Abstract
The effect of microemulsion on the biodegradation of total petroleum hydrocarbons (TPH) in nitrate-induced bioremediation of marine sediment was investigated in this study. It was shown that the microemulsion formed with non-ionic surfactant polyoxyethylene sorbitan monooleate (Tween 80), 1-pentanol, linseed oil, and either deionized water or seawater was stable when subjected to dilution by seawater. Desorption tests revealed that microemulsion was more effective than the Tween 80 solution or the solution containing Tween 80 and 1-pentanol to desorb TPH from marine sediment. In 3 weeks of bioremediation treatment, the injection of microemulsion and NO3 − seems to have delayed the autotrophic denitrification between NO3 − and acid volatile sulfide (AVS) in sediment compared to the control with NO3 − injection alone. However, after 6 weeks of treatment, the delaying effect of microemulsion on the autotrophic denitrification process was no longer observed. In the meantime, the four injections of microemulsion and NO3 − resulted in as high as 29.73 % of TPH degradation efficiency, higher than that of two injections of microemulsion and NO3 − or that of four or two injections of NO3 − alone. These results suggest that microemulsion can be potentially applied to enhance TPH degradation in the nitrate-induced bioremediation of marine sediment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum hydrocarbons are an important group of organic contaminants occurring in the environment. They mainly consist of numerous hydrophobic hydrocarbons, including linear (normal or n-), branched (iso- or i-), and cyclic alkanes and alkenes, mono- and polyaromatic compounds, resins, and asphaltenes (Frysinger et al. 2003) and have been demonstrated to be bioaccumulative (Rowland et al. 2001; Muijs and Jonker 2010) and toxic (Brils et al. 2002; Jonker et al. 2006). The contamination of marine sediment by petroleum hydrocarbons is widespread in many coastal areas due to oil spills, natural seepage, shipping, offshore drilling, and other human activities (Richardson et al. 2001; Richardson et al. 2003; Jonker et al. 2006).
In anaerobic sedimentary environments, a preferred option for stimulating the degradation of organic contaminants is to add some electron acceptors to provide microorganisms with a more energetically favorable mechanism for hydrocarbon oxidation (USEPA 2005). Nitrate-induced bioremediation has been shown to be an attractive option for remediating the contaminated marine sediment (Hutchins 1991; Wilson et al. 1997; Hutchins et al. 1998; Shao et al. 2010). Nitrate, as an electron acceptor in nitrate-induced bioremediation, is more than ten thousand times more soluble than oxygen in terms of electron-accepting capacity, and it can yield free energy almost as much as that under aerobic conditions (Lu et al. 2011). Previous studies have revealed that NO3 − stimulated the growth of both heterotrophic and autotrophic denitrifiers, and the former can out-compete sulfate-reducing bacteria for organics degradation (Eq. 1), while the latter can oxidize sulfide (Eq. 2) (Zhang et al. 2009; Mbadinga et al. 2011). The coexistence of heterotrophic and autotrophic denitrification processes can result in the reduction of sulfide in sediment and thus suppression of odor as well as the biodegradation of organics such as petroleum hydrocarbons in sediment (Shao et al. 2009; Zhang et al. 2009; Shao et al. 2011). However, it was reported that the denitrification process using NO3 − as the electron acceptor is mostly useful for the biodegradation of short-chain alkanes rather than long-chain alkanes (Coates et al. 1997; Eriksson et al. 2003; Lei et al. 2005; Yuan and Chang 2007). Also, previous studies have demonstrated that low molecular weight (LMW) PAHs, such as naphthalene and phenanthrene, can be easily biodegraded under a nitrate-reducing condition, but there was almost no enhancing effect on the removal of high molecular weight (HMW) PAHs (Johnson and Ghosh 1998; Lei et al. 2005).
One of the possible reasons that lead to the low biodegradation efficiency of petroleum hydrocarbons especially the high molecular weight fractions is their low solubility (Villemur et al. 2000; Lu et al. 2011), which makes them present as non-aqueous pollutant liquids (NAPL) (Martienssen and Schirmer 2007). The organic pollutants can be mainly degraded by microorganisms or some specific enzymes present in solutions, where they are available for microbial action. Previous studies have revealed that the contact of petroleum hydrocarbons with microorganisms for biodegradation only takes place at the interface of the NAPL and water (Puig-Grajales et al. 2000), indicating that the petroleum hydrocarbons in a sediment phase are potentially less bioavailable for the microbial community (Slater et al. 2005; Martienssen and Schirmer 2007). To release petroleum hydrocarbons from the sediment phase into an aqueous phase and thus make them available for microbial degradation, many kinds of surfactants including synthetic surfactants (Kim and Weber 2003; Zhu and Aitken 2010), natural surfactants (Kommalapati et al. 1997), and biosurfactants (Mulligan 2005; Zhao et al. 2011) have been used to promote the solubility of petroleum hydrocarbons and hence improve the biodegradation process in sediment.
