Abstract
Groundwater particularly drinking water contamination with metals has created an environmental disaster in Bangladesh. Manganese (Mn), an essential trace element, plays a key role in the development and function of the brain. Excess Mn exposure is reported to be associated with complex neurological disorders. Here, we have found a notably large extent of Mn above the permissive limit in the tube-well water of Rajshahi and Naogaon districts in Bangladesh. Higher levels of Mn in hair and nail samples, and a decreasing level of butyrylcholinesterase (BChE) activity were detected in plasma samples of the human subjects recruited from Naogaon district. Mn concentrations in water, hair, and nails were negatively correlated with the plasma BChE levels in Mn-exposed populations. To compare and validate these human studies, an animal model was used to determine the in vivo effects of Mn on neurobehavioral changes and blood BChE levels. In elevated plus maze, the time spent was significantly reduced in open arms and increased in closed arms of Mn-exposed mice compared to control group. The mean latency time to find the platform was declined significantly in control mice compared to Mn-treated group during 7 days in Morris water maze test, and Mn-exposed group also spent significantly less time in the desired quadrant as compared to the control group in probe trial. BChE activity was significantly reduced in Mn-exposed mice compared to control mice. Taken together, these results suggest that plasma BChE levels may serve as reliable biomarker of Mn-induced neurotoxicity related to behavioral changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic exposure to metal is a major threat to the public health in many countries in the world and people are exposed to these metals via water, air, and soil (Calderón et al. 2003). Tube-well water is used as the main source of drinking water in Bangladesh which has been contaminated with naturally occurring inorganic arsenic (As), as well as other metals. The presence of toxic level of As in groundwater is one of the worst natural disasters in Bangladesh and West Bengal of India (Hussam et al. 2003). Furthermore, unsafe levels of Mn, lead (Pb), nickel (Ni), and chromium (Cr) were detected along with As in groundwater in some areas of Bangladesh (Frisbie et al. 2002). Water samples from a large number of deep and shallow wells also contain excess amount of Mn beyond permissible limits in Bangladesh (BGS and DPHE 2001). Around three quarters of the 3534 wells have higher concentrations of Mn than permissible limits of WHO recommendation in 61 districts out of 64 of Bangladesh (Tasneem and Ali 2010).
In humans, as a cofactor of several enzymes involved in neurotransmitter synthesis and metabolism, including glutamine synthetase, arginase, pyruvate decarboxylase, and mitochondrial superoxide dismutase, Mn plays an important role in the development and functioning of the brain (Takeda 2003; Shishova et al. 2009; Zwingmann et al. 2004; Hearn et al. 2003). But high intakes of Mn through both inhalation and drinking water have been shown to be toxic and can produce cognitive deficits in humans. In animal models, neurobehavioral studies focusing on locomotor activity have shown that overexposure to Mn can lead to progressive, permanent neurodegenerative damage (Bardullas et al. 2009; Correa et al. 2004; Rodriguez et al. 2001; Crossgrove and Zheng 2004). Excessive Mn causes cognitive impairment resulting from injury to the central nervous system within the hippocampus (Liang et al. 2016). Many recent studies have shown that alterations in the biology of gamma-aminobutyric acid-ergic (GABAergic), glutamatergic, and dopaminergic systems are involved in the etiology of Mn-induced neurotoxicity (Fordahl and Erikson 2014; Erikson et al. 2002; Sidoryk-Wegrzynowicz and Aschner 2013; Karki et al. 2015; Robison et al. 2015).
Excessive accumulation of Mn in brain affects the cholinergic system and its dysfunction is related to Alzheimer’s disease (AD) (Yousefi et al. 2014; Calabresi et al. 2001). Acetylcholinesterase (AChE) and BChE hydrolyze the cholinergic neurotransmitter acetylcholine in the circulatory system. BChE is a nonspecific cholinesterase enzyme that hydrolyses many different choline-based esters and involved in cholinergic regulation both in the central and peripheral nervous systems (Reid et al. 2013; Loewenstein-Lichtenstein et al. 1995). BChE is found in blood, liver, and also in central and peripheral nervous systems (Santarpia et al. 2013). BChE is the major acetylcholine-hydrolyzing enzyme in the circulation and maintained the normal cholinergic pathway in AChE knockout mice (Hartmann et al. 2007). In addition, it was reported that BChE activity changes in the plasma in pregnancy, liver disease, poor nutrition, cancer, and AD patients’ brain (Bono et al. 2014; Lockridge 2015). BChE is used as an organophosphate poisoning biomarker and its activity reveals the strength of the cholinergic anti-inflammatory response and in the changes of sympathetic/parasympathetic balance (Stefanidou et al. 2009; Zhang et al. 2015; Shenhar-Tsarfaty et al. 2014).
