Abstract
Biochar was carbon-rich and generated by high-temperature pyrolysis of biomass under oxygen-limited conditions. Due to the limitations of surface functional groups and the weakness of surface activity in the field of environmental remediation, the raw biochar frequently was chemically modified to improve its properties with a new performance. In this study, a kind of high-efficiency and low-cost amino biochar modified by nano zero-valent iron (ABC/NZVI) was synthesized and applied to paddy soil contaminated with arsenic (As). Dynamic changes of soil properties, arsenic speciations and rhizosphere microbial communities have been investigated over the whole growth period of rice plants. Pot experiments revealed that the ABC/NZVI could decrease the arsenic concentration in rice straw by 47.9% and increase the content of nitrogen in rice straw by 47.2%. Proportion of Geobacter in soil with ABC/NZVI treatment increased by 175% in tillering period; while Nitrososphaera decreased by 61 and 20% in tillering and maturity, respectively, compared to that of control. ABC/NZVI promotes arsenic immobilization in rhizosphere soil and precipitation on root surface and reduces arsenic accumulation in rice. At the same time, ABC/NZVI would inhibit Nitrososphaera which is related to ammonia oxidation process, and it would have a promising potential as soil amendment to reduce nitrogen loss probably.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paddy fields were contaminated by arsenic (As) widespread around the world which leads to the risk of the area where rice was consumed as the staple food (Fendorf et al. 2010; Wang et al. 2014). Rice is particularly efficient in accumulating As compared with other crops (Williams et al. 2007; Su et al. 2010). Many researches revealed that the daily ingestion of high As from rice products would be the main cause of chronic and acute diseases (Yang et al. 2007; Xu and Wang 2014). It is necessary to remediate the paddy field and decrease the bioavailability of As in soil. However, ex situ remediation technology is unsuitable because of the need of agricultural production, and it is an emergency to find a way to reduce As absorption during crop growth and to minimize health risk.
In previous studies, biochar improved soil physical and chemical properties (Glaser et al. 2001; Glaser et al. 2002; Mann 2002; Lehmann et al. 2006; Lehmann 2007), such as increasing the pH of acid soils or improving nutrient retention through cationic adsorption. Furthermore, biochar was also helpful in immobilizing heavy metals and reducing their bioavailability in soil (Hossain et al. 2010; Fellet et al. 2011; Méndez et al. 2012). Studies had shown that biochar could transform Cd into less bioavailable forms (Bian et al. 2013; Houben et al. 2013; Bian et al. 2014), while its performance on As transformation remained unclear (Khan et al. 2013; Wang et al. 2017). Recent studies tended to use biochar compound or mixture to achieve better improvement.
Nano zero-valent iron (NZVI) is an effective adsorbent for arsenic removal from drinking water, and elemental iron and iron (hydr)oxides are widely applied as the adsorbents for arsenic removal (Driehaus et al. 1998; Guo and Chen 2005; Guan et al. 2008). The biochar (BC) modified with Fe0 nanoparticles would improve the aggregation of NZVI and maintain reactivity. NZVI@BC had better stability and mobility than NZVI and could effectively reduce the bioavailability and bioaccumulation of Cr in cabbage mustard seedlings (Su et al. 2016). Amino modification is a promising method for enhancing the adsorption efficiency of metal ions on biochar because of complex functional group in the amino moiety, which would efficiently complex with heavy metals (Vogel and Mendham 1989; Buttry et al. 1999).
In our previous study, amino biochar modified by nano zero-valent iron (ABC/NZVI) was developed to remove Cd(II) from aqueous solutions. ABC/NZVI has significant adsorption performance (Yang et al. 2016). Before the application in farmland, it should be understood how the modified biochar influenced the physicochemical properties and microbial communities of soil and the bioavailability of As. In this study, a pot experiment was established for investigating the impacts of ABC/NZVI on soil microecology during the whole growth period of rice.
