Abstract
This study evaluated the effects of two surfactants (i.e., Tween 80 and SDS) on biodegradation of crude oil by mixed bacterial consortium in soil-aqueous system. The mixed bacterial consortium was domesticated from the activated sludge of cooking plant through a progressive domestication process. High-throughput sequencing analysis revealed that Rhodanobacter sp. was the dominant bacteria. The higher CMCeff value for two surfactants was observed in soil-aqueous system compared with that in aqueous system, which was likely due to their adsorption onto soil particles. Either Tween 80 or SDS can be utilized as carbon source and promote the growth of mixed bacterial consortium. Further findings evidenced that the degradation of crude oil can be enhanced by adding either Tween 80 or SDS. The performance of Tween 80 was generally superior to SDS for the crude oil degradation. The highest crude oil degradation efficiency was 42.2 and 31.0% under the conditions of 5 CMCeff of Tween 80 and 2 CMCeff of SDS, respectively. Furthermore, the degradation efficiency of crude oil in remediation experiment (i.e., 77%) evidenced that the integration of adding Tween 80 and inoculating mixed bacterial consortium was effective for crude oil-contaminated soil decontamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil contamination by crude oil is a serious environmental problem, which mainly arises from accidental spills and discharges of oil or oily waste, posing a potential risk to human health (Urum et al. 2006; Lai et al. 2009). Therefore, numerous studies have been conducted to explore effective technologies to remove them from the contaminated soil (Vasudevan and Rajaram 2001). Among these technologies, bioremediation has a great future due to its non-invasive and cost-effective characteristics (Harayama et al. 2004; Zhang et al. 2015b).
At present, a number of crude oil-degrading bacteria have been isolated and reported in previous studies (Chaillan et al. 2004). A single type of strain normally can only degrade a certain group of hydrocarbons (Van Hamme et al. 2003). However, the degradation of crude oil with numerous fractions requires multi-step reactions carried out step by step and a variety of microorganisms involved (Tzarkova and Groudeva 2000). Some researchers previously reported that biodegradation induced by mixed bacterial consortium was more effective than that by pure strain (Sugiura et al. 1996; Chhatre et al. 1996; Leahy and Colwell 1990). Thus, it becomes of necessity to find a suitable way to obtain mixed bacterial consortium. And such mixed bacterial consortium should be capable of adjusting the complicated conditions, and consequently, a highly efficient degradation efficiency of crude oil could be achieved.
Apart from the isolation of suitable mixed bacterial consortium, the degradation of crude oil is also limited by their low solubility in aqueous phase (Kraakman et al. 2011). This is because that low solubility of crude oil makes them present in soil or other environment as non-aqueous pollutant liquids (NAPLs). But, the organic pollutants can be mainly degraded by microorganisms or some specific enzymes present in aqueous phase where they are available for microbial action (Martienssen and Schirmer 2007). However, most of the current studies focus only on a single issue (exploring high-degradation-efficient technology such as selecting more effective hydrocarbon-degrading consortium or dealing with the properties of the contaminants themselves such as improving their solubility). Recently, some researchers considered both issues and employed some integrated technologies to remediate contaminated soil. The employments of such integrated technologies have achieved satisfactory performance (Deng et al. 2016; Wang et al. 2016). Based on the abovementioned facts, this study was thus performed in which the bioaugmentation technology and the employment of surfactant were combined in order to induce effective consortium and at the same time improve the solubility of crude oil. On the one hand, the isolation of mixed bacterial consortium as well as the feasibility of combining the isolated mixed bacterial consortium with surfactant for crude oil degradation needs to be investigated. On the other hand, although extensive studies about the effect of surfactants on crude oil degradation have been conducted, the results are often contradictory (Celik et al. 2008; Joo and Kim 2013). This implies that relevant research about the effects of different surfactants on crude oil degradation is still not enough to obtain a clear picture.
In this study, the mixed bacterial consortium was obtained throughout the progressive domestication process from long-term benzene-contaminated activated sludge. Nonionic surfactant polyoxyethylene sorbitan monooleate (Tween 80) and anionic surfactant sodium dodecyl sulfate (SDS) have been demonstrated to be effective for treating crude oil contamination (Tian et al. 2016). In order to better understand the role of different surfactants in enhancing crude oil degradation, the effects of both surfactants on crude oil degradation were investigated in order to provide more reference data on how to choose a suitable surfactant with proper dosage to enhance crude oil degradation. Subsequent remediation experiment was conducted to further evaluate the feasibility of simultaneously integrating mixed bacterial consortium and surfactant on remediating crude oil-contaminated soil. It is hoped that the present study could provide environmental engineers with a more effective option for crude oil-contaminated soil remediation in field application.
