Abstract
This study investigated the toxicity of rats exposed to lead acetate (AcPb) during the second phase of brain development (8–12 days postnatal) in hematological and cerebral parameters. Moreover, the preventive effect of zinc chloride (ZnCl2) and N-acetylcysteine (NAC) was investigated. Pups were injected subcutaneously with saline (0.9% NaCl solution), ZnCl2 (27 mg/kg/day), NAC (5 mg/kg/day) or ZnCl2 plus NAC for 5 days (3rd–7th postnatal days), and with saline (0.9% NaCl solution) or AcPb (7 mg/kg/day) in the five subsequent days (8th–12th postnatal days). Animals were sacrificed 21 days after the last AcPb exposure. Pups exposed to AcPb presented inhibition of blood porphobilinogen-synthase (PBG-synthase) activity without changes in hemoglobin content. ZnCl2 pre-exposure partially prevented PBG-synthase inhibition. Regarding neurotoxicity biomarkers, animals exposed to AcPb presented a decrease in cerebrum acetylcholinesterase (AChE) activity and an increase in Pb accumulation in blood and cerebrum. These changes were prevented by pre-treatment with ZnCl2, NAC, and ZnCl2 plus NAC. AcPb exposure caused no alteration in behavioral tasks. In short, results show that AcPb inhibited the activity of two important enzymatic biomarkers up to 21 days after the end of the exposure. Moreover, ZnCl2 and NAC prevented the alterations induced by AcPb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is naturally found in the lithosphere and is typically released by erosion and volcanic activity [1]. Pb is a ubiquitous environmental contaminant due to its wide utilization in batteries, lead foil, ceramic, lead-based paint, and fishing sinkers [2]. This metal can be released by gasoline containing lead compounds [3, 4]. Population exposure occurs due to the intake of contaminated food and water as well as the inhalation of polluted air. Recent studies have shown that heavy metals, such as Pb, are found as contaminants in teas [5] and herbal preparations [6]. Furthermore, Kasperczyk et al. [7] demonstrated that employees who worked with Pb presented approximately 45 μg/dL of Pb in their blood.
Pb is a critical environmental pollutant and induces a wide range of physiological and biochemical dysfunctions [8]. Among such dysfunctions, Pb is known to cause neurological, hematological, immunological, renal, and hepatic alterations [1, 9, 10]. Additionally, Pb may cross the blood-brain barrier and accumulate in the brain [11], causing alterations in the function of cerebral enzymes, cognitive ability, and behavioral changes [12] in both human and experimental animals [13]. However, the biochemical and molecular mechanisms of Pb toxicity are not well elucidated despite numerous studies.
Pb exposure inhibits several enzymes involved in hemoglobin (Hb) biosynthesis such as porphobilinogen-synthase (PBG-synthase) [14], which is an important biomarker of Pb exposure [15]. This enzyme has sulfhydryl (-SH) groups that present high affinity to bivalent metals [16–19]. The PBG-synthase catalyzes the formation of porphobilinogen (PBG), which is a precursor of heme group [20–22]. Another enzyme that also is used as a biomarker of intoxication, mainly to organophosphates, is acetylcholinesterase (AChE). This enzyme plays an important role in the central nervous systems (CNS) by controlling synaptic transmission through the hydrolysis of the neurotransmitter acetylcholine (ACh) [23], ending the nerve impulses in the cholinergic synapses. AChE has -SH groups in its structure, which are a Pb target [24–26].
Organisms in development are extremely vulnerable to extern insults [16, 27]. In rodents, brain development is divided into three main stages: 0–6, 8–12, 17–23 days old [28, 29]. The second phase (8–12 days old) seems to be more susceptive to toxic agents, which may be due to accelerated myelin protein, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) synthesis [28].
Cases of poisoning by toxic metals, including Pb, have been treated with chelating agents such as ethylenediaminetetraacetic acid (EDTA), 2,3-dimercaptopropanol (BAL), and meso-2,3-dimercaptosuccinic acid (DMSA). These chelators bind to metals and decrease their toxicity [30]. However, due to their low specificity, they can bind to essential metals thus causing a homeostasis disturbance [31]. Therefore, studies have sought alternatives for treating Pb poisoning.
