Abstract
This study aimed to determine effects of biochar derived from wheat straw at 500 °C on arsenic immobilization in a soil-Brassica campestris L system. When the soils amended with 4% modified biochar (MBC), 0.5% Fe grit as zero-valent iron (ZVI), 0.5% Fe grit + 4% MBC (ZMBC), 0.5% ZVI + 4% biochar (ZBC), 4% biochar (BC), and control (without amendments), it confirmed that available arsenic concentration in soils occurred in the following order: ZMBC < MBC < ZVI < ZBC < Control < BC. Water-soluble As (WSAs) was reduced by 89.74% and 92.30% in MBC- and ZMBC-amended soils, respectively, compared to the control. When MBC applied into soil, As uptake of shoot and root decreased by 44.55% and 45.40%, respectively, and ZMBC resulted in 74.92% and 71.80% reduction in shoot and root As of Brassica campestris L. Immobilization effect of As in ZBC was also observed though BC elevated plant As uptake significantly. The immobilization effect of MBC was mainly attributed to Fe2O3 impregnation illustrated by x-ray diffraction (XRD) and scanning electron microscopy (SEM) images through sorption, precipitation, and coprecipitation. Such Fe containing complexes might impede As translocation from root to shoot and subsequently reduce As accumulation in the plant with modified biochar amendment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a ubiquitous metalloid found in the environment that is highly toxic to human, and its contamination in soils is a significant concern. In some countries such as North–East USA, Sonora (Mexico), Pamplonian Plain (Argentina), West Bengal (India), and Northern Chile, especially in South and Southeast Asia, many As toxicity incidents have been reported (Argos et al. 2010; Zhu et al. 2008), mainly due to the consumption of As-contaminated soil, As-contaminated water, and As-contaminated agricultural products. China is one of the most seriously affected countries, and people in many regions like inner Mongolia, Hunan, Guangxi, Guangdong, and West Taiwan coastal regions are suffering from the As-contaminated water, agricultural soil, vegetables, and grain, and the human health is threatened seriously (Li et al. 2014, 2017a; Zhao et al. 2014). In Shantou abandoned tungsten mine region in Guangdong Province, it has been found to contain As concentrations of up to 1226.5 mg kg−1 in soils (Liu et al. 2005). In the industrial districts of Chenzhou City, Southern China, influenced by high As-containing water, it was found that As in agricultural soils and rice grain reached 1217 mg kg−1 and 7.5 mg kg−1 (dry weight), respectively; arsenic concentrations in 95% of the total human hair samples in the contaminated districts were above the critical value 1.0 mg kg−1, set by the World Health Organization (Liao et al. 2005). Similarly, in the mining area of Shimen County, Hunan Province, affected by smelting activities of local realgar ores, the highest level was up to 932.10 mg As kg−1 in surface agricultural soils, 0.84 mg As kg−1 in rice, and 0.79 mg As kg−1 fresh weight in vegetables, and corresponding health risk index was much higher than that of recommend by WHO (Li et al. 2010). In the past 20 years, accumulation of As in farmlands was reported occasionally, especially for those under intensive cultivation due to abundant application of livestock manure containing As in China (Zeng et al. 2007). Remediation of As-contaminated farmland and control of As transfer in soil-plant system are necessary to meet the needs of increasingly enhanced population, land shortage, degradation of cultivated soil quality, and food safety in China.