Materials and methods
Chemicals and soils
The crude oil was obtained from China Petrochemical Corporation Pipeline Storage and Transport Company (Xuzhou, China). It contained alkane, cycloalkanes, aromatics, and other non-hydrocarbon compounds. The proportions were 52, 26, 14, and 8%, respectively. Tween 80 and SDS were obtained from Hanschemical (Nantong, China). Both surfactants were used as received from the supplier without further purification. Chemicals including NH4Cl, KH2PO4, and K2HPO4 were purchased from Shanghai Zhangyun Chemical Co., Ltd. (Shanghai, China). Other chemicals such as MgSO4·7H2O, FeCl3, and CuSO4·5H2O were ordered from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China).
The uncontaminated top soil used in this study was cinnamon soil, which was sampled from the campus of China University of Mining and Technology in May, 2016 (Yu et al. 2007). The GPS coordinates of the soil sampling point are 117°9′10″E and 34°13′4″N. The soil was sampled from 10 to 20 cm below the soil surface using a shovel. The sampled soil was sealed in polyethylene bags and then transported to the laboratory. After air-drying for 5 days, the bulk soil was homogenized and screened using a 60-mesh sieve to remove large particles and debris. An aliquot of 50 g of crude oil was artificially added to 1 kg of soil through a 5-mL methanol carrier, followed by air-drying and homogenization again. Then, the soil was stored in a glass jar for soil aging. The aging period was about 50 days. The concentration of total petroleum hydrocarbon (TPH) and other physical-chemical characteristics of artificial soil are illustrated in Table 1.
Microorganism domestication and identification
Mixed bacterial consortium was screened by the progressive domestication process. The progressive domestication process was referenced from the journal article of Wang et al. (2006) and further modified by adding intermediate substrate catechol. We collected the activated sludge from the coking plant in Xuzhou City Wastewater Treatment System. The activated sludge has been contaminated by crude oil for at least 3 years. Domestication schedule and conditions are shown in Table 2. Activated sludge suspension was diluted with saline, aerated in a plastic bucket for 24 h, and liquid phase acclimation process began. Inorganic nutrient solution and substrate were added every day. The inorganic nutrient solution contained 2 g/L NH4Cl, 2.5 g/L KH2PO4, 0.5 g/L K2HPO4, 1 g/L MgSO4·7H2O, 120 mg/L FeCl3, 50 mg/L H3BO3, 10 mg/L CuSO4·5H2O, 10 mg/L KI, 45 mg/L MnSO4·H2O, 20 mg/L NaMoO4·2H2O, 75 mg/L ZnCl2·4H2O, 50 mg/L CoCl2·6H2O, 20 mg/L AlK(SO4)2·12H2O, 13 mg/L CaC12·2H2O, and 10 mg/L NaCl. The substrate contained toluene, catechol, and crude oil.
After 10 days’ domestication when the crude oil was used as the sole carbon, the structure of bacterial community was tested by high-speed DNA-sequencing. Specifically, 5 mL of inoculum samples were taken for DNA rapid extraction using TIANamp Bacteria DNA Kit in triplicate. The concentration and purity of DNA (OD260/OD280 and OD260/OD230) were determined by UV light meter (754, Shanghai Chengguang Co., Ltd). The DNA was diluted for 10-fold by TE buffer and further used for PCR amplification. Primers were 338F (5-ACTCCTACGGGAGGCAGCAG) and 806R (5-GGACTACVSGGGTATCTAAT-3) (Yang et al. 2012). Among the three samples, the best extraction sample was delegated to Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China) carrying on high-throughput sequencing and sequencing platform is Illumina Miseq. Sequences reported in the present study were submitted to GenBank, and the accession number is SRP083938.
CMC of Tween 80 and SDS
Critical micelle concentration (CMC) values of Tween 80 and SDS in aqueous and soil-aqueous (1:10 w/v) system were measured in a series of Erlenmeyer flasks, in which Tween 80 and SDS at different dosages (i.e., 25, 50, 100, 150, 300, 600, 1200, and 2500 mg/L) were added into the two systems. Meanwhile, the groups without addition of either Tween 80 or SDS addition were used as control. All Erlenmeyer flasks were shaken for 48 h. The surface tension of the two systems was analyzed by a surface tension meter (JYW-200A, Chengde Jinhe Instrument Manufacturing Co., Ltd., China). As for a given surfactant concentration, the plotted surface tension value was recorded when stable reading was achieved by at least three consecutive measurements having nearly the same value. The CMC value of each surfactant was obtained through a conventional plot of the surface tension versus the logarithm value of the surfactant concentration, over a wide concentration range. All measures were conducted in triplicate at room temperature.