Material and methods
Biochar modification
The original biochar was produced by pyrolysis process. Dried palm shell was pyrolyzed at 500 °C for 8 h under oxygen-limited conditions. Then, biochar was grounded through a 200-mesh sieve and stirred with hydrochloric acid to remove salt. Surface amination (Yang and Jiang 2014) was achieved by nitration and reduction, and the main steps associated with were presented as follows:

Iron was reduced (Zhu et al. 2009) and loaded on amino-modified biochar according to Eq. (1):
After modification, amino group concentration of ABC/NZVI was 0.18 mmol/g, the content of carbon and iron was 29.6 and 28.1%, respectively, specific surface area was 244 m2/g and nitrogen content was doubled compared with pre-modified.
Soil sampling and characterization
Soil used in pot experiments was collected from the top 0–20 cm of farmland surrounding the Lianhuashan mining area, Guangdong Province (23°38′30.4″N, 116°50′4.7″E), which had been one of the largest tungsten mines in southern China (Liu 2010). The mining operation was closed in 1991. However, acid mine drainages (AMDs) from the mine tailing still flood in the rainy season and lead to the deposition of huge amounts of heavy metals in the farmlands. Previous studies demonstrated that As was the most important metal contaminant in these areas (Liu et al. 2012).
The soil sample was sieved through a 2-mm sieve for pot experiment. Physical and chemical properties of soil are given in Table 1. The total As content of the soils used in pot experiments is 36.5 mg kg−1; the soil was contaminated with As at a level exceeding the UK soil guideline value (SGV) on “safe” soil As concentrations (20 mg kg−1) (Environment Agency 2004), and also higher than the maximum allowable concentration (MAC) of total As for paddy soils in China (25 mg kg−1 in pH 6.5–7.5) (China 1995). ABC/NZVI was amended at a rate of 0.1% (w/w) for the experiment group (R1) and 0% for the control group (R0) before rice (Oryza sativa L.) transplantation.
Rice cultivation
Rice seedling of UU128 (from the Rice Research Institute of Guangdong Agricultural Academy) was transplanted into pots prefilled with soil (10 kg soil per pot), which was then flooded with water to 3 cm during rice growth. Soil samples at a depth of 0–10 cm surrounding rice root were collected in certain times. Growth of rice plants continued for 4 months. Soil samples for chemical and microbial analyses were collected in two periods: tillering period (30 days after seedling) and maturity period (125 days after seedling). Part of soil samples was sieved through 80 mesh for chemical analysis, and another was stored at −80 °C for DNA extraction. Rice plants were washed with distilled water and dried at 65 °C to constant weight for analysis.
Soil analysis
The pH of soil was determined with a soil to water ratio of 1:2.5 using a precision pH meter (Mettler–Toledo Instruments Co. Ltd., Shanghai, China). Soil organic matter content was detected by high-temperature thermal with K2Cr2O7 oxidation. Total nitrogen (TN) was analyzed with Kelvin-distillation titration. Available nitrogen was analyzed by method of alkaline hydrolysis diffusion (FeSO4 reduction and NaOH hydrolysis). Soil available phosphorus was extracted with hydrochloric acid-ammonium fluoride and determined by Mo-Sb colorimetry. Soil available potassium was extracted using ammonium acetate for measurement with flame photometry. Available sulfur was extracted with phosphate acetate for measurement with barium sulfate turbidimetry. Available iron was extracted using DTPA for measurement with flame atomic absorption spectrophotometry. Cationic exchange capacity was determined by method of ammonium acetate (soil treated with CH3COONH4 and then distillation and absorbed with H3BO3, HCl titration). The plant samples were digested by the mixed acids of HF, HClO4 and HNO3 then filtered by 0.45-μm membranes for measurement.
