Abstract
Extensive green roofs are urban construction systems that provide thermal regulation and sound proofing for the buildings involved, in addition to providing an urban heat island mitigation or water retention. On the other hand, policies towards reduction of energy consumption, a circular economy and sustainability are core in the European Union. Motivated by this, an experimental study was carried out to evaluate the environmental risk assessment according to release levels of polluting elements on leachates of different green roof substrate mixtures based on recycled aggregates from construction and demolition waste through (i) the performance in laboratory of two procedures: compliance and percolation tests and (ii) an upscaled experimental leaching test for long-term on-site prediction. Four plots were built on a building roof and covered with autochthonous Mediterranean plants in Córdoba, South of Spain. As growing substrate, four mixtures were used of a commercial growing substrate with different proportions of a fine mixed recycled aggregate ranging from 0 to 75% by volume. The results show that these mixtures were classified as non-hazardous materials according to legal limits of the Landfill Directive 2003/33/CE. The release levels registered in extensive green roofs were lower compared to the laboratory test data. This shows how laboratory conditions can overestimate the potential pollutant effect of these materials compared to actual conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
People living in urban areas accounted for 54% of the world population in 2014. In 2050, it is estimated that this figure will reach 66%. Taking into account the global population estimate at that date, this means that, in 2050, 6.5 billion people will be living in cities, two-thirds more than in 2014 (United Nations 2014). This is a growing evidence of the challenging problems attached to this matter, making it crucial to tackle environmental issues in cities.
Green roofs are urban construction systems that are able to provide multiple ecosystem services in order to protect not only buildings involved but especially the environment. The rise of green roofs due to their multiple benefits is widely studied (Getter and Rowe 2006). Green roof benefits include runoff water mitigation, water and air quality improvement, carbon storage and sound proofing, but especially thermal regulation of buildings and urban heat island mitigation. Extensive green roofs are those which are partially or fully covered by a thin and light layer with vegetation and a growing medium over a waterproofing membrane (Santamouris 2014).
On the other hand, construction materials at the end of their useful life become waste that can cause serious environmental problems. The recycling and reuse of this construction and demolition waste (CDW) as new materials contribute to sustainability. These new materials are then called recycled aggregates (RA), which are mainly composed by concrete, natural aggregates, bricks, and some lesser extent other constituents such as gypsum, wood, glass and plastics. The most common applications of these RA are in civil works such as bases and subbases of roads and backfilling, and others such as mortars, concrete and beds of pipes (GERD 2012). In terms of RA nature, the two major RA from CDW are recycled concrete aggregates, which are produced by crushing concrete, and mixed recycled aggregates (MRA), which contains a significant percentage of masonry rubble. In Southern European countries, many architectural interior building elements are ceramic. In Spain, MRA represents over 70% of the total RA production (GERD 2012). In terms of RA particle size, while each CDW treatment plant offers their own size, three types are generally distinguished: fine, course, and the most common, graded aggregate. In a study carried out in Andalusia, Spain, it was found that the fine fraction was only 13.5% of the total RA produced (Public Works Agency of the Regional Government of Andalusia 2015). This fine fraction is underused and is disposed in dumps in the CDW treatment plants in Spain or is used in pipe bending with low embodied energy.
European Union policies towards a circular economy and sustainability are core on taking a resource life cycle approach: to reducing the negative environmental impacts of resource use and to increasing eco-efficiency. These have been established through Directive 2008/98/EC, the legal framework for waste, from generation to disposal, with emphasis on re-use, recycling and other recovery in order to reach 70% by weight of the CDW in these actions by 2020 in the European member states.
The use of RA encompasses environmental risks, however. RA applied on-site in contact with external agents, mainly rainwater, can contaminate ground and surface water (Van der Sloot and Dijkstra 2004; Eikelboom 2006). Total content of a pollutant is not the decisive factor but rather its capacity to be incorporated into the water under certain conditions. Therefore, leaching tests are the established experimental procedures for assessing the environmental risk (Townsend et al. 2003; Wahlström et al. 2000). The European Union Landfill Directive 2003/33/CE, hereinafter called as LD, lays down criteria and procedures for the acceptance of waste at landfills, and the limit values established therein are used as reference for comparison with the results obtained with leaching tests.