A higher level of Mn in drinking water (> 300 μg/l) is associated with reduced intellectual function in children (Wasserman et al. 2007). Although there is evidence for neurotoxicity in children due to the presence of higher levels of Mn in the drinking water, but currently, there is no ideal blood biomarkers for evaluating the neurotoxicity associated with Mn exposure. Furthermore, the cholinergic system is reported to be affected with Mn but the BChE activity in Mn exposure is not clearly documented. Therefore, this study has been designed to establish the BChE as a biomarker in Mn-induced neurobehavioral changes through human and animal experiments.
Materials and methods
Study areas and subjects
Two villages in Naogaon and Rajshahi districts were selected as study areas. From these two villages, we randomly collected 98 tube-well water samples. Thirty-five human subjects were selected from the village in Naogaon district as study subjects. All sorts of confidentialities and rights of the study subjects were strictly maintained. Residents (18–60 years of ages) of this area were enrolled for this study who had lived for at least 5 years in the same area and given their written consent. Pregnant and lactating mothers as well as the individuals who had a history of hepatitis B positive, surgical operation, drug addiction, chronic alcoholism, hepatic, renal or severe cardiac diseases, and the farmers exposed to any kind of agricultural/home insecticides within last 1 month have been excluded from this study. Ethical approval (No: 661/320/IAMEBBC/IBSc) for human study was taken from the Institutional Animal, Medical Ethics, Biosafety and Biosecurity Committee (IAMEBBC), Institute of Biological Sciences, University of Rajshahi, Bangladesh.
Collection of drinking water and analysis of Mn
The tube-well water is the primary source of drinking water and also used for cooking and other household purposes. Water samples from 98 tube-wells were collected in acid-washed containers after the well was pumped for 5 min as described in Van Green et al. (2008). An yttrium solution (10 ppb in 1.0% nitric acid) was added to all water samples as an internal standard for ICP-MS analysis. Mn concentrations in water samples were measured by ICP-MS (Agilent 7700x, Japan) and the detection limit for Mn was 0.005 ppb. The ion signals for Mn and yttrium were monitored at m/z values of 54 and 79, respectively. River water (NMIJ CRM 7202-a No. 347 National Institute of Advanced Industrial Science and Technology, Japan) was used as a certified reference material (CRM). Mean average value (mean ± SD) of Mn in river water of ICP-MS analysis was 4.79 ± 0.05 μg/l (reference value, 5.03 μg/l). All water samples were determined in triplicate and the average values of Mn were used for the data analysis.
Collection of hair and nail samples and analysis of Mn
We measured hair and nail Mn concentrations as exposure metrics, together with our assessment of the water Mn concentrations. Hair and nails were collected from the study subjects as described previously (Ali et al. 2010). Samples were immersed in 1% Triton X-100, sonicated for 20 min, and then washed five times with Milli-Q water. The washed samples were allowed to dry at 60 °C overnight in a drying oven. The hair and nail samples were then digested with concentrated nitric acid using a hot plate at 70 °C and at 115 °C for 15 min and measured the concentration of Mn following the method described in previous section. The Mn measurement accuracy was confirmed by using the CRM “human hair” (GBW09101, Shanghai Institute of Nuclear Research Academia Sinica, China). The average value of Mn in “human hair” measured by an ICP-MS analysis was 2.93 ± 0.24 μg/g (reference value, 2.94 μg/g).
Collection of blood plasma samples and biochemical assay
Fasting blood samples were collected from each individual by venipuncture into the EDTA-containing blood collection tubes and kept instantly on ice and subsequently centrifuged at 5000 rpm for 15 min at 4 °C. Plasma supernatant was then collected and samples were stored at − 80 °C. BChE activity was measured using BChE kit according to the manufacturer’s protocol (RANDOX, UK). All human samples were examined in duplicate, and mean values were taken.
Animal maintenance
Swiss Albino male mice (average body weight 25–30 g) were purchased from International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) and housed at the Animal Research Laboratory, Department of Biochemistry and Molecular Biology, University of Rajshahi. Prior to use, animals were acclimatized for 7 days and divided into two groups: (a) control and (b) Mn-exposed. Exposed and control groups comprised of eight mice each. Manganese chloride was given to the mice through drinking water (10 mg/kg body weight) daily for 60 days before the behavioral test and the control group received normal drinking water. The dose of Mn was selected on the basis of previously published studies (Liang et al. 2016; Aktar et al. 2017). All experimental mice had free access to food pellets and water ad libitum throughout experimental periods with 12/12 light/dark cycle. Institutional ethical approval was taken for the animal experiment from the Institute of Biological Sciences, University of Rajshahi, Bangladesh (No: 67/320/IAMEBBC/IBSc.).
Assessing anxiety in the elevated plus maze
The elevated plus maze (EPM) test is a paradigm for assessing anxiety responses of rodents. The EPM consisted two open arms (50 × 10 cm) and two enclosed arms (5 × 10 × 40 cm) elevated to the height of 50 cm as described previously (Aktar et al. 2017). Arms of the maze form a cross with two open arms being opposite to each other. After each test, 20% ethanol was used to clean the maze. Mice were placed individually on the center of platform facing one of the closed arms and allowed to explore the maze for 5 min. The assessment in the open arms compared to closed arms indicates the level of anxiety (Carobrez and Bertoglio 2005).