Microemulsions are clear, thermodynamically stable, isotropic liquid mixtures of oil, water, and surfactant, frequently in combination with an alcohol (Testard and Zemb 1998). In microemulsions, the surfactant molecules may form a monolayer at the interface between the oil and water, with the hydrophobic tails of the surfactant molecules dissolved in the oil phase and the hydrophilic head groups in the aqueous phase (Zheng et al. 2011). In addition, the addition of alcohol further enhances contaminants’ solubilization by lowering the surface tension between the surfactant tails and the oil (Testard and Zemb 1998). It is already revealed that, in microemulsions, oil provides hydrophobic cores for solubilizing hydrophobic contaminants, while the interfacial film formed by alcohol and surfactant can stabilize the microemulsion system and meanwhile increase the degree of solubilization of hydrophobic contaminants (Zheng et al. 2011, 2012a). Thus, microemulsions have some special properties including ultralow interfacial tension between the oil and water phases, larger interfacial area, and higher solubilizing capacity for hydrophobic compounds over surfactant solutions (Zheng et al. 2011, 2012a). Previous studies have revealed that microemulsions can enhance the contact between hydrophobic substances and degradative microbes, thereby accelerating the degradation of hydrophobic substances (Zheng et al. 2012b). However, it is still unclear whether the microemulsions can enhance the biodegradation of TPH during nitrate-induced bioremediation of marine sediment, especially when the microemulsion is injected into the marine sediment, as it would be subjected to dilution by pore water during its diffusion in the sediment. Therefore, it is also necessary to investigate whether the microemulsion is still stable after being diluted with seawater, which is the pore water of marine sediment.
The aims of the present study were to (1) study how pore water in marine sediment influences the stability of microemulsions through investigating their droplet size change, (2) investigate whether microemulsions can enhance the desorption of total petroleum hydrocarbons (TPH) from the sediment phase to the aqueous phase, and (3) explore the feasibility of using microemulsions with NO3 − injection to enhance the biodegradation of TPH in sediment through a column study.
Materials and methods
Sediment and seawater
In the present study, the sediment sample was collected from 10 to 100 cm below the seawater/sediment interface in the southern part of Kowloon in Hong Kong during March. The collection was conducted using a corer hammered into the sediment up to the required depth. Upon retrieval, the sediment was extruded from the corer by inserting a plunger into the top of the corer and pushing the sediment out of the bottom. The duplicate sediment cores with a total amount of 10 kg wet weight of the sediment sample were obtained. The rocks and shells in the sediment were manually removed during collection. Then, the collected sediment was sealed in polyethylene bags to preclude the possibility of sediment oxidation, transported to the laboratory, and stored in a dark cold room at 4 °C (without sediment freezing) before use. The particle size distribution of the sediment was determined by a combination of wet sieving and hydrometer methods (Lacey et al. 1999), and the sediment was classified as sandy silt sediment according to the measurement results (Table 1) and the Shepard diagram (Shepard 1954). The sediment was further characterized by acid volatile sulfide (AVS) content, oxidation-reduction potential (ORP) value, and SO4 2− and NO3 − concentrations, and selected physicochemical properties are shown in Table 1. Briefly, the AVS content in sediment was measured following the USEPA protocol (1991). The ORP value of the sediment was determined using a Multi 3420 meter (WTW, Germany) equipped with Sen Tix® pH and ORP electrode probes. NO3 − and SO4 2− in sediment pore water were analyzed using ion chromatography (HIC-20A super, Shimadzu). The eluent was a bicarbonate buffer (1.8 Mm Na2CO3 and 1.7 Mm NaHCO3), as described elsewhere (Kleikemper et al. 2002).
Seawater was also collected from the contaminated site and filtered through a 0.22-μm cellulose nitrate membrane (Advantec MFS, CA, USA) before use. The pH and ORP values were 7.03 and 233.6 mV, the salinity was 35.6 ppt, and the concentrations of SO4 2− and NO3 − in the seawater were 794.43 mg/L and 1.79 mg NO3 −-N/L, respectively.
Microemulsion preparation and its stability investigation
A microemulsion precursor containing surfactant, alcohol, and oil was prepared by weighing and mixing 14.00 g of polyoxyethylene sorbitan monooleate (Tween 80), 4.60 g of 1-pentanol, and 1.40 g of linseed oil (Zheng et al. 2011, 2012a). The precursor was gently mixed with 80.00 g of deionized water or seawater to obtain microemulsion with a concentration of 20 % (w/w). The prepared microemulsion was a transparent dispersion in a single phase. No separation of oil phase and aqueous phase was observed for several weeks at a room temperature of 20 ± 1 °C, suggesting a good stability of microemulsion in terms of time course. The ratio of the surfactant to alcohol and the ratio of the surfactant to oil in the microemulsion were 3:1 (w/w) and 10:1 (w/w), respectively (Zheng et al. 2011). In addition, it should be noted that the concentration of microemulsion represents the total weight of Tween 80, 1-pentanol, and linseed oil present.
After preparation, microemulsion with a concentration of 20 % (w/w) was diluted with deionized water or seawater to obtain microemulsion covering a wide concentration range from 0.001 to 10 % (w/w). The diameter of microemulsion droplets in each microemulsion was determined using dynamic light scattering (DLS) (Zetaplus, LaborScience S.A.) to assess the stability of the microemulsion.