Leaching test results in laboratory conditions should not be transferred directly to on-site conditions owing to the circumstances that surround the applied RA. These include the degree of compaction, temperature, contact time with water, aging effect (carbonation) and others (Van der Sloot 2000). The relation between laboratory and on-site leaching test results has been studied by several authors (Schreurs et al. 2000; Engelsen et al. 2012; Izquierdo et al. 2008).
Different studies (Galvín et al. 2012; GEAR 2012; Del Rey et al. 2015) have been carried out in Andalusia, Spain, about the environmental risk assessment of RA from different CDW treatment plants through leaching tests. They found that chromium and sulphate were the most critical elements according to the LD criteria. This was in accordance with Butera et al. (2014) and Van der Sloot (2000), who found that the most conflicting elements were the aforementioned elements and chloride. Galvin et al. (2014) evaluated the effect of compaction on leaching in MRA, concluding that the levels of chromium and sulphate were reduced in a newly designed leaching percolation test under compaction compared to those in a conventional, uncompacted leaching test.
The use of different inert recycled materials in extensive green roof has been studied by several authors. Eksi and Rowe (2016) studied the use of crushed porcelain obtained from demolition projects including broken sinks, toilets, tiles and dishes. It was processed in a crushing plant in order to be used as aggregate concluding that its use could greatly reduce the embodied energy required to construct a green roof and divert waste from landfills. Krawczyk et al. (2017) studied the use of silica waste, a by-product from metallic ferrosilicon alloys, as a growth substrate, resulting in a positive impact on plant growth. Molineux et al. (2009) studied the substitution of the crushed clay brick, typically used in extensive green roofs in United Kingdom as a part of the growing media of vegetation, by alternative recycled materials such as sewage sludge, waste clay, fly ash, paper ash and quarry fines. These materials were mixed with commercial compost in different proportions: 15 and 25%, resulting in a pH decrease of 2.71 units by average. In this research, it was found that the performance of certain substrates could be as good as the crushed clay brick. Their results support the principle that locally sourced recycled materials can provide economically viable alternatives. This leads to the idea that future green roof substrates should be manufactured locally with suitable local secondary materials. Molineux et al. (2015) mentioned that future studies should monitor extensive green roofs using novel recycled substrates. Mickovski et al. (2013) used as growing medium for vegetation a mix of an aggregate from CDW (20%) with inert loam and compost. This laboratory study found no proof of contamination of the water drained through the designed substrate and that green roof drainage water may be suitable for non-potable purposes.
The aim of this research was to evaluate the release levels of polluting elements (12 heavy metals and 3 anions, e.g. sulphate) in leachates from extensive green roofs with fine mixed recycled aggregate (FMRA) as growth substrate. This assessment was made through (i) a compliance and percolation test to verify the material behaviour and (ii) an upscaled experimental leaching test for long-term on-site prediction. Four plots were built on a building roof in Córdoba (south of Spain) (37°54′58.7″N 4°42′55.0″W) and planted with autochthonous Mediterranean plants. As growth substrate, four different mixtures with different proportions of a FMRA, from a nearby CDW treatment plant, and a commercial substrate (CS) were used. To the best of our knowledge, this is the first upscaled experimental leaching study regarding the use of RA in extensive green roof. This on-site verification test for long-term prediction was motivated by the European Committee for Standardization (CEN/TC 292). The percolation leaching test results enable the use of these materials.
Experimental details, materials and test methods
Experimental details of the extensive green roof plots
In this experimental extensive green roof study, four plots were built on a building roof (Fig. 1); each one occupied 14.78 m2 and a thickness of the growth substrate of 10 cm. Each plot was covered by a waterproofing and root barrier membrane, an egg-cup shaped drainage layer, a nonwoven filter fabric and a coir-based erosion control blanket. Twelve autochthonous Mediterranean plants selected by their adaptation to withstand drought stress, intense lighting and extreme heat, shallow and poor substrates due to harsh conditions in urban Mediterranean ecosystems, were distributed to almost 16 plants/m2, following the recommendation of the German Guideline of green roof execution and maintenance (FLL 2008). A drip irrigation system was installed to supply approximately 4 l per m2 for all the plots equally, during the summer (May/June to October). While the details about plant emergency and survival are the subject of ongoing research, all substrates are suitable for plant growth. As can be seen in Fig. 1, an acceptable vegetative cover was reached in all plots after 6 months.