Speciation analysis
The Community Bureau of Reference (BCR) sequential extraction method was used to extract the speciation of heavy metals in soil, and the kinds of speciation were separated into exchangeable, reducible, oxidizable and residual fractions separately (Van Herreweghe et al. 2003). The extracted suspensions were filtered by 0.45-μm membranes, and the concentration of arsenic was determined by Atomic Fluorescence Spectrometer (AFS-9130, Beijing Titan Instruments Co., Ltd.). Soil redox potential was measured by in situ measurement of automatic ORP analyzer FJA-6 (Nanjing Teai-de Instrument & Equipment Co., Ltd.). Soil samples for BCR sequential extraction were collected in tillering period (30 days after seedling), elongation period (60 days after seedling), heading period (85 days after seedling) and maturity period (125 days after seedling).
DNA extraction and data processing
DNA extractions from soil samples were performed with the PowerSoil® DNA isolation kit (MO BIO Laboratories, Inc., Carlsbad, CA) according to the manufacturer’s instruction. Primers 515F (5′-CCGGACTACVSGGGTATCTAAT-3′); 907R (5′-CCGTCAATTCMTTTRAGTTT-3′) and 519F (5′-CAGCCGCCGCGGTAA-3′); 915R (5′-GTGCTCCCCCGCCAATTCCT-3′) which target the V4,V5 hypervariable regions of the bacterial and archaeal 16S rRNA genes, respectively, were selected. 18s-F(5′-TTAGCATGGAATAATRRAATAGGA-3′) and 18s-R (5′-TCTGGACCTGGTGAGTTTCC-3′) target the V5 hypervariable regions of the fungi 18S rRNA genes.
The target fragment was amplified by PCR according to standard procedures of NEBNext® Ultra™ DNA Library Prep Kit for Illuminato build database. High-throughput sequencing was completed on IlluminaMiseq PE300. Obtained sequences after quality control were classified according to 97% sequence similarity clustering, selecting representative sequences, comparing with RDP and Greengene database in order to acquire classification information of representative sequences at the same time.
Quality control and sequence assembly of bacteria, archaea and fungi sequencing analysis that will be made by the Perl script to remove the 3′end of a continuous quality value read less than 20 bases, then turned barcode sequence, double-ended fragment assembly with Mothur to obtain 16S rRNA and 18S rRNA gene sequences of V4 + V5 and V5 hypervariable region, respectively.
Results
Physical and chemical properties
As shown in Table 2, pH of soil with ABC/NZVI amendment was slightly higher than that of control in tillering and maturity periods. It means that ABC/NZVI can improve pH of soil, which might be due to the high pH of the biochar (Chan et al. 2007; Atkinson et al. 2010) and alkaline amino group on the surface of the modified biochar. The content of available nitrogen in soil with ABC/NZVI treatment increased by 1.5% in tillering and 1.7% in maturity period, and amino groups were introduced on biochar after modification which is probably the reason for available nitrogen increase. Available sulfur of R1-treated samples increased by 71.9% compared to control in the tillering period. The concentration of iron in soil with ABC/NZVI mixture increased by about 9.1% over the untreated one, while available iron in soil with ABC/NZVI amendment (R1) decreased by 18.17% in tillering and decreased by 5.69% in maturity, respectively, compared to the control (R0), indicating that large amount of available iron in R1 was transformed, which can be explained by enhanced pH and sulfur that promote the precipitation of Fe2+/Fe3+.
Arsenic fractions and redox potential
Heavy metals exist in several speciations after entering into the soil, and the migration ability and biological toxicity of different speciations are significantly different (Khan et al. 2013). The speciations of soil heavy metals are separated into weak acid extractable (exchangeable), reducible, oxidizable and residual fractions according to the BCR sequential extraction method. Exchangeable fraction can be directly absorbed by living things because of its strong migration, and it is considered as available fraction. Reducible and oxidizable fractions can transform to acid soluble species which can be utilized by living things under a certain condition, so they are considered as slow-release fractions. The residual fraction is fixed in soil lattice and hardly utilized by living things, and it is considered as an invalid fraction. The more the exchangeable fraction exists, the more active the soil heavy metal is and then the higher the bioavailability of heavy metal is (O'Dell et al. 2007). In contrast, the more the reducible, oxidizable and residual fractions exist, the lower the bioavailability is for the heavy metal.