TPH desorption from sediment to aqueous phase by microemulsion
The change of TPH desorption in a sediment-water system with reaction time was performed in 20-mL glass vials. One gram of freeze-dried sediment was added to each vial, and then, 10 mL of microemulsion, prepared with deionized water or seawater, at a concentration of 1 % was added to the vials. 0.02 % of NaN3 was added to the mixture as a microbial growth inhibitor. All vials were shaken on a rotary shaker at 250 rpm in the dark at 25 °C. During the incubation, duplicate vials were sacrificed at 12-h intervals and the mixtures were filtered through 0.22-μm glass fiber filter paper. A 5-mL aliquot of the filtered solution was then carefully withdrawn with a volumetric pipette and extracted in heptane. The TPH concentration was analyzed using an Agilent 7890 series gas chromatograph equipped with a flame ionization detector (FID) and a RTX-5MS column (30 m × 0.25 mm ID, 0.25-μm film thickness). A splitless injection method was used. The injection volume and injection temperature were 1 μL and 330 °C, respectively. Helium was used as the carrier gas (1 mL/min). The temperature program was 10 min at 50 °C, 25 °C/min between 50 and 320 °C, and then held at 320 °C for 15 min. The amount of TPH was determined as the sum of the resolved and unresolved components eluted from the GC capillary column between the retention times of n-decane and n-tetracontane (CEN 2004; Saari et al. 2007a). It should be noted that the term TPH refers to all components eluting from the gas chromatograph between the boiling range of n-alkane standards (n-decane and n-tetracontane) without the identity of the sample component, which includes a wide variety of mixtures containing hundreds to thousands of hydrocarbon compounds (Korda et al. 1997; Saari et al. 2007b). The desorption efficiency of TPH was then calculated according to the following equation:
Desorption of TPH in the sediment-water system as a function of different concentrations of the microemulsion was also performed in 20-mL glass vials. One gram of freeze-dried sediment was added to each vial, and then 10 mL of (1) Tween 80 solution, (2) solution containing Tween 80 and 1-pentanol, in which the ratio of Tween 80 to 1-pentanol was 3:1 (w/w), or (3) microemulsion with a wide range of concentrations of 0.5, 1, 2, and 5 % was added to the vials. All the solutions and microemulsions were prepared with either deionized water or seawater. 0.02 % of NaN3 was also added to the mixture as a microbial growth inhibitor. The samples were shaken on a rotary shaker at 250 rpm in the dark at 25 °C. After equilibration, all vials were sacrificed and the mixtures were then filtered through 0.22-μm glass fiber filter paper. The extraction of TPH from the filtered solution, the determination of TPH concentration, and the calculation of TPH desorption efficiency were the same as described before.
Bioremediation experiment
A series of identical plexiglass columns with height of 50 cm and inner diameter of 7 cm and with four evenly distributed injection ports (10 cm apart) were employed in this column study (Fig. 1). The volume of each column was 1.92 L, and each column was packed layer by layer with increments of 10 cm with wet sandy silt sediment, lightly tapping the column to compress the sediment until the whole column was filled. Before and after filling up each layer of the column, the sediment was agitated using a wooden dowel to avoid creating a distinct layer of packed sediment, as described elsewhere (Merkel et al. 2002). Each column was packed with about 2.9 kg of the sediment. The bulk density of the sediment in each column was 1.51 g/cm3, and the particle density was assumed to be 2.65 g/cm3. Total porosity (φ T) was then calculated from the following equation: φ T = 1 − ρ B/ρ P, where ρ B is bulk density and ρ P is particle density (Li and Shao 2006; Price et al. 2010). The calculated porosity was about 0.43, which is close to the values reported in the literature (Lisle et al. 1997; Kubo and Nakajima 2002).
An amount of 1010 g of Ca(NO3)2 · 4H2O was dissolved in 550 g of filtered seawater to get a 45 % (w/w) Ca(NO3)2 solution. The density of this Ca(NO3)2 solution was about 1.43 g/mL, and the concentration of NO3 − was 119.46 g of NO3 −-N/L. Microemulsions with a concentration of 20 % (w/w) were prepared with deionized water according to the method described previously. Two kinds of injection patterns were employed: one having equal amounts of injection in all four ports (i.e., four injections) and the other having equal amounts of injection in ports 2 and 4 only (i.e., two injections). The detailed arrangements are shown in Table 2. For each injection pattern, there were five columns, including three treatment columns with the same injection of microemulsion and NO3 − and two control columns with the injection of NO3 − alone.
Firstly, 72.8 mL of microemulsion was injected into each treatment column, and after 24 h, during which time the TPH can be desorbed from the sediment phase to the aqueous phase as determined in the present study, a total volume of 40.2 mL of NO3 − solution was injected into each control or treatment column. After injection, one control column and one treatment column in each injection pattern were incubated at a room temperature of 20 ± 1 °C for 3 weeks, and the other one control column and two treatment columns in each injection pattern were incubated at the same temperature for 6 weeks. After incubation, the corresponding column was frozen at −20 °C and the sediment in each column was sectioned into ten equal pieces along the column, each 5 cm in length. As soon as thawed, each sectioned sediment sample was analyzed for the determination of pH, ORP, AVS, NO3 −, and SO4 2− concentrations following the respective standard methods as mentioned above. After that, 20 g of each sectioned sediment sample was freeze-dried for TPH and Tween 80 measurements. TPH in corresponding sediment samples were extracted by the modern closed vessel microwave-accelerated extraction method (Saari et al. 2007a). CEM PlusTM vessels (XP-1500 Plus) and the CEM MDS 81D-system (CEM Corp.) were used for microwave-assisted extraction. Briefly, 5 g of freeze-dried sediment sample was extracted in a temperature-controlled microwave system at 150 °C for 15 min, with a solvent mixture (20 mL of acetone and 20 mL of n-heptane). All the extracts were then washed twice with 100 mL of water to remove acetone from the extracts, which is in accordance with the standard method of the European Committee for Standardization (CEN 2004). The determination of TPH concentration using GC-FID was the same as described before. The total concentration of TPH in the column after the treatment could be calculated by averaging the corresponding concentrations in each sectioned sediment sample. The residual concentration of Tween 80 was also measured to assess the biodegradation of Tween 80 in the sediment after 6 weeks of treatment. A mass of 5 g of freeze-dried sediment in each sectioned piece along the column was collected and mixed with 40-mL deionized water. The mixture was shaken on a horizontal shaker at 200 rpm for 4 h and then centrifuged at 8000 rpm for 10 min. A volume of 10 mL of the supernatant was collected and filtrated through a 0.22-μm membrane filter. The concentration of Tween 80 in the filtrate was then analyzed using the Waters model 2690 chromatographic system (Waters Corp, Milford, USA), as described elsewhere (Zheng et al. 2012a). All measurements were conducted in triplicate, and the experimental data shown in Figs. 2, 3, 4, 5, 6, and 7 are the mean values with standard deviation to show their reproducibility and reliability.