Evaluation of spatial memory and learning in the Morris water maze
The Morris water maze is widely used for the evaluation of learning and spatial memory test of rodents (D’Hooge and De Deyn 2001). The maze consisted of a circular water pool with black interior (150 cm diameter and 60 cm height) and filled with water at the depth of 30 cm and water temperature was 25 ± 2 °C. A black colored platform of 28 cm height was fixed in a constant place in the middle of the North-East (NE) approximately 1 cm below the water surface. Mice were given a maximum time of 60 s (cutoff time) to find the hidden platform and were allowed to stay on it for 20 s. Each mouse was given two trials per day for 7 consecutive days, and escape latency was recorded in each trial as the time mice took to locate the platform. Mean escape latency of two trials was averaged and plotted to see the results. Animals were excluded as they did not meet the learning criteria within 7 days of learning. A significant decrease in latency time as compared with the first session was considered as successful learning. The probe test was conducted 24 h after completion of the spatial task recording without platform and was recorded for 60 s. More time spent during the probe trial in the exact quadrant indicated better performance.
Estimation of Mn concentration in brain tissue
Whole brain tissue samples (about 400–500 mg) from experimental mice were digested with concentrated nitric acid using a hot plate at 70 °C for 15 min, and again at 115 °C for 15 min. After cooling, the samples were diluted with 1.0% nitric acid containing yttrium (10 ppb), and concentrations of Mn (54) and Y (79) were determined by ICP-MS (Agilent 7700x, Japan).
Biochemical assays
Blood was collected from the thoracic arteries of the mice under diethyl ether anesthesia. For coagulation, blood was kept in the EDTA-containing blood collection tubes. After centrifugation at 4000 rpm for 15 min at 4 °C, plasma was separated and stored at − 80 °C until the experiments were performed. Plasma BChE activity was measured by using commercially available kits according to the manufacturer’s protocol. All plasma samples were analyzed in duplicate and then mean values were taken.
Statistical analysis
Statistical analyses for this study were performed with Statistical Package for the Social Sciences (SPSS for Windows, Version 21.0. Chicago, SPSS Inc.). Spearman’s correlation coefficient test was used to assess the correlations of Mn exposure metrics with BChE levels. The statistical significance of different study groups was analyzed employing independent sample t test. Values of p < 0.05 were considered statistically significant. Linear regression analysis was also performed to examine the effect of age on the association between Mn exposure metrics and BChE levels.
Results
Presence of Mn in the collected drinking water
A total of 98 tube-well water samples from our study areas in the districts of Rajshahi and Naogaon were analyzed. Results presented in Fig. 1a showed that 39.80% of water samples had higher Mn content than the permissive limit set by WHO. In a total of 98 collected water samples, 39 samples contained the average value of Mn 1250.36 ± 1083.16 ppb, which is more than twofold higher than the permissive limit of Mn in the drinking water. By contrast, 59 samples contained the average value of Mn 265.22 ± 113.41 ppb, which is lower than the permissive value (Fig. 1b). These data showed that some people in the study area were chronically exposed to higher amount of Mn through contaminated tube-well water.
Distribution and average drinking water Mn levels of the 98 study samples collected from the study areas of Rajshahi and Naogaon districts, based on the permissive upper limit. a Percentage of water samples containing Mn below or above the permissive limit. b Average Mn concentration in the collected water samples below or above the permissive limit. Blank and black columns represent Mn levels (mean ± SE) of the tube-well water below or above the permissive limits (Mn < 500), respectively
Demographic characteristics and Mn exposure levels of the study subjects
Seventeen out of 35 study subjects were males and the rest were females. The average age and BMI of the male and female subjects were 34.03 ± 9.90 and 20.79 ± 3.37, respectively. The average levels of Mn in the subjects’ drinking water, hair, and nail were 1204.61 ± 1236.38 ppb, 14.86 ± 17.17 μg/g, and 32.19 ± 33.83 μg/g, respectively (Table 1). Results showed that the average concentrations of Mn in water, hair, and nail were higher than those of the recommended values.
Association of subjects’ Mn exposure with plasma BChE activity
Figure 2 shows the association of Mn exposure with plasma BChE activity. A significant decrease in plasma BChE activity was observed with the increasing concentration of Mn in drinking water (rs = − 0.392, p < 0.05) (Fig. 2a). Similar associations were also observed between BChE levels and hair Mn concentrations (rs = − 0.514, p < 0.01), and between BChE levels and nail Mn concentrations (rs = − 0.495, p < 0.01) (Fig. 2b, c). In regression analyses, Mn exposure showed significant (p < 0.01 for water, p < 0.05 for hair and nail) inverse association with BChE activity even after adjustment with age (Table S1). Age also showed a significant inverse association with BChE activity along with Mn concentrations in water, hair, and nails.