The soil As fractions at the different rice growth stages were presented in Fig. 1c. Although soil arsenic speciations of experiment (R1) and control (R0) treatments had no obvious difference in different rice growth periods, the variation trend of soil arsenic speciations in R0 and R1 was consistent from the whole growth period of rice. Residual component significantly increased from tillering to elongation, and weak acid extractable, reducible and oxidizable components decreased correspondingly. However, the proportion of residual component decreased from elongation to heading, and weak acid extractable, reducible and oxidizable fractions were significantly improved. The proportion of residual fraction rose again from heading to maturity, and weak acid extractable, reducible and oxidizable components significantly reduced.
Soil redox potential value at depth of 5 (a) and 10 cm (b) (Ag-AgCl reference electrode). Redox potential was measured from the beginning of tillering to maturity. Tillering period corresponds to time 1–10 days, elongation period 10–40 days, heading period 40–66 days and maturity period 66–78 days; R0–5 and R1–5 are on behalf of the redox potential of control and experiment treatments at the depth of 5 cm. R0–10 and R1–10 are on behalf of the redox potential of control and experiment treatments at the depth of 10 cm. Fractions of arsenic in soil in different periods (c); WAS, weak acid extractable; RED, reducible; OXI, oxidizable; RES, residual; As(%), content ratio of arsenic of different fractions
Soil redox potential is presented in Fig. 1a,b, and soil samples collected in tillering, elongation and heading corresponded to 7, 37 and 62 days, respectively. There is a certain relationship between the arsenic fraction and the redox potential. Proportions of weak acid extractable, reducible and oxidizable fractions in tillering and heading are relatively higher than elongation and maturity periods. Redox potential of samples collected for BCR sequential extraction in tillering and heading was near 10 and 58 days (Fig. 1a,b), and the redox potential at these two time points was relatively lower. So, arsenic components were reduced with the decrease of redox potential, and the oxidizable and weak acid extractable increased at the same time. It can be concluded that increase in the redox potential can reduce the leaching toxicity of arsenic.
Microbial beta diversity
The Clone libraries of bacteria, archaea 16S rRNA and fungi 18S rRNA were determined by high-throughput along rice growth. Mothur trim.seqs program served to remove low-quality sequences and obtained representative classified information. A 97% similarity cut-off was used to group OTUs for downstream analysis.
We selected sequences from each sample and then calculated the β diversity (Fig. 2) and the similarity of bacteria, archaea and fungi sequences analyzed by weighted UniFrac library. The difference between R0.ma and R1.ma from bacteria (Fig. 2) was not significant based on Fast UniFrac P test with 500 permutations (P > 0.2), while differences among the other samples of bacterial, archaeal and fungal libraries were significant (P < 0.05), which shows that ABC/NZVI has a significant impact on rhizosphere microbial communities.
Principal coordinate analysis (PCoA) plots based on (a) bacterial, (b) archaeal and (c) fungal Clone libraries. PCoA plots were generated using Fast UniFrac analysis based on the weighted algorithm with normalization. Values in parentheses show the percentage of community variation explained by each coordinate. Black-filled squares represent the target community in tillering period, while the red solid round of maturity period. R0.ti, R1.ti represent the control group (R0) and experiment group (R1) in tillering period; R0.ma, R1.ma represent the control group (R0) and experiment group (R1) in maturity period
Microbial community composition
Bacteria
Figure 3 shows the phylum-level distribution of clones obtained from the libraries targeting bacteria, archaea 16S rRNA and fungi 18S rRNA. Proportions of the phyla Chloroflexi and Acidobacteria in R0 and R1 were increased from tillering to maturity. Proportions of the phyla Bacteroidetes, Firmicutes, Cyanobacteria and Plactomycetes were decreased from tillering to maturity. In addition, the relative abundance of Actinobacteria in R1 treatment was 2.3 and 0.9% lower than that of control in tillering and maturity periods, respectively. An interesting phenomenon was that the proportions of the phyla OD1, OP11 and WS6 showed a cooperative reaction to the ABC/NZVI (Fig. 3), generally higher than that of control in tillering, while the relative abundances of OP11 and WS6 were below 1% in maturity period. Proportions of the genera Kaistobacter, Flavisolibacter, Anaerolinea and Nitrospira in R0 and R1 were slumped from tillering to maturity in the level of genus classification (Fig. 4a), and that of Leptolyngbya was below 1% in maturity period.