Results and discussion
Microemulsion stability during its dilution
The droplet diameter of the 20 % (w/w) microemulsion formed with 14.00 g of Tween 80, 4.60 g of 1-pentanol, 1.40 g of linseed oil, and 80.00 g of deionized water was 86.7 nm, and the microemulsion droplet size was in the range of 57–84 nm when it was diluted to as low as 0.001 % with deionized water (Fig. 2), which is consistent with previous studies that microemulsion prepared with deionized water was very stable during dilution (Zheng et al. 2012a). When the same microemulsion was diluted with seawater to a concentration of 0.005 % (w/w), the droplet diameter of the microemulsion was in the range of 81.7–248.3 nm. The droplet diameter of the 20 % (w/w) microemulsion formed with 14.00 g of Tween 80, 4.60 g of 1-pentanol, 1.40 g of linseed oil, and 80.00 g of seawater was 316.6 nm, and the droplet size varied from 195.2 nm to 444.8 nm when diluted with seawater. These results indicate that the presence of seawater instead of deionized water makes the microemulsion droplets much bigger. Despite the droplet diameter of the microemulsion having increased, the appearance of the sample was still transparent with no separation of oil phase and aqueous phase observed and the droplet diameter was still maintained in the typical range of 100–600 nm (Sharma et al. 2010), indicating that the microemulsion prepared with either deionized water or seawater is stable during dilution with seawater.
TPH desorption from sediment to aqueous phase
After the marine sediment was mixed with 1 % microemulsion formed with Tween 80, 1-pentanol, linseed oil, and either deionized water or seawater, the desorption efficiency of TPH increased rapidly in the first 24 h to about 32.9 and 27.6 %, respectively (Fig. A.1). However, further extension of the incubation time could not further enhance the desorption efficiency. Thus, an equilibration period of 24 h is sufficient for desorbing TPH from the marine sediment phase into the aqueous phase by microemulsion.
When microemulsions prepared with deionized water were applied in four concentrations, 0.5, 1, 2, and 5 %, to desorb TPH from the sediment, all tested microemulsions were more effective than the same concentration of Tween 80 solution or the solution containing Tween 80 and 1-pentanol in desorbing TPH from the marine sediment (Fig. 3a), exhibiting superiority over either the Tween 80 solution or the solution containing Tween 80 and 1-pentanol in enhancing TPH desorption (Fig. 3a). The desorption efficiency of TPH reached 29.8 % when using 0.5 % microemulsion, but only 5.1–6.6 % of TPH was desorbed when using the same concentration of Tween 80 solution or the solution containing Tween 80 and 1-pentanol. In addition, about 92.5 % of TPH desorption efficiency was achieved when using 5 % microemulsion, while about 26.4–29.32 % of TPH was desorbed when using the 5 % Tween 80 solution or the solution containing Tween 80 and 1-pentanol. The superiority of microemulsions over either the Tween 80 solution or the solution containing Tween 80 and 1-pentanol indicates that the oil phase solubilized in the core of microemulsion plays an important role in enhancing TPH desorption. This is probably because the linseed oil in the microemulsion can effectively provide hydrophobic cores for solubilizing TPH (Testard and Zemb 1998, 1999). In addition, it was previously reported that the application of microemulsions in soil washing or bioremediation was advantageous over surfactant solutions in loam soil or sandy soil which both have low sorption capacities for oil molecules and is not applicable for clay soil possessing much higher absorbing capacity for oil molecules (Zheng et al. 2012a). From this point of view, the tested sandy silt sediment may have a low sorption capacity for linseed oil in microemulsions. When the deionized water used in the microemulsion, Tween 80 solution, and solution containing Tween 80 and 1-pentanol was replaced by seawater, the microemulsion was still the most effective in desorbing TPH from the marine sediment among these three types of solutions (Fig. 3b). The microemulsion prepared with deionized water was more effective than the corresponding counterparts prepared with seawater in desorbing TPH at concentrations of 0.5, 1, and 2 %, while the microemulsion prepared with either deionized water or seawater achieved a similar TPH desorption efficiency of about 95 % when the microemulsion concentration was 5 %. Therefore, microemulsion prepared with either deionized water or seawater is effective in desorbing TPH from the sediment, suggesting a possibility of enhancing the bioavailability of TPH in marine sediment using microemulsions. However, considering the higher efficiency of microemulsions prepared with deionized water at low concentrations (0.5–2 %) than that with seawater, microemulsions prepared with deionized water should be used in practice for bioremediation of marine sediment. Therefore, the microemulsion prepared with deionized water was used in the following column study for further investigation of the feasibility of using microemulsions with NO3 − injection for enhancing the biodegradation of TPH during the bioremediation process.