Assessing anxiety of experimental mice in elevated plus maze test
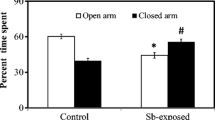
The results of EPM test of metal-exposed (Mn-treated) mice and control mice groups are presented in Fig. 3. Percentage of time spent in the open arms or in closed arms was chosen to index anxiety in EPM. The percentages of time spent in open arms of control mice and mice treated with Mn were 43.33 ± 1.10 and 33.00 ± 0.35 (mean ± SE), respectively, whereas the percentages of time spent in closed arms of the two groups were 56.66 ± 1.10 and 67.00 ± 0.35, respectively. Results showed that Mn exposure significantly (p < 0.05) reduced the time spent in open arms while increased the time spent in closed arms as compared to control mice.
Percent time spent in open and closed arms of experimental mice in elevated plus maze. Percent time spent in open and closed arms of control and Mn-exposed were expressed as mean ± SE, where n = 8 for each group of mice. *Significantly different from control group at p < 0.05 for open arms and #significantly different from control group at p < 0.05 for closed arms. p values were from independent sample t test
Evaluation of spatial memory and learning of experimental mice in the Morris water maze
Figure 4 shows the effects of Mn exposure on spatial memory and learning in mice. The Morris water maze test was conducted on day 60 from the day when the mice started to receive manganese chloride solution (MnCl2.4H2O) through drinking water to observe the effect on learning and spatial memory. Spatial acquisition for learning in the Morris water maze is presented in Fig. 4a which illustrates the escape latency to reach the platform. In the first day of spatial acquisition trials, all mice tended to swim around the edges looking for an escape, but after being placed on the platform at the end of each trial, mice gradually learned that there was an escape platform into the pool. In the Morris water maze test results, the mean latency time (mean ± SE) of control group to find the platform was 36.82 ± 1.90 s on the first day, which decreased rapidly during the learning for 7 days, and the mean latency time was 14.64 ± 1.75 s on the seventh day. On the other hand, the mean latency time of Mn-exposed mice was decreased slightly, but increased transiently as compared to control group during 7 days of learning. The mean latency time of Mn-exposed group was 34.64 ± 1.63 s on the first day and 21.60 ± 1.58 s on the seventh day, which was significantly increased (p < 0.05) than that of control group. After 7 days of trial, each mouse performed one probe trial, in which the platform was removed from the pool. In this test, the time (mean ± SE) spent in the desired quadrant of the control and Mn-exposed groups were 43.16 ± 1.49 and 35.87 ± 1.41 s, respectively (Fig. 4b). The results showed that the control group spent more times in the desired quadrant as compared to Mn-exposed group, where they found the platform during the previous trials. Mn-exposed mice spent less time in the desired quadrant compared to control group which was significantly different from control group (p < 0.05).
Changes in latency time and Morris water maze probe in mice. a Changes of mean latency time of the experimental mice in Morris water maze test. Latency time of control and Mn-exposed were expressed as mean ± SE, where n = 6 for each group of mice. Mice were trained two trials per day. *Significantly different from control group at p < 0.05 in independent sample t test. b Morris water maze probe trial of experimental mice. Time spent in NE quadrant of control and Mn-exposed were expressed as mean ± SE, where n = 6 for each group of mice. *Significantly different from control group at p < 0.05 in independent sample t test
Accumulation of Mn in brain of experimental mice
Figure 5 shows the levels of Mn accumulation in control and Mn-treated mice. The levels of Mn (mean ± SE) in control and Mn-exposed groups were 0.1556 ± 0.0264 ng/g and 3.0432 ± 0.04567 ng/g, respectively. Mn concentration was significantly increased (p < 0.05) in the Mn-exposed mice compared to control mice.
Effects of Mn on plasma BChE activity in mice
The plasma BChE activities (mean ± SE) of the control and Mn-exposed mice were 13,449.43 ± 417.08 and 7375.63 ± 508.03 U/l, respectively. BChE activity was significantly (p < 0.05) reduced in Mn-exposed mice compared to the control group (Fig. 6).
Discussion
Excess amount of Mn is present in the tube-well water in some areas in Bangladesh (Tasneem and Ali 2010). In this study, we selected two areas in Rajshahi and Naogaon districts and the concentration of Mn detected is more than two times higher than the permissive limit (Fig. 1a, b). Although Mn is an essential trace element, it was reported that excessive exposure to Mn (6 months to 2 years) is associated with adverse psychiatric, cognitive, and motor disorders (Pal et al. 1999; Olanow 2004; Perl and Olanow 2007).
In the present study, we have analyzed the blood BChE activity in human subjects naturally exposed to Mn along with Mn-exposed experimental mice. We have found that plasma BChE activity is negatively and significantly (rs = − 0.392, p < 0.05 for water; rs = − 0.514, p < 0.01 for hair; and rs = − 0.495, p < 0.01 for nail) associated with the increasing exposure to Mn in humans (Fig. 2a–c). The associations are significant (p < 0.01 for water and p < 0.05 for hair and nail) after adjustment with age (Table S1). BChE activity is also found to be significantly associated with age and this result is in agreement with previous findings (Calderon-Margalit et al. 2006). In experimental mice, we have found that blood BChE levels were lower in Mn-exposed mice than the control mice. Mn exposure is associated with the behavioral changes related to spatial memory and learning, and accumulation of Mn in brain of the Mn-exposed mice is higher than the control group. These results imply that blood BChE activity could be a promising biomarker for Mn-induced peripheral neurotoxicity.