Relative abundance distributions of the clones at phylum resolution in tillering and maturity periods. Unidentified genus is not shown here. Sequences were classified using the RDP and Greengene Classifier with a threshold level of 97%. R0.ti, R1.ti represent the control group (R0) and experiment group (R1) in tillering period; R0.ma, R1.ma represent the control group (R0) and experiment group (R1) in maturity period
Relative abundance distributions of the clones at genus resolution in tillering and maturity. (a) Targeting bacterial 16S rRNA at genus level; (b) targeting archaeal 16S rRNA at genus level; (c) targeting fungal 18S rRNA at genus level. Unidentified genus is not shown here. Sequences were classified using the RDP and Greengene Classifier with a threshold level of 97%
The relative abundance of Geobacter in soil with ABC/NZVI treatment was twice as much as that of control in tillering, and Geobacter species are anaerobic respiration bacterial species which have capabilities that use iron oxide or other available metals as electron acceptors (Childers et al. 2002). They have been found in anaerobic conditions in soil and aquatic sediment. Nano zero-valent iron on ABC/NZVI-enhanced soil iron oxides was probably the reason for Geobacter increase.
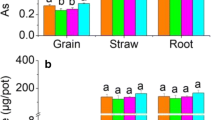
In comparison to the control, iron contents in rice straw and root (Table 3) with ABC/NZVI amendment increased by 167.3 and 5.2%, respectively, while arsenic in straw decreased by 47.9% and root increased by 7.4%, indicating that As was not directly absorbed by rice, but had settled on the root. Iron and arsenic might be adsorbed and precipitated on the layer of iron plaque which is thought to be generated by the excretion of oxygen and oxidants into the rhizosphere by physiologically active rice roots (Wu et al. 2012), suggesting that iron plaque is enhanced by the addition of ABC/NZVI and arsenic settled in iron plaque is not easily absorbed by rice, probably because arsenic is immobilized in iron plaque.
Archaea
Microbial communities of archaea in rhizosphere were dominated by Euryarchaeota, Crenarchaeota and Parvarchaeota from tillering to maturity (Fig. 3). ABC/NZVI induced a decrease of Crenarchaeota while Parvarchaeota increased. The relative abundances of archaea communities higher than 1.0% at genus level in samples were Themogymnomonas, Methanobacterium, Methanosaeta and Nitrososphaera in tillering period (Fig. 4b). However, proportions of the genera Themogymnomonas, Methanobacterium and Methanosaeta decreased to lower than 1% in maturity period.
The relative abundance of Nitrososphaera in R0 and R1 was 16.2 and 6.2% in tillering and 29.3 and 23.4% in maturity period, respectively, suggesting that ABC/NZVI has a great influence on Nitrososphaera which is a kind of ammonia-oxidizing archaea (AOA) (Tourna et al. 2011). Ammonia oxidation process is mainly controlled by ammonia-oxidizing archaea (Leininger et al. 2006; Pester et al. 2012). Nitrososphaera decrease probably lead to ammonia oxidation process be suppressed, resulting in NH4+-N increase and NO3−/NO2-N decrease.