Zinc (Zn) and N-acetylcysteine (NAC) have protective functions against toxic agents. They have direct (NAC) [32] and indirect (Zn) [33, 34] action in the chelation of metals, in addition to being important in the antioxidant system [33, 35]. Zinc, which is an essential metal, is involved in the induction of glutathione [36], metal-binding proteins, and metallothionein (MT) biosynthesis [34, 37, 38]. Moreover, studies of our research group have shown the important preventive effect of Zn against mercury toxicity in rats [27, 33, 38–40]. Regarding NAC, it is a low molecular weight thiol-containing molecule that has the role of a free-radical scavenger, in addition to being a glutathione precursor [38, 41]. Recently, Sisombath and Jalilehvand [42] demonstrated that NAC molecules form a complex with the Pb(II) ion. Moreover, NAC has the ability to sequester metal cations such as Hg2+, Pb2+, Cd2+, and Zn2+ [43].
Since rats in early stages of development have high susceptibility to external aggression that can harm their development, this study aimed to investigate the toxicity of Pb in rats exposed during the second phase (8–12 days postnatal) of brain development in hematological, cerebral, and behavioral parameters. Furthermore, this work investigated the possible Zn and/or NAC preventive effects on alterations induced by Pb, since Zn and NAC properties would help in the protection of the toxic effects caused by Pb.
Material and Methods
Chemicals
Zinc (ZnCl2), lead (AcPb), N-acetylcysteine (NAC), sodium chloride (NaCl), dibasic (K2HPO4) and monobasic (KH2PO4) potassium phosphate, dibasic sodium phosphate (Na2HPO4), sucrose, ortho-phosphoric acid, absolute ethanol, nitric acid (HNO3), and chloridric acid (HCl) were obtained from Merck (Rio de Janeiro, RJ, Brazil); acetylthiocholine iodide (ATC), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), Tris [Tris (hydroxymethyl-d3) amino-d2-methane], Coomassie brilliant blue G, and bovine serum albumin were obtained from Sigma (St. Louis, MO, USA). P-dimethylaminobenzaldehyde was obtained from Riedel (Seelze, Han, Germany). The commercial kits for biochemical dosages were obtained from Labtest Diagnóstica S.A. (Lagoa Santa/MG/Brazil).
Animals
Pregnant Wistar rats obtained from the General Animal House of the Federal University of Santa Maria were transferred to our breeding colony and maintained at a 12 h light/dark cycle and controlled temperature of 22 ± 2 °C. All animals had free access to food (Guabi, RS, Brazil) and tap water. One day after birth, the number of pups of each litter was reduced to 8, and pups were kept with the dam until weaning. The total number of eight litters was used in the experimental procedure. Procedures involving animals were carried out in strict accordance with the guidelines of the Committee on Care and Use of Experimental Animal Resources of the Federal University of Santa Maria, Brazil (Process number-5320270415). All efforts were made to minimize the number of animals used and their suffering.
Exposures
The pups from each litter were randomly distributed into eight groups. The animals were subcutaneously (s.c.) exposed to 0.9% NaCl (saline solution), NAC (5 mg/kg), ZnCl2 (27 mg/kg) or ZnCl2 plus NAC for five consecutive days, and to saline or AcPb (7 mg/kg) for another five consecutive days. The compounds (Zn, Pb, and NAC) were dissolved in saline solution at a volume of 10 mL/kg body weight. The animals were weighed daily in order to adjust the dose. Each litter contained one rat for each treatment. Pup rats were submitted to protocol of exposure from the 3rd to the 12th postnatal day of their life as described in Table 1. Twenty-one days after the last dose of Pb, animals were weighed and subsequently anesthetized with isoflurane inhalation and euthanized by decapitation.
Behavioral Tests
Eight litters were submitted to negative geotaxis task, beaker test, tail immersion, and open field task. Each litter contributed only with one experimental n in each treatment.
Negative Geotaxis Task
On postnatal days 3, 5, 7, 9, 11, and 13, the animals were submitted to negative geotaxis reflex test that was carried out on a 30-cm-long and 20-cm-wide platform with an inclination of 30°. Pups were placed with the head down. The maximum latency for the reflex of negative geotaxis was 60 s for each session. Each trial consisted of the mean latency of five consecutive sessions. The trials were made before solution administration. Latency decrease of negative geotaxis reflex was considered as the improvement of the motor reflex response [44].
Tail Immersion Test
On postnatal days 13, 20, and 27, nociception was assessed in the tail immersion test as described in Franciscato et al. [27], with some changes. Rats were wrapped in a towel and 3.5 cm of tail was immersed in water bath (48 ± 1 °C). The time needed for the animal to deflect the tail was used as latency immersion. A cut-off time of 10 s was used to avoid tail tissue damage.