Results of the present study were novel, as the average value of Mn concentration was markedly higher than the recommended value in hair and nail, and BChE activity was declined in the human blood samples that are exposed to Mn-contaminated drinking water (Table 1). Previously, we have shown that plasma BChE activity in humans is reduced with accumulative levels of As in the drinking water from As-contaminated areas in Bangladesh (Ali et al. 2010). However, the presence of plasma BChE activity in Mn-exposed human subject has never been studied. BChE is primarily expressed in white matter and glia as well as in distinct populations of neurons in the human brain areas that are important in cognition and behavior (Darvesh 2016). Lower level of blood BChE activity is associated with neurotoxicity as well as Aβ plaque formation in AD (Patlolla and Tchounwou 2005; Mesulam and Geula 1994). It has been reported that serum BChE activity is dysregulated due to metal toxicity in Parkinson’s disease as well as Alzheimer’s-like syndrome (Peres et al. 2016). BChE activity reduction is linked with the severity of Parkinson’s disease-related dementia (Dong et al. 2017). In this study, we have noted that plasma BChE activity was significantly reduced in mice exposed to Mn when compared to that of control mice and more importantly in the Mn-exposed human samples. The changes in plasma BChE activity of Mn exposure and association of BChE with Parkinson’s disease-related dementia and Alzheimer’s-like syndrome inspired us to investigate the relationship between BChE activity in Mn exposure and neurobehavioral changes. However, there are limitations to carry out neurobehavioral analyses in Mn-exposed human subjects although a few studies reported Mn exposure and poor cognitive performance, and impairments in attention (Bowler et al. 2007a; Bowler et al. 2007b; Klos et al. 2006). To validate the results of our human study, we have performed neurobehavioral studies related to anxiety-like behavior, learning and memory impairment as well as the activity of plasma BChE in Mn-exposed mice. These studies suggest that exposure to Mn perturbs anxiety-like behavior, learning and memory impairment in addition to changes in plasma BChE activity in mice.
Behavioral procedures have been used in pharmacology and toxicology for many years to measure the effects of chemicals on the nervous system. Anxiety is a normal emotional response but when it is inappropriate then constitutes an anxiety disorder (Weinberger 2001). EPM test was performed for assessing anxiety-like behavior in the experimental mice and it was found that Mn-treated mice significantly (p < 0.05) decreased the time spent in open arms and increased the time spent in closed arms compared to the control mice (Fig. 3). It has also been shown that a change of anxiety-like behavior was observed in metal-treated mice (Aktar et al. 2017; Tanu et al. 2018). Here, a neuropsychological test through a maze was used to assess learning and memory impairment in Mn-exposed mice. This current study suggests that the latency time of Mn-exposed group of mice is prolonged to find the hidden platform in comparison with control mice almost all through the trials in a 7-day period. Outcomes of probe test showed that the Mn-treated group spent significantly less time compared with the control group in the desired quadrant (Fig. 4a, b). In addition to anxiety-like behavior, learning and memory impairment also observed in Mn-exposed mice in the Morris water maze tests suggesting a risk associated with humans exposed to contaminated drinking water in many areas in Bangladesh. In the present study, we could not explain exactly how Mn-induced neurobehavioral changes occurred. However, it is reported that Mn accumulated in brain basal ganglia particularly in the striatum, globus pallidus, and substantia nigra (Dorman et al. 2006; Guilarte et al. 2006). In this study, we also found a significantly higher concentration of Mn accumulated in the brain of Mn-exposed mice (Fig. 5).
Brain tissues are the important primary target of Mn toxicity as it induces oxidative stress in the brain (Crossgrove and Zheng 2004; Erikson et al. 2004). It is also known that Mn produces reactive oxygen species with the depletion of antioxidant defense mechanisms (Stredrick et al. 2004). Additionally, Song et al. (2016) reported that DR1 and NMDR played important roles in Mn-induced learning and memory dysfunction of striatum. Moreover, the excessive accumulation of Mn in the brain can lead to the development of a syndrome whose symptoms are similar to those of Parkinson’s disease and AD (Tong et al. 2014; Normandin and Hazell 2002; Aschner et al. 2007). BChE is associated with Aβ plaque deposition in human AD brain tissue as well as in AD mouse models (Mesulam and Geula 1994; Darvesh and Reid 2016; Reid and Darvesh 2015) and Aβ plaque deposition affects the subcortical regions, such as the amygdala, basal ganglia, thalamus, and brainstem (Diamant et al. 2006).