The nitrogen concentrations increased by 47.2% in the straw and 5.5% in the root of the rice plant in soil with ABC/NZVI amendment compared to that of control (Table 3). NO3−/NO2−-N could not be well-utilized by rice because of the lack of nitrate reductase in the root of rice; therefore, NH4+-N in soil is more available for rice compared with NO3−/NO2−-N. So, ABC/NZVI increase of the nitrogen content in rice tissues might be mainly due to it suppressing the process of NH4+-N transformation to NO3−/NO2−-N by reducing the abundances of Nitrososphaera associated with ammonia oxidization. Moreover, due to denitrification, nitrate in the soil is easily lost, and ABC/NZVI can be used to reduce nitrogen loss and improve nitrogen uptake in rice.
Fungi
The dominated phyla of fungi in rhizosphere were Chytridiomycota, Basidiomycota and Ascomycota from tillering to maturity (Fig. 3c). The relative abundance of Chytridiomycota in ABC/NZVI-amended soil was doubled from tillering to maturity, and that of Basidiomycota was nearly four times as much as that of control. The proportion of Ascomycota in ABC/NZVI-treated soil was 26.4 and 19.5%, respectively, lower than control in tillering and maturity periods. Proportions of the genera (Fig. 4c) Endogone and Nuclearia were below 0.1% in tillering, and Camarops disappeared in maturity period.
Discussions
Fe0 is greatly susceptible to corrosion in aqueous media, being oxidized to Fe2+ (fast process) and Fe3+ (slower process) (Tosco et al. 2014). In natural waters, the preferred oxidant is the dissolved oxygen, whose presence results in a rapid corrosion according to Eq. (2). Fe2+ could be furthermore oxidated to Fe3+ by dissolved oxygen (Eq. (3)) with the precipitation of the less soluble ferric hydroxide (rust). Moreover, corrosion could occur also under anaerobic conditions by using water as the oxidant (Eq. (4)):
Under the natural conditions of the neutral pH environment, nano zero-valent iron on the surface of ABC/NZVI in rice soil will generate Fe(II)/Fe(III) compounds (amorphous Fe, including oxides and hydroxides, also known as hydrated iron). These unstable iron oxides recrystallize to form new stable minerals (such as ferrihydrite to goethite, lepidocrocite to magnetite) (Jeon et al. 2003; Hansel et al. 2005):
At the same time, ferrous iron (Fe2+) can catalyze the recrystallization of iron oxides (hematite, goethite and magnetite) (Latta et al. 2012; Handler et al. 2014), such as hematite forming amorphous ferric oxide (AFO) in the presence of ferrous iron, which further formed more complex minerals under different conditions.
Researchers believed that both As(III) and As(V) can be adsorbed by iron oxides, and surface complexation is the key to these processes (Gao and Mucci 2003; Waychunas et al. 2005). Arsenate adsorption at the surface of goethite (> FeOH) occurs in the following reaction:
Under anaerobic conditions in rice rhizosphere, FeS minerals in soil are capable of adsorbing arsenate and arsenite (Bostick and Fendorf 2003; Bostick et al. 2004):
ABC/NZVI’s huge surface area greatly improves the adsorption capacity, and arsenic adsorption and fixation occur in the iron oxide layer. It can be concluded that the role of nano zero-valent iron on the surface of ABC/NZVI is mainly responsible for arsenic settling and immobilization.
Conclusions
This study suggests a potential role of amino biochar supported by zero-valent iron (ABC/NZVI) in arsenic stabilization in paddy soil. ABC/NZVI decreased the accumulation of As and increased nitrogen absorption in rice, which also improved soil pH and available nitrogen. Microbial community abundances of Nitrososphaera associated with nitrogen transformation were suppressed under the amendment of ABC/NZVI, indicating that the potential application of ABC/NZVI reduces soil nitrogen loss and improves nitrogen utilization for rice. Furthermore, as balanced nutrient capital of ABC/NZVI for rice soil is uncertain, the quantity, quality and complementarity of ABC/NZVI, together with additional inputs (fertilizers, composts etc.), would need to be evaluated.