Beaker Test
The animals were submitted to the beaker test on postnatal days 17 to 20 (sessions 1 to 4) with an interval of 24 h between sessions as described in Peixoto et al. [45]. In this task, the capability of the rats to balance and move along the rim of a 2-L polypropylene beaker was observed. This beaker, which was 19.5 cm high and 14 cm in diameter, had an outward-curving top edge. The apparatus was placed on a workbench 1 m from the room floor. A dark refuge box, which had the inner dimensions 9.5 cm × 5.5 cm × 3.5 cm high and an 5 cm × 5.5 cm entrance platform, was clamped in order that the platform could rest on the spout of the beaker. Each rat was placed on the rim of the beaker facing the refuge at the furthest distance from it. Time points to reach the refuge, fall, or jump off the rim were recorded. A cut off time of 90 s was used for each session [46]. Results are presented as mean of latency to access to refuge.
Open Field Task
Open field task was carried out as described in Peixoto et al. [45], with some changes. The animals were submitted to open field at 30 days of age. The exploratory behavior was investigated in a rectangular open field, 57 cm of length × 45 cm of width × 43 cm of high wall, and was situated on the room floor. Each rat was placed in the lower left corner with its face to the wall, in a way that the animal could not to see the observer. During a period of 5 min, the following behaviors were recorded: latency to leave the initial area, crossing (number of areas entered), and rearing (incidence of head-lifting on the hind legs either against a vertical surface or unsupported). The behavior of exiting the initial area and crossing was considered when the animal placed the four paws inside another area.
Biochemistry Assays
PBG-Synthase Activity
Blood samples (collected with anticoagulant) were transferred to a recipient with distilled water, at 1:4 (v/v) proportions, and under constant agitation in ice bath for 10 min for full hemolysis. The technique was performed according with Sassa [47] by measuring the rate of product formation (porphobilinogen, PBG), as previously described by Peixoto et al. [39]. The incubation medium contained 2.2 mM δ-ALA and 76 mM potassium phosphate buffer (pH 6.8). After adding 200 μL of hemolyzed blood, the incubation was initiated and carried out for 120 min at 39 °C. The reaction was stopped by the addition of 10% trichloroacetic acid (TCA) containing 0.05 M HgCl2 and the PBG formation was measured with Ehrlich’s reagent using the molar absorption coefficient of 6.1 × 104 for Ehrlich-PBG salt. The specific enzymatic activity was expressed as nmol PBG formed/h/mg protein.
AChE Activity
Cerebrum and cerebellum were homogenized (1:10, w/v) in 10 mM Tris-HCl buffer, pH 7.2 with 160 mM sucrose. The homogenate was centrifuged for 10 min at 1000 g and the supernatant were frozen at −20 °C until analysis. AChE activity was determined by the method of Ellman et al. [48] modified as described by Pereira et al. [49]. The mixture assay contained 1.04 mM DTNB, 24 mM potassium phosphate buffer pH 7.2, and 25 μL of enzymatic material. It was pre-incubated for 2 min at 30 °C and the reaction was started with the addition of 0.83 mM ATC. The product from the reaction of thiocholine with DTNB was determined at 412 nm every 30 s for 2 min with a molar absorption coefficient of 1.36 × 104. The specific activity was expressed as μmol ATC hydrolyzed/h/mg protein.
Hb Content
For Hb level determination, 10 μL of blood (collected with anticoagulant and diluted 1:20 v/v in deionized water) was added to a medium containing 200 mM phosphate buffer, 120 mM potassium ferricyanide, 150 mM potassium cyanide, and surfactant (Triton X-100) for 5 min at room temperature. The Hb content was determined spectrophotometrically at 540 nm. The levels were expressed as g/dL.
Urea
Serum was separated from the total blood after centrifugation at 1050 g for 10 min. The incubation at 37 °C for 5 min was started by adding 10 μL of serum sample to a medium containing 19.34 mM phosphate buffer, pH 6.9, 58.84 mM sodium salicylate, 3.17 mM sodium nitroprusside, and urease (≥12.63 UK/L) using a Labtest commercial kit. The reaction was stopped by adding oxidant solution (final concentrations, 0.07 M NaOH and 3.01 mM sodium hypochlorite) and the mixture was incubated for 5 min in order to achieve color development. Urea levels were determined at 600 nm and expressed as mg/dL.
Creatinine
Serum was separated from the total blood after centrifugation at 1050 g for 10 min. Creatinine level estimation was carried out by measuring the quantity of product formed, creatinine picrate, and by using creatinine as standard using a Labtest commercial kit. The reaction was carried out in a medium containing 20.2 mM picric acid and 145.4 mM NaOH at 37 °C with 50 μL of serum. Creatinine levels were determined at 510 nm and expressed as mg/dL.