Culture growth in the presence of surfactant
In order to explore whether surfactants could be utilized as carbon sources by bacteria and whether surfactants at high concentrations would cause inhibition effects on culture growth, growth experiment of mixed bacterial consortium was determined in Erlenmeyer flasks with surfactant under three conditions: (i) without addition of any surfactant, (ii) addition of Tween 80, and (iii) addition of SDS. A total of 100 ml of sterilized surfactant solution (Tween 80 or SDS) with various concentrations (50, 100, 300, 600, and 1200 mg/L) was poured into a series of 250-mL Erlenmeyer flasks. Meanwhile, mixed bacterial consortium was inoculated to the solution and the bacterium suspension density was kept at around 0.1 g/L. LB medium contained 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl. After inoculation, the flasks were incubated in a rotary shaker (180 rpm) set at 25 °C. An aliquot of 3 mL was withdrawn after culturing for 6, 12, 18, 24, 36, 48, 60, and 72 h before absorbance measurement. Meanwhile, the value obtained before culturing was used as the control. In this study, mixed liquor volatile suspended solids (MLVSS) value was used to refer to the bacterial suspension density. The measurement of absorbance (OD600) was performed at 600 nm using a visible spectrophotometer (722, Shanghai Sunny Hengping Scientific Instrument Co., Ltd., China) (Wiegand et al. 2008). The bacterial density was calculated according to the corresponding absorbance between OD600 and MLVSS.
Biodegradation experiments in the presence of surfactant
In order to select suitable surfactant and the corresponding optimum concentration for maximizing crude oil degradation efficiency, the effects of surfactant addition on crude oil biodegradation were evaluated in soil-aqueous system amended with surfactant at 0.25, 0.5, 1, 2, and 5 CMC. The bacterium suspension density was kept at 0.1 g/L in the experiment. The soil-aqueous system was prepared by adding 10 g of sterilized soil into 100 ml of deionized water. Tween 80 and SDS were added right after inoculation, respectively. Then, the flasks were further incubated in a rotary shaker (180 rpm) at 25 °C for 72 h. Crude oil concentration analysis in liquid and soil phases was measured by Fourier transform infrared (FTIR) spectrometer (Thermo iS5, Nicolet Instrument Corporation, USA). Crude oil samples were extracted with CCl4, and then were transferred to 4 cm cuvette. Using CCl4 as the reference solution, the absorbance (A2930, A2960, and A3030) of crude oil was measured by FTIR at the wavenumber of 2930, 2960, and 3030 cm−1 (Huang et al. 2014). The crude oil concentration was calculated from the following equation:
where C (mg/L) is the crude oil concentration in the sample; X, Y, Z, and F are the correction factors; A2930, A2960, and A3030 are the absorbance of liquid extract at corresponding wavenumber, V0 (mL) is the volume of extract solvent; V W (mL) is the volume of sample; and D is the dilution ratio of extract. Degradation efficiency of crude oil was calculated from the following equation:
where C0 (mg/L) is the initial crude oil concentration in contaminated soil, and C t (mg/L) is the crude oil concentration at the incubation time t.
Remediation experiments of crude oil-contaminated soil
During the bioremediation practice, the degradation efficiencies of organic contaminant in soil can be always affected by the bioavailability of nutrients (Oh et al. 2001). C/N/P ratio of 100:4:0.67 has been verified to be most suitable for our remediation experiments, which was determined by our preliminary study (Fig. A1). Under the circumstances, remediation experiments of crude oil-contaminated soil were conducted in the container under four conditions: untreated soil as control (S0), soil amended with mixed bacterial consortium inoculation (S1), soil amended with mixed bacterial consortium inoculation and Tween 80 (S2), and soil amended with Tween 80 (S3). For each experiment group, 1 kg of crude oil-contaminated soil with an initial crude oil concentration of 5% (w/w) was added, and 10% (v/w) of bacterial solution was inoculated. The experiment was conducted at 30–35 °C and the pH was 7–8. The nutrient solution was added every 5 days. The soil was turned over once a week in order to ensure sufficient oxygen content throughout the experiment. The crude oil concentration was measured at 1-day interval using FTIR spectrometer as described above. All experiments were performed in triplicate in order to gain reliable data. The data presented here and in tables and figures is the arithmetic mean value of the triplicates. The linear relationship is defined by the coefficient of determination (R2) equal or greater than 0.990.