References
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Bian R, Chen D, Liu X, Cui L, Li L, Pan G, Xie D, Zheng J, Zhang X, Zheng J, Chang A (2013) Biochar soil amendment as a solution to prevent Cd-tainted rice from China: results from a cross-site field experiment. Ecol Eng 58:378–383
Bian R, Joseph S, Cui L, Pan G, Li L, Liu X, Zhang A, Rutlidge H, Wong S, Chia C, Marjo C, Gong B, Munroe P, Donne S (2014) A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J Hazard Mater 272:121–128
Bostick BC, Chen C, Fendorf S (2004) Arsenite retention mechanisms within estuarine sediments of Pescadero, CA. Environ Sci Technol 38:3299–3304
Bostick BC, Fendorf S (2003) Arsenite sorption on troilite (FeS) and pyrite (FeS2). Geochim Cosmochim Acta 67:909–921
Buttry DA, Peng JCM, Donnet J-B, Rebouillat S (1999) Immobilization of amines at carbon fiber surfaces. Carbon 37:1929–1940
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of greenwaste biochar as a soil amendment. Soil Res 45:629–634
Childers SE, Ciufo S, Lovley DR (2002) Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767–769
China, N. S. (1995) Environmental quality standard for soils. GB 15618-1995. National Environmental Protection Agency of China
Driehaus W, Jekel M, Hildebrandt U (1998) Granular ferric hydroxide—a new adsorbent for the removal of arsenic from natural water. J Water Supply Res Technol AQUA 47:30–35
Environment Agency (2004) https://www.gov.uk/government/organisations/environment-agency
Fellet G, Marchiol L, Delle Vedove G, Peressotti A (2011) Application of biochar on mine tailings: effects and perspectives for land reclamation. Chemosphere 83:1262–1267
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in south and southeast Asia. Science 328:1123–1127
Gao Y, Mucci A (2003) Individual and competitive adsorption of phosphate and arsenate on goethite in artificial seawater. Chem Geol 199:91–109
Glaser B, Haumaier L, Guggenberger G, Zech W (2001) The ‘Terra Preta’ phenomenon: a model for sustainable agriculture in the humid tropics. Naturwissenschaften 88:37–41
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils 35:219–230
Guan X-H, Wang J, Chusuei CC (2008) Removal of arsenic from water using granular ferric hydroxide: macroscopic and microscopic studies. J Hazard Mater 156:178–185
Guo X, Chen F (2005) Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ Sci Technol 39:6808–6818
Handler RM, Frierdich AJ, Johnson CM, Rosso KM, Beard BL, Wang C, Latta DE, Neumann A, Pasakarnis T, Premaratne WAPJ, Scherer MM (2014) Fe(II)-catalyzed recrystallization of goethite revisited. Environ Sci Technol 48:11302–11311
Hansel CM, Benner SG, Fendorf S (2005) Competing Fe(II)-induced mineralization pathways of ferrihydrite. Environ Sci Technol 39:7147–7153
Hossain MK, Strezov V, Yin Chan K, Nelson PF (2010) Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 78:1167–1171
Houben D, Evrard L, Sonnet P (2013) Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.) Biomass Bioenergy 57:196–204
Jeon B-H, Dempsey BA, Burgos WD (2003) Kinetics and mechanisms for reactions of Fe(II) with iron(III) oxides. Environ Sci Technol 37:3309–3315
Khan S, Chao C, Waqas M, Arp HPH, Zhu Y-G (2013) Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environ Sci Technol 47:8624–8632
Latta DE, Gorski CA, Scherer MM (2012) Influence of Fe2+-catalysed iron oxide recrystallization on metal cycling. Biochem Soc Trans 40:1191–1197
Lehmann J (2007) A handful of carbon. Nature 447:143–144
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adapt Strateg Glob Chang 11:395–419
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Liu C-P, Luo C-L, Gao Y, Li F-B, Lin L-W, Wu C-A, Li X-D (2010) Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern China. Environ Pollut 158:820–826