Determination of MT Levels
For MT assays, blood (collected with anticoagulant) was diluted, and liver and cerebrum tissues were homogenized in four volumes of cold 20 mM Tris-HCl buffer, pH 8.6 containing 0.5 mM PMSF as antiproteolytic agent, and 0.01% β-mercaptoethanol as a reducing agent. The homogenate was then centrifuged at 16,000 g at 4 °C for 30 min in order to obtain a supernatant containing metallothioneins. Aliquots of 1 mL of supernatant were added with 1.05 mL of cold (−20 °C) absolute ethanol and 80 μL of chloroform; the samples were then centrifuged at 6000×g for 10 min. The collected supernatant was combined with three volumes of cold ethanol (−20 °C), maintained at −20 °C for 1 h and centrifuged at 6000×g for 10 min. The metallothionein-containing pellets were then rinsed with 87% ethanol and 1% chloroform and centrifuged at 6000×g for 10 min. The pellet was resuspended in 150 μL 0.25 M NaCl and subsequently 150 μL 1 N HCl containing 4 mM EDTA was added to the sample. MT content was assayed as described in Peixoto et al. [39] using the colorimetric method with Ellman’s reagent at 412 nm [48]. Metallothionein concentration was estimated utilizing cysteine as a reference standard and expressed as μg of SH/g of tissue.
Protein Determination
Protein levels were determined by method of Bradford [50] using bovine serum albumin as standard.
Determination of Zn and Pb Levels
Metal levels were determined by inductively coupled plasma atomic emission spectrometry (ICPE-9000; Shimadzu Scientific Instruments). The samples of wet tissue were placed in vials and frozen at −20 °C until analysis. Samples were digested as previously described by Ineu et al. [51]. After digestion, samples were diluted with deionized water and the metals determined by ICPE-9000. The analytical standard of the metals (Merck®) was used to make the curve. The detection limit was considered 0.0025 ppm, which was the minimum measurable quantity for this method.
Statistical Analysis
Results were analyzed by one- (biochemistry assays and metal levels) or two-way ANOVA (behavioral tasks) followed by Duncan’s multiple range test when necessary. Effects were considered significant when p ≤ 0.05.
Results
Weight
Body, Cerebrum, and Cerebellum Weight
The body, cerebrum, and cerebellum weight were not altered by the treatments (Table 2).
Behavioral Tests
Negative Geotaxis Task
The negative geotaxis responses are shown in Fig. 1a. All groups presented the negative geotaxis reflex. Two-way ANOVA (eight treatments × six sessions) showed a significant effect of session [F(5280) = 301.43; p < 0.001], since all groups presented better performance throughout the sessions. The treatments did not interfere in the appearance of this reflex.
Latency of negative geotaxis reflex (a) and latency of access to refuge in the beaker test (b) of rats treated as described in Table 1. The results are presented as mean ± SEM (n = 8)
Tail Immersion Test
The tail immersion test latency was not altered by treatments (data not shown).
Beaker Test
Latency to access the refuge is shown in Fig. 1b. Two-way ANOVA (eight treatments × four sessions) revealed a significant effect of the session on latency to access to refuge [F(3, 168) = 112.19; p < 0.001], since all groups presented a better performance throughout the sessions. The treatments did not alter the performance of the animals.
Open Field Task
The performance of rats in the open field task was not altered by treatments (Table 3).
Biochemistry Assays
PBG-Synthase Activity
PBG-synthase activity is shown in Fig. 2. Pups exposed to AcPb exhibited a significant decrease in enzyme activity [F(7, 32) = 6.512; p < 0.001]. Zinc alone partially prevented the inhibitory effect of Pb, but NAC and Zn + NAC association did not prevent the AcPb inhibitory effect on enzyme activity.
Blood PBG-synthase activity of rats treated as described in Table 1. The specific activity is expressed as nmol PBG/h/mg protein. The results are presented as mean ± SEM (n = 5). Duncan’s multiple range test: different letters confer significant statistical difference among groups at least p ≤ 0.05
AChE Activity
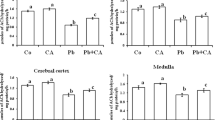
The cerebrum and cerebellum AChE activity are presented in Fig. 3a, b, respectively. Pups showed a significant decrease in cerebrum AChE activity [F(7, 40) = 4.005; p < 0.02] as a result of Pb exposure. All preventive treatments (Zn, NAC, and Zn + NAC association) protected the Pb inhibitory effect.
Cerebrum (a) and cerebellum (b) AChE activities of rats treated as described in Table 1. The specific activity is expressed as μmol ATC hydrolyzed/h/mg protein. The results are presented as mean ± SEM (n = 5–6). Duncan’s multiple range test: different letters confer significant statistical difference among groups at least p ≤ 0.05
Hb Content and Urea and Creatinine Levels
These parameters were not altered by treatments (Table 4).