Results and discussion
Identification of microorganism
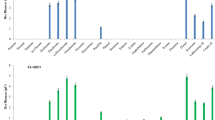
In this study, activated sludge from coking wastewater treatment plant was employed as inoculation source in which the bacterial communities were identified using high-throughput sequencing technology. A total number of 278 OTUs were found in the class level, and these OTUs revealed a diverse bacterial community. Alphaproteobacteria and gammaproteobacteria were the dominant classes and the corresponding percentages of them were 42 and 35%, respectively (Fig. 1). These results were in line with previous studies, in which Alphaproteobacteria and Gammaproteobacteria-related species were commonly found in the environment long-term contaminated by crude oil (Zhang et al. 2015a). The bacteria from these two classes were further identified at the genus level (Fig. 1). Among them, Rhodanobacter sp. was the dominant species, which accounted for 34%. Rhodanobacter has been previously reported to be one of the dominant species in crude oil plus dispersant (Corexit 9500A) microcosms after 30 days of incubation (Al-Jawasim et al. 2015). Apart from Rhodanobacter-related species, Mycobacterium sp. and unclassified rhizobiales were also detected to be abundant in the soil, and the percentages of them were 12 and 11%, respectively. Both bacteria were also reported to be capable of utilizing petroleum as carbon sources (Vila et al. 2001; Zhao et al. 2011). It can be seen that the bacteria which were capable of degrading crude oil already exists in the activated sludge. It is reasonable since the indigenous microorganisms would have been naturally selected in the environment long-term exposed to the contaminants (Xiang et al. 2009). This confirms the feasibility of employing the activated sludge as inoculation source to obtain functional mixed bacterial consortium for soil remediation.
Selection of surfactants for enhancing crude oil degradation in soil
CMC of Tween 80 and SDS in aqueous and soil-aqueous system
CMC value is known as one of the key properties of surfactants, since numerous studies have shown that the solubilization of hydrophobic organic compounds cannot be effectively enhanced until the concentration of surfactant is higher than CMC (Zhong et al. 2016; Iglesias et al. 2014; Bramwell and Laha 2000). Therefore, it is highly necessary to consider the CMC of Tween 80 and SDS before selecting suitable one for solubilizing crude oil from contaminated soil.
The changes of surface tension with different concentrations of Tween 80 and SDS in aqueous and soil-aqueous system are shown in Fig. 2. All conditions reflected a common law that surface tension gradually decreased and then tended to be gentle with increasing the concentration of surfactants. The inflection point exactly corresponded to the CMC of surfactants. CMC and CMCeff have been usually used to describe the CMC of surfactant in aqueous and soil-aqueous system, respectively (Mouton et al. 2009). Thus, in aqueous system, the CMC values of Tween 80 and SDS were 25 and 1250 mg/L, respectively. While in the soil-aqueous system, their CMCeff values reached 75 and 2500 mg/L, respectively. Regardless of either aqueous or soil-aqueous system, a huge difference was detected between Tween 80 and SDS. This is mainly because that anionic surfactant (i.e., SDS) has to overcome the repulsion force between ions so that the micelle was not easy to form (Panya et al. 2005). In the soil-aqueous system, soil particles are negatively charged, and the anionic surfactant SDS is also negatively charged after ionization. The repulsion between them is unfavorable to the adsorption of SDS on soil particles (Rao et al. 2006). Therefore, SDS exhibits lower adsorption compared to Tween 80. On the other hand, the results show that the CMCeff values of either Tween 80 or SDS in soil-aqueous system were higher than the corresponding ones in aqueous system. When surfactants entered the soil-aqueous system, a certain amount of surfactant would be inevitably adsorbed by soil particles, which means that there was a certain loss of surfactant due to the adsorption by soil. That was to say, the more amount of surfactant was adsorbed, consequently, the less amount of surfactant was conducive to the solubilization of organic compounds. Therefore, compared with crude oil-contaminated water, higher dosage of surfactants is needed in order to achieve the similar solubilization efficiency on crude oil in contaminated soil.
Growth condition of bacterial colony in different concentrations of Tween 80 and SDS
The changes of bacterial density with different concentrations of Tween 80 and SDS are shown in Fig. 3. In the control group without any addition, bacteria gradually declined with incubation time (Fig. 3a). When the concentrations of Tween 80 were 50 and 100 mg/L, the bacterial density sharply increased from 0.10 g/L to 0.20 and 0.24 g/L, respectively, after 6 h of incubation. The bacterial density further increased to 0.24 and 0.26 g/L when the incubation period prolonged to 24 h and then gradually decreased until the end of the experiment (i.e., 72 h). Interestingly, when the concentration of Tween 80 was equal or above 300 mg/L, the growth of bacterial colony was not as good as that with Tween 80 concentrations of 50 and 100 mg/L until the incubation period prolonged to 18–24 h. However, after passing the adaptation period, bacterial density fleetly increased to 0.47 g/L and then kept stable throughout the whole incubation period. In general, the results illustrate that the bacterial growth can be stimulated with the addition of Tween 80 alone. And the bacterial density increased with raising the Tween 80 concentration. This suggests that the bacteria can utilize Tween 80 as carbon source. Further, the delaying effect of Tween 80 on bacterial growth was observed when high concentration of Tween 80 (i.e., 300, 600, and 1200 mg/L) was introduced to the bacteria-water mixed solution but extending the incubation time could overcome such delaying effect.