TPH removal during bioremediation process
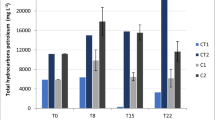
It can be seen from Fig. 4 that four injections or two injections of NO3 − alone resulted in 6.18 and 5.03 % of TPH biodegradation after 3 weeks of treatment. When the incubation period was prolonged to 6 weeks, the TPH biodegradation efficiency in the columns with four injections and two injections of NO3 − alone was 8.04 and 6.69 %, respectively. The relatively evener injection of NO3 − appears to be more beneficial for TPH degradation. It is also worthy of note that although indigenous sulfate and injected NO3 − were copresent in the marine sediment during nitrate-induced bioremediation, the heterotrophic denitrification process can out-compete the sulfate reduction process for TPH degradation since NO3 − is a more preferential terminal electron acceptor than sulfate (Zhang et al. 2009; Shao et al. 2011). Interestingly, the injection of microemulsion and NO3 − in both four injections and two injections led to higher TPH biodegradation efficiencies compared to the controls with the injection of NO3 − alone. It can also be seen from Fig. 4 that after 6 weeks of incubation, 29.73 % of TPH was removed in the columns with four injections of microemulsion and NO3 −, and 17.18 % of TPH biodegradation was achieved in the columns with two injections of microemulsion and NO3 −. The higher TPH biodegradation efficiencies induced by the presence of microemulsions may result from the enhanced bioavailability of TPH by microemulsions. Although the addition of both NO3 − and microemulsion exhibited a positive effect on TPH degradation, a high level of TPH was still detected in the column after 6 weeks of treatment. This is likely due to the reason that the low mass transfer of NO3 − and microemulsions in sediment matrix may limit the TPH degradation efficiency (Bosma et al. 1997; Fang et al. 2008). Besides, the incubation period (i.e., 6 weeks) may still be inadequate for more substantial biodegradation of TPH since some of the TPH components with condensed structures (e.g., high-molecular-weight branched alkanes, cycloparaffins, asphaltenes, and polynuclear aromatic hydrocarbons) are very recalcitrant (Salanitro 2001; Maletic et al. 2012), and thus, a longer time may be required for their biodegradation. Furthermore, the injection of microemulsions seems not to suppress the growth of heterotrophic denitrifying bacteria, since the TPH biodegradation efficiencies after 3 weeks of treatment in the columns with four injections and two injections of microemulsion and NO3 − were still as high as 14.23 and 12.01 %, respectively. Thus, about 2356.5 mg/kg dry weight (dw) of TPH was successfully removed by the four injections of microemulsion and NO3 − after 6 weeks of treatment. The calculated biodegradation rate of TPH was 56.11 mg TPH/kg/day, which is about two times of the reported value of 28 mg TPH/kg/day when using NO3 − as the terminal electron acceptor and without the addition of any surface active agent (Hasinger et al. 2012). Therefore, it can be concluded that the introduction of microemulsions into marine sediment successfully promoted the biodegradation of TPH by heterotrophic denitrifying bacteria with enhancing the bioavailability of TPH. In addition, most of Tween 80, the major component of the microemulsion, was also degraded by heterotrophic denitrifying bacteria as a carbon source. After 6 weeks of treatment, the concentration of residual Tween 80 in the sediment was found to be lower than 2504.75 mg/kg dw at all depths in the columns with four injections of microemulsion and NO3 −, compared with the initial Tween 80 concentration of 6739 mg/kg dw after injection. More than 63 % of Tween 80 was degraded during the 6 weeks of the bioremediation treatment, and a much lower concentration of residual Tween 80 can be expected if the treatment period is further prolonged. The high biodegradation potential of Tween 80 observed in the present study can, to some extent, evidence the environmental safety of using microemulsions to enhance TPH biodegradation during nitrate-induced bioremediation.