MT Levels
Blood, cerebrum, and liver MT levels were not altered by treatments (Table 5).
Cerebrum and Blood Metal Levels
Lead and Zn levels are presented in Table 6. Pups exposed to AcPb presented Pb accumulation in the blood [F(7, 24) = 7.211; p < 0.001] and cerebrum [F(7, 16) = 80.213; p < 0.001]. The preventive treatments protected this Pb accumulation in both tissues. Zinc levels were similar between the treatment groups.
Discussion
This work investigated the sensitivity of young rats to AcPb exposure in biochemical and behavioral parameters as well as the protective effects of ZnCl2 and NAC. In this study, Pb exposure caused inhibition of blood PBG-synthase activity and cerebrum AChE activity. Zinc partially prevented the inhibition of blood PBG-synthase activity and all pre-treatments (Zn, NAC, and Zn plus NAC) prevented the cerebrum AChE activity decrease. Indeed, Pb exposure caused accumulation of this metal in the blood and cerebrum, and Zn, NAC, as well as their combination prevented such accumulation.
The enzyme PBG-synthase is an important biomarker of toxic effects induced by divalent metals [16, 40], mainly because of its -SH groups, which have high affinity to heavy metals [52]. Studies have shown that Pb decreases PBG-synthase activity. It is likely that Pb binds to the enzyme -SH groups thus interfering in the interaction of the substrate with the active site [17, 53]. In our work, Pb intoxication decreased about 60% of blood PBG-synthase activity. This result is in agreement with other studies that reported inhibition of this blood enzyme of rodents due to Pb exposure [54, 55], when investigated immediately after exposure. Additionally, this inhibition persists even several days after exposure, which confirms that the PBG-synthase activity measurement is a good marker of blood Pb exposure.
It is known that PBG-synthase is responsible for the second step of heme biosynthesis and consequently influences Hb synthesis [14]. However, considering that only 20% of the PBG-synthase activity is necessary for heme synthesis [56], the blood enzyme inhibition verified here was not enough to alter Hb levels.
Our study also shows a decrease in cerebrum AChE activity. Studies have shown that the central nervous system is a target of Pb [57]. This metal may cause dysfunction in the cholinergic system [24, 25, 58], which would be related to behavioral manifestations [2, 59]. In fact, one possible mechanism of Pb neurotoxicity is the inhibition of AChE attributed to high Pb affinity for -SH groups [26]. Lead may bind to AChE and cause the enzyme inhibition followed by the accumulation of ACh in the synaptic cleft [59].
In this work, we assessed the motor function necessary for the animal to respond to the negative geotactic reflex [44], muscular strength and cerebellar function necessary to balance in the rim and access to refuge in beaker test [45], the locomotor and exploratory activity in the open field task [45], and pain sensitivity in tail immersion test [27], in which the hyperalgesia response is generally attributed to central mechanisms [60]. Animals treated with Pb showed no alterations in behavioral activities in the tests performed. In the studies by Phyu and Tangpong [2] and Liu et al. [25], mice treated with drinking water with 1 g Pb/L for 2 months and 0.5 g Pb/L for 3 months, respectively, presented AChE inhibition and an impairment in water maze swimming test [2] and open field test [25] performance.
Animals treated with Pb presented blood and cerebrum Pb accumulation. This occurred in parallel to blood PBG-synthase and cerebrum AChE activity inhibition, respectively, as commented above. After absorption, Pb is distributed and accumulates in several tissues of the body, most notably in the bones, which is called storage compartment [21]. In humans, the half-life of Pb in the blood is approximately 30 days, although the total body Pb is, in great percentage, accumulated in the bone, with a half-life of decades [61]. In our study, analyses were performed 21 days after the last Pb administration, and the metal may have been moved to the bone and partly excreted in the feces and urine. Therefore, despite blood and cerebrum levels being 1.7 and 6 times higher than the control group, Pb levels are low when considered the high-dose injected.
Zinc prevented partially PBG-synthase inhibition. Zinc, NAC, and the association (Zn + NAC) prevented the decrease in the AChE activity and promoted a decrease in Pb levels in the blood and cerebrum. Studies have related that the supplementation with Zn decreases gastrointestinal absorption and distribution of Pb [34, 62, 63]. Moreover, Zn is known as an important inductor of metal-bind proteins, such as metallothionein, which has chelating properties [37, 38]. This property may explain the protective effects of Zn on Pb intoxication; however, the Zn groups did not present high MT content. Regarding NAC, Nehru and Kanwar [64] demonstrated that this may be a GSH precursor molecule, since NAC exposure restored GSH cerebrum levels depleted by Pb exposure. Moreover, NAC protective effect may be due to Pb(II) ion complex with the NAC molecule as suggested by Sisombath and Jalilehvand [42] and Cardiano et al. [43].