Materials
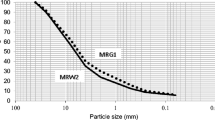
As growth substrate, four mixtures with different proportions of a fine mixed recycled aggregate (FMRA), from a nearby CDW treatment plant, and a commercial growth substrate (CS) were used. The properties of both materials are summarised in Table 1. Physical properties are similar to those studied by Graceson et al. (2014), who used mixtures of inorganic substrates and composted green waste as growth substrate in their extensive green roofs. They presented a maximum size of 4 mm. The granulometric analysis is plotted in Fig. 2.
Four mixtures were elaborated: one composed by CS on its totality, called S100, and others with different percentage of substitution in volume of CS by FMRA: 25% (S75), 50% (S50) and 75% (S25).
To fill the plots with the corresponding amount of growth substrate, the amount of CS and FMRA in the mixtures was determined previously (Table 2). The initial moisture of the materials CS and FMRA were 100 and 6%, respectively. The material of each, once mixed, was not pressed down in any way, but levelled by hand to ensure a substrate thickness of 10 cm.
Leaching test methods
The four substrate mixtures were subjected to three different leaching test methods: two of them performed in laboratory and one leaching test implemented in field.
First, the two raw materials (before mixing) were subjected to the Spanish standard NLT 115/1999 (1999) and the standard UNE-EN 1744–1 (2013) to determine sulphate and chloride content (by Mohr method). Next, the four mixed substrate materials, obtained from mixing different proportions of the raw materials, were subject to laboratory and field testing. The two tests carried out in the laboratory were the Compliance Test (UNE-EN 1744–1 2013) and the Percolation test (NEN 7343: 1994). The test performed in the extensive green roof plots was an upscaled experimental percolation leaching test described below. Thanks to this setup, percolation test results carried out under laboratory and field conditions could be compared and discussed.
The Compliance laboratory leaching test (UNE-EN 12457-3: 2003) procedure consists of a two-step batch leaching test resulting in two liquid/solid ratios (L/S). The dry mass used of the material is 175 g with a particle size < 4 mm. The first step is carried out by stirring for 6 ± 0.5 h with an L/S of 2 l/kg the solution of the material dry mass plus 350 l of deionised water. During the second step, deionised water is added to establish an L/S of 10 l/kg, and the solution is then stirred for another 18 ± 0.5 h. After each step, the samples were filtered (0.45 μm membrane filters), and a subsample of 40 ml of eluate is collected for testing.
The percolation laboratory leaching test (standard NEN 7343: 1994) is designed to simulate the leaching behaviour of a material by relating the accumulated pollutant release (expressed as mg/kg leached) to the L/S ratio. The procedure consists of a seven-step batch leaching test, but in this case, six L/S ratios (0.1, 0.2, 0.5, 1, 2 and 5 l/kg) were carried out. The column (inner diameter of 5 cm and length of 20 cm) is filled with the test material (maximum particle size of 4 mm) and the dry mass used is measured. The deionised water quantity for each step is calculated from the dry matter and the L/S relationship. In the first step, a peristaltic pump (flow rate of 18 ml/h) fills the column with deionised water until the material is saturated, the eluate passes through two filters (a 1.5-μm prefilter and a 0.45-μm filter) to prevent entrainment of fine particles, and a collection flask picks up the leachate corresponding to each L/S ratio. From each flask, a sample of 20 ml of eluate is collected for testing. Conductivity and pH were measured at 22.5 °C (± 2.5 °C).
To perform an upscaled experimental percolation test, in a similar way to standard NEN 7343: 1994, a system was installed to collect the leachate from the different green roof plots in different tanks based on a free drainage system in order to allow quick drainage of excess percolation water from each plot (Fig. 3). Pipes, 40 mm in diameter, were connected to the tanks. The analysed samples were extracted from these tanks. The first five liquid to solid ratios of the column test: 0.1, 0.2, 0.5, 1 and 2 l/kg were sampled and analysed. The amount water needed to reach these L/S is given in Table 3. Samples of 20 ml of eluate were extracted from each tank (Fig. 3), in similar way than in percolation test (standard NEN 7343: 1994), for testing. Conductivity and pH were measured at 22.5 °C (±2 .5 °C).