Liu C-P, Luo C-L, Xu X-H, Wu C-A, Li F-B, Zhang G (2012) Effects of calcium peroxide on arsenic uptake by celery (Apium graveolens L.) grown in arsenic contaminated soil. Chemosphere 86:1106–1111
Mann CC (2002) The real dirt on rainforest fertility. Science 297:920–923
Méndez A, Gómez A, Paz-Ferreiro J, Gascó G (2012) Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 89:1354–1359
O'Dell R, Silk W, Green P, Claassen V (2007) Compost amendment of Cu–Zn minespoil reduces toxic bioavailable heavy metal concentrations and promotes establishment and biomass production of Bromus carinatus (Hook and Arn.) Environ Pollut 148:115–124
Pester M, Rattei T, Flechl S, Gröngröft A, Richter A, Overmann J, Reinhold-Hurek B, Loy A, Wagner M (2012) amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ Microbiol 14:525–539
Su H, Fang Z, Tsang PE, Zheng L, Cheng W, Fang J, Zhao D (2016) Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J Hazard Mater 318:533–540
Su Y-H, McGrath SP, Zhao F-J (2010) Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 328:27–34
Tosco T, Petrangeli Papini M, Cruz Viggi C, Sethi R (2014) Nanoscale zerovalent iron particles for groundwater remediation: a review. J Clean Prod 77:10–21
Tourna M, Stieglmeier M, Spang A, Könneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci U S A 108:8420–8425
Van Herreweghe S, Swennen R, Vandecasteele C, Cappuyns V (2003) Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples. Environ Pollut 122:323–342
Vogel, A. I., and J. Mendham (1989) Vogel's textbook of quantitative chemical analysis. Longman Scientific and Technical. John wiley, New York
Wang Z-X, Hu X-B, Xu Z-C, Cai L-M, Wang J-N, Zeng D, Hong H-J (2014) Cadmium in agricultural soils, vegetables and rice and potential health risk in vicinity of Dabaoshan Mine in Shaoguan, China. J Cent South Univ 21:2004–2010
Wang N, Xue X-M, Juhasz AL, Chang Z-Z, Li H-B (2017) Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic. Environmental Pollution 220(Part A):514–522
Waychunas GA, Kim CS, Banfield JF (2005) Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms. J Nanopart Res 7:409–433
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41:6854–6859
Wu C, Ye Z, Li H, Wu S, Deng D, Zhu Y, Wong M (2012) Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? J Exp Bot 63:2961–2970
Xu P, Wang Z (2014) A comparison study in cadmium tolerance and accumulation in two cool-season turfgrasses and Solanum nigrum L. Water Air Soil Pollut 225:1938
Yang G-X, Jiang H (2014) Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res 48:396–405
Yang JE, Kim HJ, Ok Y-S, Lee J-Y, Park J (2007) Treatment of abandoned coal mine discharged waters using lime wastes. Geosci J 11:111–114
Yang, C., T. X. Ma, Z. Zhang, Z. Dang., C. L. Guo, G. N. Lu, and X. Y. Yi (2016) Iron-based amino composite modified charcoal material as well as preparation and application. in S. C. U. o. Technology, editor. State Intellectual Property Office, China
Zhu H, Jia Y, Wu X, Wang H (2009) Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J Hazard Mater 172:1591–1596
Acknowledgments
This study was funded by the National High Technology Research and Development Program of China (2013AA06A209) and the Science and Technology Planning Project of Guangdong Province, China (2016B020242004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Liu, S., Lu, Y., Yang, C. et al. Effects of modified biochar on rhizosphere microecology of rice (Oryza sativa L.) grown in As-contaminated soil. Environ Sci Pollut Res 24, 23815–23824 (2017). https://doi.org/10.1007/s11356-017-9994-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9994-1