Aretha CW, Geddes TJ (1999) Peroxynitrite inactivation of tyrosine hydroxylase: mediation by sulfhydryl oxidation, not tyrosine nitration. J Neurosci 19:10289–10294
Li G, Jiang W, Tian J, Qu G, Zhu H, Fu F (2010) In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis. Phytomed 17:282–288
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin Phenol Reagent. J Biol Chem 193:269–275
Lukas J, Lukas C, Bartek J (2011) More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nature Cell Boil 13:1161–1169
Machado DG, Cunha MP, Neis VB, Balen GO, Colla A, Bettio LEB, Oliveira Á, Pazini FL, Dalmarco JB, Simionatto EL, Pizzolatti MG, Rodrigues ALS (2013) Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem 136:999–1005
Malo C, Gil L, Gonzalez N, Martinez F, Cano R, Blasde I, Espinosa E (2010) Anti-oxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 61:142–147
Maran E, Fernandez M, Barbieri P, Font G, Ruiz MJ (2009) Effects of four carbamate compounds on antioxidant parameters. Ecotoxicol Environ Saf 72:922–930
Marouani N, Tebourbi O, Hallégue D, Mokni M, Yacoubi MT, Sakly M, Benkhalifa M, Rhouma KB (2017) Mechanisms of chromium hexavalent-induced apoptosis in rat testes. Toxicol Ind Health 33:97–106
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 34:1–32
Mukesh K, Raikwar P, Manoj S, Anand S (2016) Toxic effect of heavy metals in livestock health. Indian Vet Res Inst Izatnagar 1:28–30
Murthy RC, Saxena DK, Gupta SK, Chandra SV (1991) Ultrastructural observations in testicular tissue of chromium-treated rats. ReprodToxicol 5:443–447
Naemura A, Ura M, Yamashita T, Arai R, Yamamoto J (2008) Long-term intake of rosemary and common thyme herbs inhibits experimental thrombosis without prolongation of bleeding time. Thromb Res 122:517–522
Naimi M, Vlavcheski F, Shamshoum H, Tsiani E (2017) Rosemary extract as a potential anti-hyperglycemic agent: current evidence and future perspectives. Nutrients 9:968
Navya K, Phani Kumar G, Chandrasekhar Y, Anilakumar KR (2018) Evaluation of potassium dichromate (K2Cr2O7) - induced liver oxidative stress and ameliorative effect of Picrorhiza kurroa extract in Wistar albino rats. Bio Trace Element Res 184:154–164
Ngo SN, Williams DB, Head RJ (2011) Rosemary and cancer prevention: preclinical perspectives. Crit Rev Food Sci Nutr 51:946–954
Opdyke DL (1974) Monographs on fragrance raw materials. Food Cos Toxicol 12:517–537
Parmar J, Sharma P, Verma P, Sharma P, Goyal PK (2011) Antitumor and anti-oxidative activity of Rosmarinus officinalis in 7,12 dimethyl benz(a) anthracene induced skin carcinogenesis in mice. Am J Biomed Sci 3:199–209
Principato GB, Asia MC, Talesa V, Rosi G, Giovannini E (1985) Characterization of the soluble alkaline phosphatase from hepatopancreas of Squilla mantis L. Comp Biochem Physiol B 985:801–804
Rajgopal A, Roloff SJ, Burns CR, Fast DJ (2019) The cytoprotective benefits of turmeric, quercetin, and rosemary blend through activation of the oxidative stress pathway. Phcog Mag 15(Suppl S3):449–454
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Saidi M, Aouacheri O, Saka S (2019) Protective effect of Curcuma against chromium hepatotoxicity in rats. Phytothérapie 18:148–155. https://doi.org/10.3166/phyto-2019-0114
Saka S, Aouacheri O (2017) The investigation of the oxidative stress-related parameters in high doses methotrexate-induced albino Wistar rats. J Bioequiv Availab 9:372–376
Samaha SR (2017) Effects of Rosemary extract supplementation on ethanol induced liver injury in adult male albino rat. Al-Azhar Med J 46:373–382
Soudani N, Ben-Amara I, Sefi M, Boudawara T, Zeghal N (2011) Effects of selenium on chromium (VI)-induced hepatotoxicity in adult rats. Exp Toxicol Pathol 63:541–548
Srivastava PA, Narain AS (1985) Catfish blood chemistry under environmental stress. Experimentia 4:855–857
Takaki I, Bersani-Amado LEE, Vendruscolo A, Sartoretto SMM, Diniz SPP, Bersani-Amado CAA, Cuman RKNK (2008) Anti-inflammatory and antinociceptive effects of Rosmarinus officinalis L. essential oil in experimental animal models. J Med Food 11:741–746. https://doi.org/10.1089/jmf.2007.0524
Tan BL, Norhaizan ME, Liew WP, Sulaiman Rahman H (2018) Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol 9:1162
Türkez H, Aydin E (2013) In vitro assessment of cytogenetic and oxidative effects of α-pinene. Toxicol Ind Health 30:1–9
Wang HL, Sun ZO, Rehman RU, Wang H, Wang YF, Wang H (2017) Rosemary extract-mediated lifespan extension and attenuated oxidative damage in drosophila melanogaster fed on high-fat diet. J Food Sci 82:1006–1011
Yarbrough JD, Chambers JE, Robinson KM (1982) Effect of chronic exposures to pesticides on animal system. In: Chambers JE, Yarbrough YD (eds) Alterations in liver structure and function resulting from chronic insecticide exposure. Raven, New York, pp 25–59
Acknowledgements
The authors gratefully acknowledge the Institute of Graduate Studies and Research, Alexandria University, where the research work was done. Also, the work was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Author information
Authors and Affiliations
Contributions
FE: conceptualization, methodology, data generation, manuscript preparation, and publication.
RA: practical work, data analysis, writing - original draft.
MA: investigation, manuscript revision.
Corresponding author
Ethics declarations
Ethical approval
This manuscript includes animal experiments, and the animal handling, housing, and care, as well as the experimental protocol, were approved by Animal Care and Ethics Review Committee at the Faculty of Medicine, Alexandria University, Alexandria, Egypt.
Consent to participate
The authors declare that they have participated in this work.
Consent for publication
The authors declare that they have agreed for the publication of this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Demerdash, F.M., El-Sayed, R.A. & Abdel-Daim, M.M. Hepatoprotective potential of Rosmarinus officinalis essential oil against hexavalent chromium-induced hematotoxicity, biochemical, histological, and immunohistochemical changes in male rats. Environ Sci Pollut Res 28, 17445–17456 (2021). https://doi.org/10.1007/s11356-020-12126-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12126-8