The samples extracted from compliance, percolation and long-term upscaled experimental percolation tests were kept under cooling conditions before being analysed by inductively coupled plasma mass spectrometry (ICP-MS) using a Perkin Elmer ELAN DRC-e spectrometer for quantifying the 12 heavy metals specified by the LD: Ni, Cr, Sb, Se, Mn, Hg, As, Pb, Cd, Cu, Ba and Zn. The sulphate, fluoride and chloride anion contents were obtained by ion chromatography according to the requirements of standard UNE-EN ISO 10304-1: 2009. These group of metals are the ones specified by the Landfill Directive (LD), and the legal limits are indicated in Table 4, where waste is classified as inert (I), non-hazardous (NH) and hazardous (H).
Results and discussion
Comparison of the laboratory data with Landfill Directive limits
The acceptance criteria of waste at landfills according to LD limits based on the potential contamination, as determined by leaching behaviour, in relation to the Compliance Test and the first eluate of the percolation test (Co) is shown in Table 4.
Table 5 shows the results of the compliance test and the first eluate of the percolation test (Co) for the different substrate mixtures. Values exceeding LD limits are shown in bold. Cd, Hg and Pb content were below the detection limit, so they were not shown. All mixtures were classified as non-hazardous materials. The high level of sulphate in the test results may come from the gypsum and ceramic content in MRA (Del Rey et al. 2015; Sanchez and Alaejos 2009) and from substrate (Vijayaraghavan et al. 2012). Regarding the metal chromium, it has already been detected at high levels in other RA of similar characteristics, specifically MRA (Martins et al. 2015). It has been demonstrated in previous studies that the ceramic particles (mainly bricks and tiles) are the origin of this element in the leachates (Galvín et al. 2013; GEAR 2012). In relation to the chloride content, Butera et al. (2014) and Van der Sloot (2000) found the chloride content as a conflicting element in RA. However, in S100 (100% of CS), the chloride content in compliance test is higher than in the rest of the mixtures, indicating that the origin of the chlorides comes from CS.
Figure 4 shows the release (according to the compliance test) of the most conflictive elements registered: chromium (Fig. 4c), chloride (Fig. 4a) and sulphate (Fig. 4b). Also, the inert limits of the LD are plotted. In the case of the chloride content, LD limits were not complied by the S100 and S75 mixtures (and in all samples for percolation test, see Table 3). In relation to sulphate levels, none of the mixtures comply with any of the limits imposed by the LD. The same happens for the percolation test. Respect to chromium content, the higher levels were measured for the first leachant of the percolation test on S50 and S25 mixtures. Therefore, none of the mixtures, including the prepared with 100% of CS, can be classified as inert but as non-hazardous materials.
Additionally, in order to determine the origin of the sulphate and chloride content, the standards NLT 315/99 and UNE EN 1744-1, respectively, were performed on the original materials constituting the substrate mixtures. FMRA and CS presented a percentage of chloride of 0.011 and 0.086% and a percentage of sulphate 3.47 and 1.31%, respectively. It means that CS presented a chloride content nearly 8 times greater than FMRA. On the other hand, FMRA presented a sulphate content nearly three times greater than CS, which can be expected based on previous research. These have shown how in FMRA, sulphate originates from gypsum and ceramic particles (Del Rey et al. 2015; Barbudo et al. 2012; Jang and Townsend 2001). Additionally, the detection of high levels of sulphate due to the presence of other CDW compounds such as mortar particles has been confirmed by authors such as Sanchez and Alaejos (2009), Ledesma et al. (2014) and De Juan and Gutiérrez (2009).
Comparison between percolation tests: laboratory versus long-term upscaled experimental conditions
In order to evaluate the effect of analysing the pollutant behaviour under actual conditions against laboratory conditions, both experimental methodologies are compared. Thus, Figs. 5, 6, 7 and 8 show the comparison between the obtained levels of cumulative release for chromium, sulphate, chloride and pH, respectively. In addition, LD limits are plotted on these figures.
In Fig. 5, it can be observed that chromium content is related to the percentage of substitution of CS by FMRA in the mixtures. CS was not a substantial source of this metal, in accordance with Berndtsson et al. (2009). Del Rey et al. (Del Rey et al. 2015) studied the performance of FMRA, among other RAs, in percolation tests. They concluded that the LD limit for the first extraction was not met, in agreement with the results presented here for the S25 mix in laboratory, which contained the greater amount of FMRA. Their data also agrees with the total release of this metal in the S25 mixture. More importantly, a general reduction of leachate contents under on-site conditions was observed compared to laboratory conditions. It can also be observed that the first extraction of the percolation test in the S25 mixture was exceeded under laboratory conditions but not under on-site conditions. That is consistent with findings described by Galvin et al. (2014) which report that the effect of controlled conditions during laboratory leaching tests affects the release of contaminants. This increases the differences with tests performed under conditions closer to on-site scenarios and makes it difficult to extrapolate data from laboratory tests (Schreurs et al. 2000).