BChE is important in the regulation of cholinergic system (Reid et al. 2013) and chronic Mn toxicity selectively affect the cholinergic mechanism of central nervous system (Finkelstein et al. 2007). High concentration of Mn in brain impaired the regulation of the cholinergic system which may attribute in learning and memory impairment (Calabresi et al. 2001). Cholinergic system impairment induced by the accumulation of large amount of Mn in the brain may be involved to enunciate anxiety-like behavior, learning and memory impairment. Collectively, we assume that the neurobehavioral deficits associated with significant brain Mn deposition may be evaluated by the BChE activity. Further study is needed to explore the pathphysiological mechanism of Mn-induced BChE activity reduction and its impact on neurobehavioral alterations. Moreover, BChE activity may serve as a biomarker to evaluate the Mn-induced behavioral changes related to anxiety-like behavior, learning and memory impairment. However, there are some limitations in this study that warrant further discussion. First, the number of human subjects was limited that may limit the generalizability of the findings of the study. Second, we did not check the effects of Mn on iron status of human samples as well as experimental mice. There might also be other metals or chemicals along with Mn in the drinking water that might have confounding effects on the association between Mn exposure levels and BChE activity. If other metals or iron status have confounding effects on the association between Mn exposure and blood BChE activity, they would follow the same concentration gradients as Mn did. It is unlikely, however, we could not exclude the possibility. Third, in animal experiment, we used only single dose of Mn to treat the mice. A concentration-dependent experiment may provide the exact kinetics of the relationship of Mn exposure with BChE activity. In spite of these limitation, the present study through human and animal experiment provides the evidence that Mn exposure decrease the BChE levels that may at least in part implicate in Mn exposure-related neurobehavioral changes.
Conclusions
Results of this human and animal study clearly suggest that prolonged drinking of Mn-contaminated water is linked with the reduction of plasma BChE activity. This study also demonstrates that consumption of Mn-contaminated drinking water induces an anxiety-like behavior and causes learning and memory impairment. Neurobehavioral changes and reduced blood BChE activity in both Mn-exposed humans and animals indicate that blood BChE level may be a plausible indicator of Mn-induced neurotoxicity.
References
Aktar S, Jahan M, Alam S, Mohanto NC, Arefin A, Rahman A, Haque A, Himeno S, Hossain K, Saud ZA (2017) Individual and combined effects of arsenic and lead on behavioral and biochemical changes in mice. Biol Trace Elem Res 177:288–296
Ali N, Hoque MA, Haque A, Salam KA, Karim MR, Rahman A, Islam K, Saud ZA, Khalek MA, Akhand AA, Hossain M, Mandal A, Karim MR, Miyataka H, Himeno S, Hossain K (2010) Association between arsenic exposure and plasma cholinesterase activity: a population based study in Bangladesh. Environ Health 9:36
Aschner M, Guilarte TR, Schneider JS, Zheng W (2007) Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol 221:131–147
Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM (2009) Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol 239:169–177
BGS and DPHE (2001) Arsenic contamination of groundwater in Bangladesh, Final report, British Geological Survey and Department of Public Health Engineering. Available at https://www.bgs.ac.uk/downloads/start.cfm?id=2251
Bono GF, Simão-Silva DP, Batistela MS, Josviak ND, Dias PF, Nascimento GA, Souza RL, Piovezan MR, Souza RK, Furtado-Alle L (2014) Butyrylcholinesterase: K variant, plasma activity, molecular forms and rivastigmine treatment in Alzheimer’s disease in a southern Brazilian population. Neurochem Int 81:57–62
Bowler RM, Nakagawa S, Drezgic M, Roels HA, Park RM, Diamond E, Mergler D, Bouchard M, Bowler RP, Koller W (2007a) Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology 28:298–311
Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M, Smith D, Gwiazda R, Doty RL (2007b) Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med 64:167–177
Calabresi P, Ammassari-Teule M, Gubellini P, Sancesario G, Morello M, Centonze D, Marfia GA, Saulle E, Passino E, Picconi B, Bernardi GA (2001) Synaptic mechanism underlying the behavioral abnormalities induced by manganese intoxication. Neurobiol Dis 8:419–432
Calderón J, Ortiz-Pérez D, Yáñez L, Díaz-Barriga F (2003) Human exposure to metals. Pathways of exposure, biomarkers of effect, and host factors. Ecotoxicol Environ Saf 56:93–103