Conclusion
Results showed that AcPb exposure during the second phase of brain development alters biochemical parameters even long time after exposure. Moreover, the potential preventive action of Zn and NAC suggests that these compounds may serve as a promising alternative treatment for Pb poisoning. However, further investigations are necessary to better understand the mechanism by which Pb causes its toxic effects as well as to understand the preventive effects of Zn and NAC.
References
Berraha AA, Nehdi A, Hajjaji N, Gharbi N, El-Fazâa S (2007) Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. C R Biol 330:581–588
Phyu MP, Tangpong J (2013) Protective effect of Thunbergia laurifolia (Linn.) on lead induced acetylcholinesterase dysfunction and cognitive impairment in mice. Biomed Res Int. doi:10.1155/2013/186098
Godwin HA (2001) The biological chemistry of lead. Curr Opin Chem Biol 5:223–227
Pande M, Flora SJS (2002) Lead induced oxidative damage and its response to combined administration of α-lipoic acid and succimers in rats. Toxicology 177:187–196
Li X, Zhang Z, Li P, Zhang Q, Zhang W, Ding X (2013) Determination for major chemical contaminants in tea (Camellia sinensis) matrices: a review. Food Res Int 53:649–658
Nagarajan S, Sivaji K, Krishnaswamy S, Pemiah B, Rajan KS, Krishnan UM, Sethuraman S (2014) Safety and toxicity issues associated with lead-based traditional herbo-metallic preparations. J Ethnopharmacol 151:1–11
Kasperczyk S, Dobrakowski M, Kasperczyk A, Romuk E, Rykaczewska-Czerwinska M, Pawlas N, Birkner E (2016) Effect of N-acetylcysteine administration on homocysteine level, oxidative damage to proteins, and levels of iron (Fe) and Fe-related proteins in lead-exposed workers. Toxicol Ind Health 32:1607–1618
Gurer H, Ercal N (2000) Can antioxidants be beneficial in the treatment of lead-poisoning? Free Radic Biol Med 29:927–945
Casado MF, Cecchini AL, Simão ANC, Oliveira RD, Cecchini R (2007) Free radical-mediated pre-hemolytic injury in human red blood cells subjected to lead acetate as evaluated by chemiluminescence. Food Chem Toxicol 45:945–952
Dewanjee S, Sahu R, Karmakar S, Gangopadhyay M (2013) Toxic effects of lead exposure in Wistar rats: involvement of oxidative stress and the beneficial role of edible jute (Corchoruso litorius) leaves. Food Chem Toxicol 55:78–91
Richetti SK, Rosemberg DB, Ventura-Lima J, Monserrat JM, Bogo MR, Bonan CD (2011) Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology 32:116–122
Ademuyiwa O, Ugbaja RN, Rotimi SO, Abama E, Okediran BS, Dosumu OA, Onunkwor BO (2007) Erythrocyte acetylcholinesterase activity as a surrogate indicator of lead-induced neurotoxicity in occupational lead exposure in Abeokuta, Nigeria. Environ Toxicol Pharmacol 24:183–188
Cory-Slechta DA, Pokora MJ, Widzowski DV (1992) Postnatal lead exposure induces super sensitivity to the stimulus properties of a D2-D3 agonist. Brain Res 598:162–172
Wang Q, Zhao H, Chen J, Hao Q, Gu K, Zhu Y, Zhou Y, Ye L (2010) δ-Aminolevulinic acid dehydratase activity, urinary δ-aminolevulinic acid concentration and zinc protoporphyrin level among people with low level of lead exposure. Int J Hyg Environ Health 213:52–58
Patrick L (2006) Lead toxicity, a review of the literature. Part 1: exposure, evalution and treatment. Altern Med Rev 11:2–22
Rocha JBT, Pereira ME, Emanuelli T, Christofari RS, Souza DO (1995) Effect of treatment with mercury chloride and lead acetate during the second stage of rapid postnatal brain growth on δ-aminolevulinic acid dehydratase (ALA-D) activity in brain, liver, kidney and blood of suckling rats. Toxicology 100:27–37
Peixoto NC, Roza T, Pereira ME (2004) Sensitivity of δ-ALA-D (E.C. 4.2.1.24) of rats to metals in vitro depends on the stage of postnatal growth and tissue. Toxicol in Vitro 18:805–809
Perottoni J, Meotti FC, Folmer V, Pivetta L, Nogueira CW, Zeni G, Rocha JBT (2005) Ebselen and diphenyl diselenide do not change the inhibitory effect of lead acetate on delta-aminolevulinate dehydratase. Environ Toxicol Pharmacol 19:239–248