Figure 6 shows the cumulative release of sulphate comparing data under laboratory and on-site conditions, and inert LD limits. Del Rey et al. (Del Rey et al. 2015) and (Galvín et al. 2014) obtained for fine mixed recycled aggregate values for total release at L/S = 10 above 10,000 mg/kg. These results are in agreement with the laboratory data presented here. This demonstrates the effect of ceramic particles of FRMA on release levels of mixtures. However, according to the results, again a lower cumulative release was observed in all mixtures tested under on-site conditions. This behaviour further confirms the effect of experimental test conditions on the release of elements. In part, this can be attributed to the significant differences between the physical conditions of materials in on-site and laboratory conditions, such as permeability, material density, liquid-solid contact, temperature or composition of the water phase (Van der Sloot and Dijkstra 2004, Tiruta-Barna et al. 2004). In addition, the confinement effect on the columns (which does not occur in the plots) and the application of water by a peristaltic pump with a constant flow rate are increasing the contact of material and the leachant liquid, which could further contribute to explain the higher release levels observed (Galvín et al. 2014).
One notable exception was observed, however. As can be seen in Fig. 7, which shows the cumulative release of chloride comparing data under laboratory and on-site conditions, and inert LD limits, after L/S = 1 l/kg, the on-site release curve was above the laboratory curve. This implies that the cumulative release of chloride did not depend on the conditions of the leaching test. This could be supported by the high mobility of chloride, due to its high solubility (Engelsen et al. 2012). Butera et al. (2014) stated that leached chloride tended to coincide with the chloride total content, confirming the availability control as the main release mechanism. The total cumulative chloride content release in the mixtures varied in L/S = 2 ratio between 420 and 1060 mg/kg, with the maximum corresponding to the S100 and the minimum to the S25. These values are consistent with observations by other authors (Butera et al. 2014; Hyks and Astrup 2009; Izquierdo et al. 2008).
Figure 8 shows the evolution of pH levels as a function of L/S for the four substrate mixtures and compares both laboratory and on-site conditions. As expected, the material S25 made of 75% of FMRA and 25% of CVS gave the most alkaline solutions. The trend of pH of the mixtures indicates that the greater incorporation FMRA was that the higher pH was observed, because of the portlandite Ca(OH)2 in FMRA concrete particles (Engelsen et al. 2012). Molineux et al. (2009) found a decrease in pH for crushed red bricks (9.7) after mixing with a commercial compost (7.6–7.8), in agreement with the results obtained.
Conclusions
In this research, the percolation leaching behaviour of the fine mixed recycled aggregates (FMRA) from CDW was studied in order to make a risk assessment for the use of this material as growth substrate for extensive green roofs. In total, four different substrates were analysed, with substitution levels of FMRA for traditional growing substrate ranging between 0 and 75%. According to the experimental methodology described, the release levels in leachates of polluting elements (12 heavy metals and 3 anions) were evaluated and the following conclusions were drawn:
-
The four mixtures analysed as growth substrate were classified as non-hazardous materials by the compliance laboratory leaching test, being the most conflictive elements: chloride and sulphate anions in all mixtures and chromium in two of them (S25 and S50).
-
Comparing the release data obtained by the percolation leaching test performed in laboratory and the test performed in the extensive green roof plots, the leaching pattern of cumulative release levels of chromium and sulphate were in all materials lower in the upscaled experimental percolation test in plots (closer to actual scenario) compared to those obtained by the laboratory leaching test. It can also be observed that the LD limit of the first leachant of the percolation test was exceeded for chloride and sulphate content in all the mixtures for laboratory conditions, but not for upscaled experimental conditions.
This study about environmental assessment by percolation leaching tests of extensive green roofs with FMRA demonstrated the significant differences between the release data of polluting elements obtained according to leaching tests in laboratory, and the release levels of the upscaled experimental leaching percolation test carried out. The results obtained in this research show how laboratory conditions can overestimate the potential pollutant effect of recycled aggregates.