Figure 3b shows that when the concentrations of SDS were 50, 100, and 300 mg/L, bacterial density increased with raising the concentration of SDS. This suggests that low concentration of SDS could also be utilized by bacteria as carbon source. When the concentration of SDS was 600 mg/L, the delaying effect of SDS on bacterial growth can also be observed, similar with the group dosing Tween 80 at concentrations above 300 mg/L. However, in contrast to Tween 80, bacterial density showed a continuous decline trend throughout the whole incubation period when the concentration of SDS was 1200 mg/L, demonstrating that a high concentration of SDS had disincentive effect on the growth of bacteria colony.
Effect of different concentrations of Tween 80 and SDS on crude oil degradation in soil-aqueous system
In this part of study, different concentrations of Tween 80 and SDS in terms of their corresponding CMC values were employed in order to explore their effects on crude oil degradation. Figure 4a shows that when the concentration of Tween 80 was below CMCeff (i.e., 0.25, 0.5, and 1 CMCeff), the crude oil degradation was inhibited in soil-aqueous system, while the crude oil degradation was promoted when the concentration of Tween 80 was higher than CMCeff (i.e., 2 and 5 CMCeff). When the concentration of Tween 80 was 5 CMCeff, the degradation efficiency of crude oil was the highest among all Tween 80 groups. The results show that Tween 80 above CMCeff could promote the crude oil degradation and a higher concentration of Tween 80 achieved a better performance of crude oil degradation. Figure 4b shows that the degradation efficiencies of crude oil in soil-aqueous system were also affected by different concentrations of SDS. When the concentration of SDS was 2 CMCeff, the degradation efficiency of crude oil was the highest among all SDS groups. While the concentration of SDS was lower or higher than 2 CMCeff, the degradation of crude oil was inhibited. To sum up, when the concentrations of Tween 80 and SDS were 5 CMCeff (375 mg/L) and 2 CMC (5000 mg/L), the degradation efficiencies of crude oil after 72 h were highest (42.2 and 31.0%, respectively). The experimental results evidently show that comparing with SDS addition, the degradation efficiency of crude oil was higher when Tween 80 was added. Meanwhile, lower dosage of Tween 80 was used. Therefore, we chose Tween 80 with the concentration of 5 CMCeff (375 mg/L) as surfactant in the subsequent remediation experiment.
The roles of Tween 80 and SDS in regulating crude oil degradation were further tentatively discussed herein. When the concentrations of Tween 80 were below CMCeff (i.e., 0.25 and 0.5 CMCeff), the degradation efficiencies of crude oil were even lower than that of control group. On account of the non-existent solubilization effect in such cases, the appearance of the results is likely due to the carbon competition between Tween 80 and crude oil. It has been found that Tween 80 can be utilized as carbon source for bacterial growth (Fig. 3a). This further confirms that the degradation of crude oil can be negatively affected by the carbon competition induced by Tween 80 at a lower dosage. When the concentrations of Tween 80 were over CMCeff (i.e., 2, 5 CMCeff), apart from the carbon competition between Tween 80 and crude oil, the solubilization effect was brought in the soil-aqueous system. Solubilization effect could promote the crude oil degradation, while carbon competition could inhibit the crude oil degradation. Under these circumstances, the degradation efficiencies of crude oil were apparently enhanced, which were much higher than that of the control group. This indicates that the promotion effect caused by solubilization was more significant than the inhibition effect caused by carbon competition. Different from Tween 80, it has been found that when the concentration of SDS was over 0.25 CMCeff (about 600 mg/L), the disincentive effect on the growth of bacteria colony caused by SDS would occur. This can be evidenced by Fig. 3b. Thus, it is reasonable that the degradation efficiencies of crude oil were even lower than that of the control group at the SDS concentrations below CMCeff (i.e., 0.25 and 0.5 CMCeff) due to both negative effects including the microbial disincentive effect and carbon competition. When the concentration of SDS was above CMCeff, solubilization effect began to appear. At the SDS concentration of 2 CMCeff, the degradation efficiency of crude oil was relatively higher among all the groups. This indicates that in such condition, the solubilization effect instead of microbial disincentive effect or carbon competition plays a predominant role in promoting the crude oil degradation. It has been found that the microbial disincentive effect increased when increasing the SDS concentration (Fig. 3b). The negative effect caused by the microbial disincentive effect and the carbon competition seems to overwhelm the solubilization effect. Although SDS could accelerate the dissolution of crude oil, the functional mixed bacterial consortium which was involved in crude oil degradation would lose the degradation function because of the microbial disincentive effect of SDS (Zhu et al. 2014). Hence, the degradation efficiency of crude oil decreased and even was lower than the control group. In conclusion, the role of the surfactant in regulating crude oil degradation was affected by the interaction of solubilization effect, carbon competition, and microbial disincentive effect (if any) (Fig. A2), likely depending on the dosage of the surfactant.