Besides heterotrophic denitrification, autotrophic denitrification is also an important process in nitrate-induced bioremediation of marine sediment, in which autotrophic denitrifying bacteria utilize AVS as an electron donor and NO3 − as an electron acceptor (Shao et al. 2009; Shao et al. 2010; Shao et al. 2011). In this study, AVS in the columns with four injections of NO3 − alone was almost removed at the depths of 0–30 cm after 3 weeks of treatment, and less than 200 mg could be detected at each sectioned depth of 30–50 cm (Fig. 5a), which indicates that a significant autotrophic denitrification occurred with NO3 − injection. However, compared with the columns with four injections of NO3 − alone, it is unexpected to find out that much higher amounts of AVS were observed at all depths after 3 weeks of treatment in the columns with four injections of microemulsion and NO3 −. This implies that the injection of microemulsion may probably lag the growth of autotrophic denitrifying bacteria in 3 weeks of treatment, thus delaying the autotrophic denitrification process. The delaying effect of the microemulsion on the autotrophic denitrification process was also observed for columns with the two injections of microemulsion and NO3 −. The two injections of microemulsion and NO3 − resulted in less AVS removal in all depths than that of two injections of NO3 − alone after 3 weeks of treatment. Based on our best knowledge, this is the first time that a lag phase of autotrophic denitrifying bacteria by applying microemulsion in nitrate-induced bioremediation has been reported. Additional study is required to understand the factor contributing to this delaying effect of microemulsion on the autotrophic denitrification process. When the incubation period was prolonged to 6 weeks, the delaying effect of the microemulsion on the autotrophic denitrification process was no longer observed as revealed from Fig. 5b in that the sediment in all the columns achieved a similar degree of AVS removal at all depths in both four injections and two injections of microemulsion and NO3 − or NO3 − alone. Therefore, although the injection of microemulsion may lag the growth of autotrophic denitrifying bacteria, the extension of the incubation period could effectively overcome the delaying effect of the microemulsion on the autotrophic denitrification process to remove AVS in marine sediment.
It can be seen from Fig. 6 that in the columns with four injections or two injections of microemulsion and NO3 −, a high amount of residue NO3 − was detected in the sediment after 3 weeks of treatment. Although the consumption of NO3 − by heterotrophic and autotrophic denitrification processes is hard to differentiate, the high amount of residue NO3 − can only be ascribed to the delayed autotrophic denitrification process between AVS and NO3 − because no suppressing effect of the microemulsion on the heterotrophic denitrification process was noted. However, when the incubation period was prolonged to 6 weeks, a very low amount of NO3 − was detected at all depths of columns with four injections, and there is no significant difference between columns receiving microemulsion and NO3 − and columns receiving NO3 − alone. A similar phenomenon was also found in the columns with two injections. After 6 weeks of treatment, the detected NO3 − decreased drastically and was mainly found in the sediment near the injection depths, and the NO3 − distribution in the treatment columns with the injection of microemulsion and NO3 − and the control column with the injection of NO3 − alone were very similar. This result also confirmed that extending the incubation period could overcome the delaying effect of microemulsion on the autotrophic denitrification process.
After 6 weeks of treatment, the pH values of sediment in all columns were in the range of 7.1 to 7.5 (Fig. 7a), which were close to the initial sediment pH value before treatment, and there was no obvious change along the sediment depth in each column. These results indicate that a neutral sediment pH could be maintained probably due to the high buffering capacity of the sediment matrix, although some protons can be produced during sulfide oxidation driven by autotrophic denitrifying bacteria (Shao et al. 2010). It was shown in Fig. 7b that the ORP of the sediment at all depths of the columns with four injections of either NO3 − alone or microemulsion and NO3 − increased to higher than −107.2 mV. For the columns with two injections of either NO3 − alone or microemulsion and NO3 −, the sediment ORP was higher than −118.1 mV at a depth of 10–30 and 35–50 cm after 6 weeks of treatment, because these depths are near the injection ports. For the portion of the sediment with no or low AVS removal (depths of 0–10 and 30–35 cm), its ORP was still of strong reducing condition (ranging from −193.3 to −240.5 mV), and this extreme reducing condition was close to the initial oxidation-reduction condition of the sediment. Therefore, the injection of NO3 − led to the increased sediment ORP, which is helpful for the growth of both heterotrophic and autotrophic denitrifying bacteria during such nitrate-induced bioremediation (Vazquez-Rodriguez et al. 2008).
Conclusions and implications
In this study, it was revealed that the microemulsion formed with Tween 80, 1-pentanol, linseed oil, and either deionized water or seawater was stable when subjected to dilution by seawater with a series of dilution to a concentration of as low as 0.005 % (w/w). The results from the desorption test showed that microemulsion prepared with either deionized water or seawater at all tested concentrations (i.e., 0.5, 1, 2, and 5 %) was effective in desorbing TPH from the sediment. Further column study found that the combination of NO3 − and microemulsion prepared with deionized water could achieve a higher TPH removal efficiency compared with the use of NO3 − alone after 6 weeks of treatment. The results obtained here suggest that the microemulsion formed with Tween 80, 1-pentanol, linseed oil, and deionized water can be a good candidate for enhancing TPH degradation during the nitrate-induced bioremediation of marine sediment. However, when microemulsions are used in the bioremediation practices, their injections in marine sediment should be as even as possible and the treatment period should be long enough so that the indigenous bacteria can adopt and grow in the presence of microemulsions.