Extensive green roofs with substitution of CS by FMRA from CDW up to 75% by volume were feasible from the point of view of release of polluting elements to leachates. Thus, this type of application could have an important environmental-friendly potential in the extensive green roof market, contributing to the circular economy and urban sustainability.
References
Barbudo A, Galvín AP, Agrela F, Ayuso J, Jiménez JR (2012) Correlation analysis between sulphate content and leaching of sulphates in recycled aggregates from construction and demolition wastes. Waste Manag 32(6):1229–1235
Berndtsson JC, Bengtsson L, Jinno K (2009) Runoff water quality from intensive and extensive vegetated roofs. Ecol Eng 35(3):369–380
Butera S, Christensen TH, Astrup TF (2014) Composition and leaching of construction and demolition waste: inorganic elements and organic compounds. J Hazard Mater 276:302–311
De Juan MS & Gutiérrez PA (2009) Study on the influence of attached mortar content on the properties of recycled concrete aggregate. Constr Build Mater 23(2):872–877
Del Rey I, Ayuso J, Galvín AP, Jiménez JR, López M, García-Garrido ML (2015) Analysis of chromium and sulphate origins in construction recycled materials based on leaching test results. Waste Manag 46:278–286
Eikelboom R (2006) Challenges Environmental evaluation and use of recycling materials. In: Proceedings of the 6th international conference environmental and technical implications of construction with alternative materials, WASCON, Belgrade
Eksi M, Rowe DB (2016) Green roof substrates: effect of recycled crushed porcelain and foamed glass on plant growth and water retention. Urban For Urban Green 20:81–88
Engelsen CJ, Wibetoe G, van der Sloot HA, Lund W, Petkovic G (2012) Field site leaching from recycled concrete aggregates applied as sub-base material in road construction. Sci Total Environ 427:86–97
FLL (2008) Guideline for the planning, execution and upkeep of green-roof sites (English ed). Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau
Galvín AP, Ayuso J, Jiménez JR, Agrela F (2012) Comparison of batch leaching tests and influence of pH on the release of metals from construction and demolition wastes. Waste Manag 32(1):8–95
Galvín AP, Ayuso J, Agrela F, Barbudo A, Jiménez JR (2013) Analysis of leaching procedures for environmental risk assessment of recycled aggregate use in unpaved roads. Constr Build Mater 40:1207–1214
Galvín AP, Ayuso J, García I, Jiménez JR, Gutiérrez F (2014) The effect of compaction on the leaching and pollutant emission time of recycled aggregates from construction and demolition waste. J Clean Prod 83:294–304
GEAR Project (2012) Spanish guide of recycled aggregates from construction and demolition waste. Spanish association of managers of construction and demolition waste. Ministry of Environment and Rural and Marine Government of Spain
Getter KL, Rowe DB (2006) The role of extensive green roofs in sustainable development. Hortscience 41(5):1276–1285
Graceson A, Hare M, Hall N, Monaghan J (2014) Use of inorganic substrates and composted green waste in growing media for green roofs. Biosyst Eng 124:1–7
Hyks J, Astrup T (2009) Influence of operational conditions, waste input and ageing on contaminant leaching from waste incinerator bottom ash: a full-scale study. Chemosphere 76(9):1178–1184
Izquierdo M, Querol X, Josa A, Vazquez E, López-Soler A (2008) Comparison between laboratory and field leachability of MSWI bottom ash as a road material. Sci Total Environ 389(1):10–19
Jang Y-C, Townsend TG (2001) Occurrence of inorganic pollutants in recovered soil fines from construction and demolition waste. Waste Manag 21:703–715
Krawczyk A, Domagała-Świątkiewicz I, Lis-Krzyścin A, Daraż M (2017) Waste silica as a valuable component of extensive green-roof substrates. Pol J Environ Stud 26(2):643–653
Ledesma EF, Jiménez JR, Fernández JM, Galvín AP, Agrela F, Barbudo A (2014) Properties of masonry mortars manufactured with fine recycled concrete aggregates. Constr Build Mater 71:289–298
Martins IM, Roque AJ, Freire AC, Neves J, Antunes ML (2015) Release of dangerous substances from construction and demolition recycled materials used in road pavements-Laboratory and field leaching tests. In III Progress of Recycling in the Built Environment. RILEM Publications SARL, pp 109–115
Mickovski SB, Buss K, McKenzie BM, Sökmener B (2013) Laboratory study on the potential use of recycled inert construction waste material in the substrate mix for extensive green roofs. Ecol Eng 61:706–714
Molineux CJ, Fentiman CH, Gange AC (2009) Characterising alternative recycled waste materials for use as green roof growing media in the UK. Ecol Eng 35(10):1507–1513
Molineux CJ, Gange AC, Connop SP, Newport DJ (2015) Using recycled aggregates in green roof substrates for plant diversity. Ecol Eng 82:596–604
NEN 7343 (1994) Leaching characteristics of solid earthy and story building and waste materials. Leaching test. Determination of the leaching of inorganic components from granular materials with the column test
NLT 115/1999 (1999) Experimental technical standard for determination of gypsum content in soils. CEDEX. General Directorate of Roads.