Evaluation of remediation performance
Before the introduction of mixed bacterial consortium, the amount of indigenous microorganisms in the crude oil-contaminated soil was determined to be 4.6 × 104 CFU/g dry soil. The integration of Tween 80 and mixed bacterial consortium were further conducted in order to evaluate the feasibility of employing this integrated approach in soil remediation. Figure 5 illustrates the degradation efficiency of crude oil content during the remediation process. For control S0 group (i.e., untreated soil), only 5.4% of crude oil was degraded which might be due to the volatility of short chain and oxidation by air. The degradation efficiency in S1 group (i.e., soil amended with mixed bacterial consortium inoculation) (46%) was significantly higher than that in S0 group during the whole remediation experiments (analyzed by ANVOA test, p < 0.05), indicating that the mixed bacterial consortium was able to quickly adapt to the new environment contaminated by oil and exhibited high capacity to degrade crude oil. The conclusion could be further confirmed by an additional short-term bioremediation experiment. In Fig. A3, the amount of bacteria increased from 5.03 × 106 to 1.43 × 108 CFU/g dry soil during a 20-day bioremediation. It can be seen that the mixed bacteria could grow well which can, in turn, facilitate the degradation of crude oil depending on their own ability. As for S2 group (i.e., soil amended with mixed bacterial consortium inoculation and Tween 80), the degradation efficiency had a further enhancement as compared with that in S0 and S1 group and reached 77% after 80 days’ treatment. Alarcón et al. (2008) found that the degradation efficiency of crude oil was highest (59%) after 80 days in a treatment, which was far below our experimental result. As for S3 group (i.e., soil amended with Tween 80), to a certain extent, the introduction of surfactant Tween 80 enhanced the crude oil degradation efficiency of indigenous bacteria (Fig. A4). The reason may be that Tween 80 could be utilized as carbon source by indigenous bacteria and promote the growth of indigenous bacteria, which was consistent with the results of Fig. 3. As discussed above, Tween 80 played an important role in enhancing the solubility of crude oil, and thus increased their bioavailability to the added mixed bacterial consortium. The rapid proliferation of microorganism might contribute to the high degradation efficiency of crude oil in S2 group. During the first 40 days of remediation, degradation efficiency of the two treatment groups increased faster. Since in early time after incubation, there was abundant supply of carbon source in contaminated soil, and microorganism could degrade long-chain alkenes or straight-chain alkenes in petroleum hydrocarbon priority (Chang et al. 2009). From day 40 to day 80 of remediation, the curve of degradation efficiency trended to be flat. Possible reasons may be as follows: (1) the crude oil which was easy to be degraded has been already completely consumed by mixed bacterial consortium (Zahed et al. 2010); (2) toxic intermediate metabolites or byproduct might be produced and accumulated (Zhu and Aitken 2010); (3) with the consumption of carbon source, the activity of mixed bacterial consortium markedly decreased (Yang et al. 2010). The variation of soil physical regime after remediation experiments of crude oil for 80 days can be seen in Fig. 6. Soil sample in S0 group is still black lumps and contaminated soil can be clearly seen in natural light. The color of soil in S2 group looked yellow and texture was loosest among all the three groups. Besides, the oil agglomeration was not observed in the soil sample in S1 and S2 groups. This further confirmed that the contamination extent of crude oil in these groups was effectively mitigated.
Comparing with S0 group, the results of S1 group showed that the domesticated mixed bacterial consortium could effectively degrade crude oil, and thus confirmed the feasibility of employing the activated sludge as inoculation source to obtain functional mixed bacterial consortium for soil remediation. Also comparing with S0 group, it is speculated from the results of S3 group (Fig. A4) that Tween 80 may serve as the carbon source of indigenous bacteria and motivate indigenous bacteria to degrade crude oil by increasing the bioavailability of crude oil. Comparing with S1 group, the results of S2 group indicate that adding Tween 80 could enhance the degradation of crude oil. It could be confirmed that during the short remediation time, the degradation effect was derived from the solubilization of Tween 80. Comparing with S0 and S1 groups, the results of S2 group demonstrated that the integration of mixed bacterial consortium and Tween 80 was a promising technology for crude oil-contaminated soil remediation.