References
Bosma TNP, Middeldorp PJM, Schraa G, Zehnder AJB (1997) Mass transfer limitation of biotransformation: quantifying bioavailability. Environ Sci Technol 31:248–252
Brils JM, Huwer SL, Kater BJ, Schout PG, Harmsen J, Delvigne GAL, Scholten MCT (2002) Oil effect in freshly spiked marine sediment on Vibrio fischeri, Corophium volutator, and Echinocardium cordatum. Environ Toxicol Chem 21:2242–2251
CEN (2004) Characterization of waste—determination of hydrocarbon content in the range of C10 to C40 by gas chromatography. vol EN 14039: 2004. European Committee for Standardization
Coates JD, Woodward J, Allen J, Philp P, Lovley DR (1997) Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl Environ Microb 63:3589–3593
Eriksson M, Sodersten E, Yu ZT, Dalhammar G, Mohn WW (2003) Degradation of polycyclic aromatic hydrocarbons at low temperature under aerobic and nitrate-reducing conditions in enrichment cultures from northern soils. Appl Environ Microb 69:275–284
Fang HHP, Zhang M, Zhang T, Chen J (2008) Predictions of nitrate diffusion in sediment using horizontal attenuated total reflection (HATR) by Fourier transform infrared (FTIR) spectrometry. Water Res 42:903–908
Frysinger GS, Gaines RB, Xu L, Reddy CM (2003) Resolving the unresolved complex mixture in petroleum-contaminated sediments. Environ Sci Technol 37:1653–1662
Hasinger M, Scherr KE, Lundaa T, Brauer L, Zach C, Loibner AP (2012) Changes in iso- and n-alkane distribution during biodegradation of crude oil under nitrate and sulphate reducing conditions. J Biotechnol 157:490–498
Hutchins SR (1991) Optimizing BTEX biodegradation under denitrifying conditions. Environ Toxicol Chem 10:1437–1448
Hutchins SR, Bantle JA, Schrock EJ (1998) Effect of nitrate-based bioremediation on contaminant distribution and sediment toxicity—column study. Environ Toxicol Chem 17:349–361
Johnson K, Ghosh S (1998) Feasibility of anaerobic biodegradation of PAHs in dredged river sediments. Water Sci Technol 38:41–48
Jonker MTO, Brils JM, Sinke AJC, Murk AJ, Koelmans AA (2006) Weathering and toxicity of marine sediments contaminated with oils and polycyclic aromatic hydrocarbons. Environ Toxicol Chem 25:1345–1353
Kim HS, Weber WJ (2003) Preferential surfactant utilization by a PAH-degrading strain: effects on micellar solubilization phenomena. Environ Sci Technol 37:3574–3580
Kleikemper J, Pelz O, Schroth MH, Zeyer J (2002) Sulfate-reducing bacterial community response to carbon source amendments in contaminated aquifer microcosms. Fems Microbiol Ecol 42:109–118
Kommalapati RR, Valsaraj KT, Constant WD, Roy D (1997) Aqueous solubility enhancement and desorption of hexachlorobenzene from soil using a plant-based surfactant. Water Res 31:2161–2170
Korda A, Santas P, Tenente A, Santas R (1997) Petroleum hydrocarbon bioremediation: sampling and analytical techniques, in situ treatments and commercial microorganisms currently used. Appl Microbiol Biot 48:677–686
Kubo YS, Nakajima T (2002) Laboratory experiments and numerical simulation of sediment-wave formation by turbidity currents. Mar Geol 192:105–121
Lacey R, Watzin MC, McIntosh AW (1999) Sediment organic matter content as a confounding factor in toxicity tests with Chironomus tentans. Environ Toxicol Chem 18:231–236
Lei L, Khodadoust AP, Suidan MT, Tabak HH (2005) Biodegradation of sediment-bound PAHs in field contaminated sediment. Water Res 39:349–361
Li YY, Shao MA (2006) Change of soil physical properties under long-term natural vegetation restoration in the Loess Plateau of China. J Arid Environ 64:77–96
Lisle TE, Pizzuto JE, Ikeda H, Iseya F, Kodama Y (1997) Evolution of a sediment wave in an experimental channel. Water Resour Res 33:1971–1981
Lu XY, Zhang T, Fang HHP (2011) Bacteria-mediated PAH degradation in soil and sediment. Appl Microbiol Biot 89:1357–1371
Maletic S, Roncevic S, Dalmacija B, Agbaba J, Watson M, Tubic A, Perovic SU (2012) Characterisation of weathered petroleum hydrocarbons during a landfarming bioremediation study. J Serb Chem Soc 77:1671–1685
Martienssen M, Schirmer M (2007) Use of surfactants to improve the biological degradation of petroleum hydrocarbons in a field site study. Environ Technol 28:573–582
Mbadinga SM, Wang LY, Zhou L, Liu JF, Gu JD, Mu BZ (2011) Microbial communities involved in anaerobic degradation of alkanes. Int Biodeter Biodegr 65:1–13
Merkel B, Planer-Friedrich B, Wolkersdorfer C (2002) Uranium in the aquatic environment: proceedings of the International Conference [on] Uranium Mining and Hydrogeology III and the International Mine Water Association Symposium, Freiberg, Germany, 15–21 September 2002
Muijs B, Jonker MTO (2010) A closer look at bioaccumulation of petroleum hydrocarbon mixtures in aquatic worms. Environ Toxicol Chem 29:1943–1949
Mulligan CN (2005) Environmental applications for biosurfactants. Environ Pollut 133:183–198
Price K, Jackson CR, Parker AJ (2010) Variation of surficial soil hydraulic properties across land uses in the southern Blue Ridge Mountains, North Carolina, USA. J Hydrol 383:256–268