Public Works Agency of the Regional Government of Andalusia (2015) Guide of recycled aggregates from construction and demolition waste in Central Andalusia. Spain [in Spanish]. Available online: Accessed on 06 September 2017
Sanchez M, Alaejos P (2009) Study on the influence of attached mortar content on the properties of recycled concrete aggregate. Constr Build Mater 23:872–877
Santamouris M (2014) Cooling the cities–a review of reflective and green roof mitigation technologies to fight heat island and improve comfort in urban environments. Sol Energy 103:682–703
Schreurs JPGM, Van der Sloot HA, Hendriks C (2000) Verification of laboratory–field leaching behavior of coal fly ash and MSWI bottom ash as a road base material. Waste Manag 20(2):193–201
Spanish Waste Management Association (GERD) (2012) Spanish guide of recycled aggregates from CDW. Madrid, Spain
Tiruta-Barna L, Imyim A, Barna R (2004) Long-term prediction of the leaching behavior of pollutants from solidified wastes. Adv Environ Res 8(3):697–711
Townsend TG, Jang YCH, Tolaymat T (2003) Leaching tests for evaluating risk in solid waste management decision making. Florida Center for Solid and Hazardous. Waste Manage Res Report No. 03-01. Gainesville, USA
UNE-EN 12457–3 (2003) Characterization of waste. Leaching. Compliance test for leaching of granular waste materials and sludges. Part 3: Two stage batch at a liquid to solid ratio of 2 l/kg and 8 l/kg for materials with high solid content and with a particle size below 4 mm (without or with size reduction)
UNE-EN 1744–1 (2013) Tests for chemical properties of aggregates - Part 1: Chemical analysis. Chloride content determination by Bohr Method
United Nations (2014) World urbanization prospects: the 2014 revision. Available online: https://esa.un.org/unpd/wup/publications/files/wup2014-highlights.Pdf Accessed on 06 September 2017
Van der Sloot HA (2000) Comparison of the characteristic leaching behavior of cements using standard (EN 196-1) cement mortar and an assessment of their long-term environmental behavior in construction products during service life and recycling. Cem Concr Res 30(7):1079–1096
Van der Sloot HA & Dijkstra JJ (2004) Development of horizontally standardized leaching tests for construction materials: a material based or release based approach? Identical leaching mechanisms for different materials. Energy Research Center of the Netherlands, Report No-. ECN-C-04-060, 44pp. & annexes
Vijayaraghavan K, Joshi UM, Balasubramanian R (2012) A field study to evaluate runoff quality from green roofs. Water Res 46(4):1337–1345
Wahlström M, Laine-Ylijoki J, Määtänen A, Luotojärvi T, Kivekäs L (2000) Environmental quality assurance system for use of crushed mineral demolition wastes in road constructions. Waste Manag 20:225–232
Acknowledgements
The authors would like to express appreciation for the support of the sponsor: the Agency of Public Works of Andalusia, Spain, who funded the project: “Optimizing the potential of green roofs for building rehabilitation: Interaction between recycled substrates, water properties and energy efficiency. Code: GGI3003IDIB” whose results are partially here presented.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
López-Uceda, A., Galvín, A.P., Ayuso, J. et al. Risk assessment by percolation leaching tests of extensive green roofs with fine fraction of mixed recycled aggregates from construction and demolition waste. Environ Sci Pollut Res 25, 36024–36034 (2018). https://doi.org/10.1007/s11356-018-1703-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1703-1