Calderon-Margalit R, Adler B, Abramson JH, Gofin J, Kark JD (2006) Butyrylcholinesterase activity, cardiovascular risk factors, and mortality in middle-aged and elderly men and women in Jerusalem. Clin Chem 52:845–852
Carobrez AP, Bertoglio LJ (2005) Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29:1193–1205
Correa M, Roig-Navarro AF, Aragon CM (2004) Motor behavior and brain enzymatic changes after acute lead intoxication on different strains of mice. Life Sci 74:2009–2021
Crossgrove J, Zheng W (2004) Manganese toxicity upon overexposure. NMR Biomed 17:544–553
Darvesh S (2016) Butyrylcholinesterase as a diagnostic and therapeutic target for Alzheimer’s disease. Curr Alzheimer Res 13:1173–1177
Darvesh S, Reid GA (2016) Reduced fibrillar beta-amyloid in subcortical structures in a butyrylcholinesterase-knockout Alzheimer disease mouse model. Chem Biol Interact 259:307–312
D'Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90
Diamant S, Podoly E, Friedler A, Ligumsky H, Livnah O, Soreq H (2006) Butyrylcholinesterase attenuates amyloid fibril formation in vitro. Proc Natl Acad Sci U S A 103:8628–8633
Dong MX, Xu XM, Hu L, Liu Y, Huang YJ, Wei YD (2017) Serum butyrylcholinesterase activity: a biomarker for Parkinson’s disease and related dementia. Biomed Res Int 2017:1524107
Dorman DC, Struve MF, Wong BA, Dye JA, Robertson ID (2006) Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol Sci 92:219–227
Erikson KM, Suber RL, Aschner M (2002) Glutamate/aspartate transporter (GLAST), taurine transporter and metallothione in mRNA levels are differentially altered in astrocytes exposed to manganese chloride, manganese phosphate or manganese sulfate. Neurotoxicology 23:281–288
Erikson KM, Dobson AW, Dorman DC, Aschner M (2004) Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ 334–335:409–416
Finkelstein Y, Milatovic D, Aschner M (2007) Modulation of cholinergic systems by manganese. Neurotoxicology 28:1003–1014
Fordahl SC, Erikson KM (2014) Manganese accumulation in membrane fractions of primary astrocytes is associated with decreased γ-aminobutyric acid (GABA) uptake, and is exacerbated by oleic acid and palmitate. Environ Toxicol Pharmacol 37:1148–1156
Frisbie SH, Ortega R, Maynard DM, Sarkar B (2002) The concentrations of arsenic and other toxic elements in Bangladesh’s drinking water. Environ Health Perspect 110:1147–1153
Guilarte TR, Mcglothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS (2006) Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol Sci 94:351–358
Hartmann J, Kiewert C, Duysen EG, Lockridge O, Greig NH, Klein J (2007) Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J Neurochem 100:1421–1429
Hearn AS, Stroupe ME, Cabelli DE, Ramilo CA, Luba JP, Tainer JA, Nick HS, Silverman DN (2003) Catalytic and structural effects of amino acid substitution at histidine 30 in human manganese superoxide dismutase: insertion of valine C gamma into the substrate access channel. Biochemistry 42:2781–2789
Hussam A, Habibuddowla M, Alauddin M, Hossain ZA, Munir AKM, Khan AH (2003) Chemical fate of arsenic and other metals in groundwater of Bangladesh: experimental measurement and chemical equilibrium model. J Environ Sci Health A Tox Hazard Subst Environ Eng 38:71–86
Karki P, Smith K, Johnson JJ, Aschner M, Lee E (2015) Role of transcription factor yin yang 1 in manganese-induced reduction of astrocytic glutamate transporters: putative mechanism for manganese-induced neurotoxicity. Neurochem Int 88:53–59
Klos KJ, Chandler M, Kumar N, Ahlskog JE, Josephs KA (2006) Neuropsychological profiles of manganese neurotoxicity. Eur J Neurol 13:1139–1141
Liang G, Zhang L, Ma S, Lv Y, Qin H, Huang X, Qing L, Li Q, Chen K, Xiong F, Ma Y, Nong J, Yang X, Zou Y (2016) Manganese accumulation in hair and teeth as a biomarker of manganese exposure and neurotoxicity in rats. Environ Sci Pollut Res Int 23:12265–12271
Lockridge O (2015) Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther 148:34–46
Loewenstein-Lichtenstein Y, Schwarz M, Glick D, Nørgaard-Pedersen B, Zakut H, Soreq H (1995) Genetic predisposition to adverse consequences of anti-cholinesterases in “atypical” BCHE carriers. Nat Med 1:1082–1085
Mesulam M, Geula C (1994) Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann Neurol 36:722–727
Normandin L, Hazell AS (2002) Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metab Brain Dis 17:375–387
Olanow CW (2004) Manganese-induced parkinsonism and Parkinson’s disease. Ann N Y Acad Sci 1012:209–223
Pal PK, Samii A, Calne DB (1999) Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology 20:227–238
Patlolla AK, Tchounwou PB (2005) Serum acetylcholinesterase as a biomarker of arsenic induced neurotoxicity in Sprague-Dawley rats. Int J Environ Res Public Health 2:80–83
Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M (2016) Manganese-inducedneurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol 17:57