Puig-Grajales L, Tan NG, van der Zee F, Razo-Flores E, Field JA (2000) Anaerobic biodegradability of alkylphenols and fuel oxygenates in the presence of alternative electron acceptors. Appl Microbiol Biot 54:692–697
Richardson BJ, Zheng GJ, Tse ESC, Lam PKS (2001) A comparison of mussels (Perna viridis) and semi-permeable membrane devices (SPMDs) for monitoring chlorinated trace organic contaminants in Hong Kong coastal waters. Chemosphere 45:1201–1208
Richardson BJ, Zheng GJ, Tse ESC, De Luca-Abbott SB, Siu SYM, Lam PKS (2003) A comparison of polycyclic aromatic hydrocarbon and petroleum hydrocarbon uptake by mussels (Perna viridis) and semi-permeable membrane devices (SPMDs) in Hong Kong coastal waters. Environ Pollut 122:223–227
Rowland S, Donkin P, Smith E, Wraige E (2001) Aromatic hydrocarbon “humps” in the marine environment: unrecognized toxins? Environ Sci Technol 35:2640–2644
Saari E, Peramaki P, Jalonen J (2007a) A comparative study of solvent extraction of total petroleum hydrocarbons in soil. Microchim Acta 158:261–268
Saari E, Peramaki P, Jalonen J (2007b) Effect of sample matrix on the determination of total petroleum hydrocarbons (TPH) in soil by gas chromatography-flame ionization detection. Microchem J 87:113–118
Salanitro JP (2001) Bioremediation of petroleum hydrocarbons in soil. Adv Agron 72:53–105
Shao MF, Zhang T, Fang HHP (2009) Autotrophic denitrification and its effect on metal speciation during marine sediment remediation. Water Res 43:2961–2968
Shao MF, Zhang T, Fang HHP (2010) Sulfur-driven autotrophic denitrification: diversity, biochemistry, and engineering applications. Appl Microbiol Biot 88:1027–1042
Shao MF, Zhang T, Fang HHP, Li XD (2011) The effect of nitrate concentration on sulfide-driven autotrophic denitrification in marine sediment. Chemosphere 83:1–6
Sharma N, Bansal M, Visht S, Sharma PK, Kulkarni GT (2010) Nanoemulsion: a new concept of delivery system. Chron Young Sci 1:2–6
Shepard FP (1954) Nomenclature based on sand-silt-clay ratios. J Sediment Petrol 24:151–158
Slater GF, White HK, Eglinton TI, Reddy CM (2005) Determination of microbial carbon sources in petroleum contaminated sediments using molecular C-14 analysis. Environ Sci Technol 39:2552–2558
Testard F, Zemb T (1998) Excess of solubilization of lindane in nonionic surfactant micelles and microemulsions. Langmuir 14:3175–3181
Testard F, Zemb T (1999) Excess of solubilization and curvature in nonionic microemulsions. J Colloid Interf Sci 219:11–19
USEPA (1991) Draft analytical method for determination of acid volatile sulfide in sediment. EPA 821/R-91/100. United States Environmental Protection Agency, Washington, DC
USEPA (2005) Contaminated sediment remediation guidance for hazardous waste sites. EPA 540/R-05/012. United States Environmental Protection Agency, Washington D.C.
Vazquez-Rodriguez GA, Beltran-Hernandez RI, Lucho-Constantino CA, Blasco JL (2008) A method for measuring the anoxic biodegradability under denitrifying conditions. Chemosphere 71:1363–1368
Villemur R, Deziel E, Benachenhou A, Marcoux J, Gauthier E, Lepine F, Beaudet R, Comeau Y (2000) Two-liquid-phase slurry bioreactors to enhance the degradation of high-molecular-weight polycyclic aromatic hydrocarbons in soil. Biotechnol Progr 16:966–972
Wilson LP, D’Adamo PC, Bouwer EJ (1997) Bioremediation of BTEX, naphthalene, and phenanthrene in aquifer material using mixed oxygen/nitrate electron acceptor conditions. United States Environmental Protection Agency, Washington, DC
Yuan SY, Chang BV (2007) Anaerobic degradation of five polycyclic aromatic hydrocarbons from river sediment in Taiwan. J Environ Sci Heal B 42:63–69
Zhang M, Zhang T, Shao MF, Fang HHP (2009) Autotrophic denitrification in nitrate-induced marine sediment remediation and Sulfurimonas denitrificans-like bacteria. Chemosphere 76:677–682
Zhao ZY, Selvam A, Wong JWC (2011) Effects of rhamnolipids on cell surface hydrophobicity of PAH degrading bacteria and the biodegradation of phenanthrene. Bioresour Technol 102:3999–4007
Zheng GY, Zhao ZY, Wong JWC (2011) Role of non-ionic surfactants and plant oils on the solubilization of organochlorine pesticides by oil-in-water microemulsions. Environ Technol 32:269–279
Zheng GY, Selvam A, Wong JWC (2012a) Enhanced solubilization and desorption of organochlorine pesticides (OCPs) from soil by oil-swollen micelles formed with a nonionic surfactant. Environ Sci Technol 46:12062–12068
Zheng GY, Selvam A, Wong JWC (2012b) Oil-in-water microemulsions enhance the biodegradation of DDT by Phanerochaete chrysosporium. Bioresour Technol 126:397–403
Zhu HB, Aitken MD (2010) Surfactant-enhanced desorption and biodegradation of polycyclic aromatic hydrocarbons in contaminated soil. Environ Sci Technol 44:7260–7265
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Zheng, G. & Lo, I.M.C. Enhancement of nitrate-induced bioremediation in marine sediments contaminated with petroleum hydrocarbons by using microemulsions. Environ Sci Pollut Res 22, 8296–8306 (2015). https://doi.org/10.1007/s11356-014-3979-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3979-0