Scheuhammer AM (1987) Erythrocyte d-aminolevulinic acid dehydratase in birds. I. The effects of lead and other metals in vitro. Toxicology 45:155–163
Shemin D (1976) 5-Aminolaevulinic acid dehydratase: structure, function, and mechanism. Phil Trans R Soc Lond B 273:109–115
Klaassen CD (1996) Heavy metals and heavy-metals antagonists. In: Hardman JG, Giman AG, Limbird LE (eds) Goodman & Gilman’s the pharmacological basis of therapeutics, 9th edn. McGraw-Hill, New York, pp 1649–1671
Goyer RA (1996) Toxic effects of metals. In: Klaassen CD (ed) Casarett & Doull’s toxicology: the basic science of poisons, 5th edn. McGraw-Hill, New York, pp 691–736
Blokland A (1995) Acetylcholine: a neurotransmitter for learning and memory? Brain Res Rev 21:285–300
Alfano DP, Petit TL, Leboutillier JC (1983) Development and plasticity of the hippocampal-cholinergic system in normal and early lead exposed rats. Brain Res 312:117–124
Liu C, Zheng G, Ming Q, Sun J, Cheng C (2013) Protective effect of puerarin on lead-induced mouse cognitive impairment via altering activities of acetylcholinesterase, monoamine oxidase and nitric oxide synthase. Environ Toxicol Pharmacol 35:502–510
Reddy GR, Devi BC, Chetty CS (2007) Developmental lead neurotoxicity: alterations in brain cholinergic system. Neurotoxicology 28:402–407
Franciscato C, Goulart FR, Lovatto NM, Duarte FA, Flores EMM, Dressler VL, Peixoto NC, Pereira ME (2009) ZnCl2 exposure protects against behavioral and acetylcholinesterase changes induced by HgCl2. Int J Dev Neurosci 27:459–468
Gottlieb A, Keydar I, Epstein HT (1977) Rodent brain growth stages: an analytical review. Biol Neonate 32:166–176
Winick M, Noble A (1965) Quantitative changes in DNA, RNA, and protein during prenatal and postnatal growth in the rat. Dev Biol 12:451–466
Domingo JL (1998) Developmental toxicity of metal chelating agents. Reprod Toxicol 12:499–510
Cantilena LR Jr, Klaassen CD (1982) The effect of chelating agents on the excretion of endogenous metals. Toxicol Appl Pharmacol 63:344–350
Aruoma OI, Halliwell B, Hoey BM, Butler J (1989) The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6:593–597
Oliveira VA, Oliveira CS, Ineu RP, Moraes-Silva L, Siqueira LF, Pereira ME (2014) Lactating and non-lactating rats differ in sensitivity to HgCl2: protective effect of ZnCl2. J Trace Elem Med Biol 28:240–246
Roney N, Colman J (2004) Interaction profile for lead, manganese, zinc, and copper. Environ Toxicol Pharmacol 18:231–234
Chen W, Ercal N, Huynh T, Volkov A, Chusuei CC (2012) Characterizing N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) binding for lead poisoning treatment. J Colloid Interface Sci 371:144–149
Lange A, Ausseil O, Segner H (2002) Alterations of tissue glutathione levels and metallothionein mRNA in rainbow trout during single and combined exposure to cadmium and zinc. Comp Biochem Physiol C Toxicol Pharmacol 131:231–243
Eaton DL, Stacey NH, Wong KL, Klaassen CD (1980) Dose-response effects of various metal ions on rat liver metallothionein, glutathione, heme oxygenase, and cytochrome P-450. Toxicol Appl Pharmacol 55:393–402
Peixoto NC, Serafim MA, Flores EMM, Bebiano MJ, Pereira ME (2007) Metallothionein, zinc and mercury levels in tissues of young rats exposed to zinc and subsequently to mercury. Life Sci 81:1264–1271
Peixoto NC, Roza T, Flores EM, Pereira ME (2003) Effects of zinc and cadmium on HgCl2-δ-ALA-D inhibition and Hg levels in tissues of suckling rats. Toxicol Lett 146:17–25
Franciscato C, Moraes-Silva L, Duarte FA, Oliveira CS, Ineu RP, Flores EM, Dressler VL, Peixoto NC, Pereira ME (2011) Delayed biochemical changes induced by mercury intoxication are prevented by zinc pre-exposure. Ecotoxicol Environ Saf 74:480–486
Ercal N, Treeratphan P, Lutz P, Hammond TC, Matthews RH (1996) N-acetylcysteine protects chinese hamster ovary (CHO) cells from lead-induced oxidative stress. Toxicology 108:57–64