Perl DP, Olanow CW (2007) The neuropathology of manganese induced parkinsonism. J Neuropathol Exp Neurol 66:675–682
Reid GA, Darvesh S (2015) Butyrylcholinesterase-knockout reduces brain deposition of fibrillar beta-amyloid in an Alzheimer mouse model. Neuroscience 298:424–435
Reid GA, Chilukuri N, Darvesh S (2013) Butyrylcholinesterase and the cholinergic system. Neuroscience 234:53–68
Robison G, Sullivan B, Cannon JR, Pushkar Y (2015) Identification of dopaminergic neurons of the substantianigra pars compacta as a target of manganese accumulation. Metallomics 7:748–755
Rodriguez VM, Carrizales L, Jimenez-Capdeville ME, Dufour L, Giordano M (2001) The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull 55:301–308
Santarpia L, Grandone I, Contaldo F, Pasanisi F (2013) Butyrylcholinesterase as a prognosticmarker: a review of the literature. J Cachexia Sarcopenia Muscle 4:31–39
Shenhar-Tsarfaty S, Berliner S, Bornstein NM, Soreq H (2014) Cholinesterases as biomarkers for parasympathetic dysfunction and inflammation-related disease. J Mol Neurosci 53:298–305
Shishova EY, Di Costanzo L, Emig FA, Ash DE, Christianson DW (2009) Probing the specificity determinants of amino acid recognition by arginase. Biochemistry 48:121–131
Sidoryk-Wegrzynowicz M, Aschner M (2013) Manganese toxicity in the central nervous system: the glutamine/glutamate-γ-aminobutyric acid cycle. J Intern Med 273:466–477
Song Q, Deng Y, Yang X, Bail Y, Xu B, Liu W, Zheng B, Wang C, Zhang M, Xu Z (2016) Manganese-disrupted interaction of dopamine D1 and NMDAR in the striatum to injury learning and memory ability of mice. Mol Neurobiol 53:6745–6758
Stefanidou M, Athanaselis S, Spiliopoulou H (2009) Butyrylcholinesterase: biomarker for exposure to organophosphorus insecticides. Intern Med J 39:57–60
Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, Lash L, Aschner M, Vrana KE (2004) Manganese-induced cytotoxicity in dopamine producing cells. Neuro Toxicology 25:543–553
Takeda A (2003) Manganese action in brain function. Brain Res Brain Res Rev 41:79–87
Tanu T, Anjum A, Jahan M, Nikkon F, Hoque M, Roy AK, Haque A, Himeno S, Hossain K, Saud ZA (2018) Antimony-induced neurobehavioral and biochemical perturbations in mice. Biol Trace Elem Res 186:199–207
Tasneem KM, Ali MA (2010) Assessment of manganese removal from groundwater using adsorptive filtration media. Proc of International Conference on Environmental Aspects of Bangladesh (ICEAB10), Japan
Tong Y, Yang H, Tian X, Wang H, Zhou T, Zhang S, Yu J, Zhang T, Fan D, Guo X, Tabira T, Kong F, Chen Z, Xiao W, Chui D (2014) High manganese, a risk for Alzheimer’s disease: high manganese induces amyloid-β related cognitive impairment. J Alzheimers Dis 42:865–878
Van Green A, Zheng Y, Goodbred JRS, Horneman A, Aziz Z, Cheng Z, Stute M, Mailloux B, Weinman B, Hoque MA, Seddique AA, Hossain MS, Chowdhury SH, Ahmed KM (2008) Flushing history as a hydrogeological control on the regional distribution of arsenic in shallow groundwater of the Bengal Basin. Environ Sci Technol 42:2283–2288
Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Zheng Y, Graziano JH (2007) Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 115:285–289
Weinberger DR (2001) Anxiety at the frontier of molecular medicine. N Engl J Med 344:1247–1249
Yousefi BV, Sadeghi L, Shirani K, Malekirad AA, Rezaei M (2014) The toxic effect of manganese on the acetylcholinesterase activity in rat brains. J Toxicol 2014:946372
Zhang QH, Li AM, He SL, Yao XD, Zhu J, Zhang ZW, Sheng ZY, Yao YM (2015) Serum total cholinesterase activity on admission is associated with disease severity and outcome in patients with traumatic brain injury. PLoS One 10:e0129082
Zwingmann C, Leibfritz D, Hazell AS (2004) Brain energy metabolism in a sub-acute rat model of manganese neurotoxicity: an ex vivo nuclear magnetic resonance study using [1–13C] glucose. Neurotoxicology 25:573–587
Funding
This work was supported by grants from the University of Rajshahi, Bangladesh (57-5/52/RABI/BINGAN-3/17-18) and we also thank MUSC Center for Global Health for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval (No: 661/320/IAMEBBC/IBSc and 67/320/IAMEBBC/IBSc) for both human and animal studies was taken from the Institutional Animal, Medical Ethics, Biosafety and Biosecurity Committee (IAMEBBC), Institute of Biological Sciences, University of Rajshahi, Bangladesh.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Table S1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Anjum, A., Biswas, S., Rahman, M. et al. Butyrylcholinesterase—a potential plasma biomarker in manganese-induced neurobehavioral changes. Environ Sci Pollut Res 26, 6378–6387 (2019). https://doi.org/10.1007/s11356-018-04066-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-04066-1