Conclusions
In this study, the integration of mixed bacterial consortium and Tween 80 demonstrated a potential for crude oil removal from the contaminated soil. The mixed bacterial consortium revealed their ability on crude oil biodegradation, in which 48.9% of crude oil was degraded within 80-day incubation. Tween 80 and SDS could be utilized as carbon source for bacterial growth. However, high concentration of SDS had disincentive effect on the growth of bacteria colony. Tween 80 was superior to SDS considering their effects on degradation efficiency of crude oil. It was further found that the predominant role of Tween 80 in crude oil was solubilization instead of competition with crude oil as a carbon source within the first 7 days of treatment. The feasibility of simultaneously employing mixed bacterial consortium and Tween 80 was confirmed, in which as high as 77% of crude oil was degraded. It was much higher than the degradation efficiency of crude oil when only mixed bacterial consortium or Tween 80 was employed. It inspires us an idea that providing more effective technique and improving contaminant natures should be considered synthetically in the real practice in order to obtain a desirable remediation performance.
References
Alarcón A, Davies Jr FT, Autenrieth RL, Zuberer DA (2008) Arbuscular mycorrhiza and petroleum-degrading microorganisms enhance phytoremediation of petroleum-contaminated soil. Int J Phytoremediat 10:251–263
Al-Jawasim M, Yu K, Park JW (2015) Synergistic effect of crude oil plus dispersant on bacterial community in a louisiana salt marsh sediment. FEMS Microbiol Lett 362. https://doi.org/10.1093/femsle/fnv144
Bramwell DAP, Laha S (2000) Effects of surfactant addition on the biomineralizationand microbial toxicity of phenanthrene. Biodegradation 11:263–277
Celik GY, Aslim B, Beyatli Y (2008) Enhanced crude oil biodegradation and rhamnolipid production by Pseudomonas stutzeri strain G11 in the presence of Tween-80 and triton X-100. J Environ Biol 29:867–870
Chaillan F, Le Flèche A, Bury E, Phantavong YH, Grimont P, Saliot A, Oudot J (2004) Identification and biodegradation potential of tropical aerobic hydrocarbon-degrading microorganisms. Res Microbiol 155:587–595
Chhatre S, Purohit H, Shanker R, Khanna P (1996) Bacterial consortia for crude oil spill remediation. Water Sci Technol 34:187–193
Chang WN, Liu CW, Liu HS (2009) Hydrophobic cell surface and bioflocculation behavior of Rhodococcus erythropolis. Process Biochem 44:955–962
Deng F, Xiong BL, Chen BS, Zheng GC, Zhang JZ (2016) Microbial degradation of endosulfan in contaminated soil with the elution of surfactants. Environ Sci Pollut Res 23:13268–13275
Harayama S, Kasai Y, Hara A (2004) Microbial communities in oil-contaminated seawater. Curr Opin Biotechnol 15:205–214
Huang MF, Xing XF, Zhao ZL, Li ZQ (2014) Dynamic monitoring of water petroleum substance using HJ-1/CCD remote sensing data. IOP Conf Ser: Earth Environ Sci 17:012104
Iglesias O, Sanromán MA, Pazos M (2014) Surfactant-enhanced solubilization and simultaneous degradation of phenanthrene in marine sediment by electro-Fenton treatment. Ind Eng Chem Res 53:2917–2923
Joo MH, Kim JY (2013) Characteristics of crude oil biodegradation by biosurfactant-producing bacterium Bacillus subtilis JK-1. J Korean Soc Appl Biol 56:193–200
Kraakman NJR, Rocha-Rios J, van Loosdrecht MCM (2011) Review of mass transfer aspects for biological gas treatment. Appl Microbiol Biotechnol 91:873–886
Lai CC, Huang YC, Wei YH, Chang JS (2009) Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater 167:609–614
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315
Martienssen M, Schirmer M (2007) Use of surfactants to improve the biological degradation of petroleum hydrocarbons in a field site study. Environ Technol 28:573–582
Mouton J, Mercier G, Blais JF (2009) Amphoteric surfactants for PAH and lead polluted-soil treatment using flotation. Water Air Soil Pollut 197:381–393
Oh YS, Sim DS, Kim SJ (2001) Effects of nutrients on crude oil biodegradation in the upper intertidal zone. Mar Pollut Bull 42:1367–1372
Panya P, Wanless EJ, Arquero OA, Franks GV (2005) The effect of ionic surfactant adsorption on the rheology of ceramic glaze suspensions. J Am Ceram Soc 88:540–546