Sisombath NS, Jalilehvand F (2015) Similarities between N-acetylcysteine and glutathione in binding to lead(II) ions. Chem Res Toxicol 28:2313–2324
Cardiano P, Foti C, Giuffre O (2016) On the interaction of N-acetylcysteine with Pb2+, Zn2+, Cd2+ and Hg2+. J Mol Liq 223:360–367
Da-Silva VA, Malheiros LR, Bueno FMR (1990) Effects of toluene exposure during gestation on neurobehavioral development of rats and hamster. Braz J Med Biol Res 23:533–537
Peixoto NC, Roza T, Morsch VM, Pereira ME (2007) Behavioral alterations induced by HgCl2 depend on the postnatal period of exposure. Int J Dev Neurosci 25:39–46
Smart JL, Dobbing J (1971) Vulnerability of developing brain. II. Effects of early nutritional deprivation on reflex ontogeny and developmental of behavior in the rat. Brain Res 28:85–95
Sassa S (1982) Delta-aminolevulinic acid dehydratase assay. Enzyme 28:133–145
Ellman GL, Courtney KD, Andress JRV, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Pereira ME, Adams AIH, Silva NS (2004) 2,5-Hexanedione inhibits rat brain acetylcholinesterase activity in vitro. Toxicol Lett 146:269–274
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ineu RP, Oliveira CS, Oliveira VA, Moraes-Silva L, Luz SCA, Pereira ME (2013) Antioxidant effect of zinc chloride against ethanol-induced gastrointestinal lesions in rats. Food Chem Toxicol 58:522–529
Rooney JP (2007) The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 234:145–156
Goering PL (1993) Lead–protein interactions as a basis for lead toxicity. Neurotoxicology 14:45–60
Nakagawa K, Asami M, Kuriyama K (1980) Inhibition of release of lysosomal enzymes in young rat brain by lead acetate. Toxicol Appl Pharmacol 56:86–92
Rendón-Ramírez A, Cerbón-Solórzano J, Maldonado-Veja M, Quintanar-Escorza MA, Calderón-Salinas JV (2007) Vitamin-E reduces the oxidative damage on δ-aminolevulinic dehydratase induced by lead intoxication in rat erythrocytes. Toxicol in Vitro 21:1121–1126
Petrucci RA, Leonardi A, Battistuzzi G (1982) The genetic polymorphism of delta-ALAD in Italy. Hum Genet 60:289–290
Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH (2003) Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: in vivo evidence for Pb and Zn competition. Int J Dev Neurosc 21:1–12
Abdel Moneim AE (2012) Flax seed oil as a neuroprotective agent on lead acetate-induced monoamineric alterations and neurotoxicity in rats. Biol Trace Elem Res 148:363–370
Reddy GR, Basha MDR, Devi CB, Suresh A, Baker JL, Shafeek A, Heinz J, Chetty CS (2003) Lead induced effects on acetylcholinesterase activity in cerebellum and hippocampus of developing rat. Int J Dev Neurosc 21:347–352
Ramabadran K, Bansinath M, Turndorf H, Puig MM (1989) The hyperalgesic effect of naloxone is attenuated in streptozotocin-diabetic mice. Psychopharmacology 97:169–174
ASTDR (Agency for Toxic Substances and Disease Registry) (2007) Toxicological profile for lead. U.S. Department Of Health And Human Services, Atlanta
Peraza MA, Ayalo-Fierro F, Barber DS, Casarez E, Rael LT (1998) Effects of micronutrients on metal toxicity. Environ Health Perspect 106:203–216
Fowler BA (1998) Roles of lead-binding proteins in mediating lead bioavailability. Environ Health Perspect 106:1585–1587
Nehru B, Kanwar SS (2007) Modulation by N-acetylcysteine of lead-induced alterations in rat brain: reduced glutathione levels and morphology. Toxicol Mech Method 17:289–293
Acknowledgements
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 311082/2014-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources, Federal University of Santa Maria, Brazil (Process number-5320270415).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pedroso, T.F., Oliveira, C.S., Fonseca, M.M. et al. Effects of Zinc and N-Acetylcysteine in Damage Caused by Lead Exposure in Young Rats. Biol Trace Elem Res 180, 275–284 (2017). https://doi.org/10.1007/s12011-017-1009-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1009-z