Rao PH, He M, Yang X, Zhang YC, Sun SQ, Wang JS (2006) Effect of an anionic surfactant on hydraulic conductivities of sodium-and calcium-saturated soils. Pedosphere 16:673–680
Sugiura K, Ishihara M, Shimauchi T, Harayama S (1996) Physicochemical properties and biodegradability of crude oil. Environ Sci Technol 31:45–51
Tian W, Yao J, Liu RP, Zhu MJ, Wang F, Wu XY, Liu HJ (2016) Effect of natural and synthetic surfactants on crude oil biodegradation by indigenous strains. Ecotoxicol Environ Saf 129:171–179
Tzarkova EK, Groudeva VI (2000) Bioremediation of petroleum-polluted soils by adsorptive immobilized corynebacterium sp. RB-96. Biotechnol Biotechnol Equip 14:72–75
Urum K, Grigson S, Pekdemir T, McMenamy S (2006) A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere 62:1403–1410
Van Hamme JD, Singh A, Ward OP (2003) Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67:503–549
Vasudevan N, Rajaram P (2001) Bioremediation of oil sludge-contaminated soil. Environ Int 26:409–411
Vila J, López Z, Sabaté J, Minguillón C, Solanas AM, Grifoll M (2001) Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp. strain AP1: actions of the isolate on two-and three-ring polycyclic aromatic hydrocarbons. Appl Environ Microbiol 67:5497–5505
Wang LP, Hua SL, Zhao YM, Zhu YL, Xu LQ (2006) Acclimation of high biodegradability consortium of benzene toluene and xylene in a biotrickling filter. J China Univ Min Technol 35:633–636
Wang LW, Li F, Zhan Y, Zhu LZ (2016) Shifts in microbial community structure during in situ surfactant-enhanced bioremediation of polycyclic aromatic hydrocarbon-contaminated soil. Environ Sci Pollut Res 23:14451–14461
Wiegand I, Hilpert K, Hancock RE (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175
Xiang TS, Liu XB, Zhang M, Liu F, Li BG, Fu HE, Zhao L, Hu WS (2009) Distribution of the indigenous microorganisms and mechanisms of their orientational activation in Daqing oilfield. Sci China Ser D Earth Sci 52:128–134
Yang JC, Zhang L, Fukuzaki Y, Hira D, Furukawa K (2010) High-rate nitrogen removal by the Anammox process with a sufficient inorganic carbon source. Bioresour Technol 101:9471–9478
Yang X, Zhang W, Li SW, Zhang MX, Liu GX, Chen T, Hu P, Wu XK, Tai XS, Chen W (2012) Study on isolation, identification and physiological characteristics of PAHs-degrading bacteria. Acta Sci Circumst 32:1033–1040
Yu FZ, You HM, Li BJ, Zhao W (2007) Analysis of physicochemistry properties of greenbelt-soil different city zones in Xuzhou City. Res Soil Water Conserv 14:85–88
Zahed MA, Aziz HA, Isa MH, Mohajeri L (2010) Enhancement biodegradation of n-alkanes from crude oil contaminated seawater. Int J Environ Res 4:655–664
Zhang Z, Lo IMC, Yan DYS (2015a) An integrated bioremediation process for petroleum hydrocarbons removal and odor mitigation from contaminated marine sediment. Water Res 83:21–30
Zhang Z, Zheng GY, Lo IMC (2015b) Enhancement of nitrate-induced bioremediation in marine sediments contaminated with petroleum hydrocarbons by using microemulsions. Environ Sci Pollut Res 22:8296–8306
Zhao DF, Liu CS, Liu LH, Zhang YB, Liu QY, Wu WM (2011) Selection of functional consortium for crude oil-contaminated soil remediation. Int Biodeterior Biodegrad 65:1244–1248
Zhong H, Yang X, Tan F, Brusseau ML, Yang L, Liu ZF, Zeng GM, Yuan XZ (2016) Aggregate-based sub-CMC solubilization of n-alkanes by monorhamnolipid biosurfactant. New J Chem 40:2028–2035
Zhu HB, Aitken MD (2010) Surfactant-enhanced desorption and biodegradation of polycyclic aromatic hydrocarbons in contaminated soil. Environ Sci Technol 44:7260–7265
Zhu CX, Guan FC, Wang C, Jin LH (2014) The protective effects of Rhodiola crenulata extracts on Drosophila melanogaster gut immunity induced by bacteria and SDS toxicity. Phytother Res 28:1861–1866
Acknowledgements
Acknowledgment attributed to National Natural Science Foundation of China (grant no. 51778612) and National Natural Science Foundation for Young Scientists of China (grant no. 51608531).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
ESM 1
(DOC 421 kb)
Rights and permissions
About this article
Cite this article
Xu, R., Zhang, Z., Wang, L. et al. Surfactant-enhanced biodegradation of crude oil by mixed bacterial consortium in contaminated soil. Environ Sci Pollut Res 25, 14437–14446 (2018). https://doi.org/10.1007/s11356-018-1604-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1604-3