Compared with conventional remediation options, such as soil excavation and dumping and ex situ and in situ soil washing/flushing, chemical stabilization has shown to be less destructive, cost-effective, and operational simplicity alternatives (Dermont et al. 2008; Shi et al. 2009; Komárek et al. 2013; Clemente et al. 2015; Sun et al. 2017). Immobilization techniques of heavy metals through sorption or precipitation reactions induced by soil amendments have gained considerable interest in recent years due to the potential in reducing metal’s mobility and bioavailability in the amended soil. Several sorbents, such as activated carbon (AC), Fe-coated granular AC and fly ash, Fe oxides-based nano-adsorbents, and Fe sulfides, have been used to remove As from water (Shakoor et al. 2016; Niazi and Burton 2016). Natural iron oxide minerals (magnetite, hematite, and goethite) have been effective in adsorbing arsenic from solution (Aredes et al. 2012). According to the research of Xiu et al. (2015), removal of As in solutions by bacterial-induced ferrihydrite was attributed to coprecipitation and adsorption, and As species transformation occurred in this process, which was expected to remediate of arsenic-polluted groundwater (Li et al. 2016). Many attempts has been made for As-polished soil remediation with immobilization amendments, and Fe-containing adsorbents were often used as amendments in reducing As uptake by farmland plants successfully, such as tomatoes, rice, and lettuce (Hartley and Lepp 2008; Lee et al. 2011). As is known to all, arsenic in soil is generally associated with Fe oxides and hydroxides (Hale et al. 1997), and iron Fe-containing materials like Fe oxides have been proven to act as amendments for the in situ immobilization of As in agricultural fields, significantly reduced As bioavailability in soil, and prevented As accumulation in crops and transfer to the food chain (Miretzky and Cirelli 2010; Madeira et al. 2012; Farrow et al. 2015). Zero-valent iron (ZVI, Fe(0)), usually in the form of Fe grit, oxidizes within the soil to form poorly crystalized Fe oxides and hydroxides that bind both As, and is often recommended to remediate As-contaminated soil. Besides, the use of Fe(0) avoids the acidification of soil caused by Fe(II) and Fe(III) sulfate additions, which can potentially mobilize cations. It should be mentioned that Fe can cause soil structure problems, such as aggregate cementation and reduced soil porosity when the application rate is higher than 5% w/w (Kumpiene et al. 2008). Similar to Fe oxides, zero-valent iron can be utilized for immobilization of As in soils provided that it can be used properly.
Recently, the application of biochar (BC) to agricultural farmland has attracted much interest for many environmental benefits like enhancing soil pH, improving plant yield, reducing greenhouse gas emission, and immobilizing heavy metal in soils (Laird et al. 2010). Especially, biochar can be used as amendments since it contains micro- to mesoporus structures, huge specific surface area, different surface functional groups, including carboxylic, hydroxyl, carbonyl, alcoholic, and lactone groups, and some inorganic mineral species (e.g., CaCO3, PO43−), which strongly affect the transport and bioavailability of contaminates in natural system (Li et al. 2017b; Zhang et al. 2016). Many investigators have reported that the bioavailability of cadmium (Cd), lead (Pb), and other heavy metals is reduced significantly following the addition of BC to soil (Venegas et al. 2015). Biochars derived from various types of feedstocks, such as pine wood and bark, walnut shell, oak wood and bark, rice husks, perilla leaf, biosolids, and animal products, have been used to remove As in aqueous solutions efficiently (Duan et al. 2017; Niazi et al. 2018a, b). However, the sorption of negative-charged arsenic in the form of oxyanions onto biochar surfaces is restricted due to the predominance of net negative charge on most biochar surfaces. Biochar amendments have been proven to enhance As reduction, resulting in the high As toxicity (Chen et al. 2016), and new techniques have been developed to produce engineered BC with enhanced sorption ability to organic and inorganic contaminates (Liu et al. 2018). With certain modifications, either physical or chemical treatment, the biochar utility can be enhanced, rendering it suitable for different environmental application (He et al. 2018; Trakal et al. 2018). Many researchers have revealed that modified biochar had excellent ability of removing arsenic in aqueous solutions (Zhang et al. 2013, 2016; Agrafioti et al. 2014). In the meanwhile, Bakshi et al. (2018) demonstrated that zero-valent iron (ZVI)–biochar complex was effective for removing As from contaminated drinking water because of Fe oxide-biochar composites produced by pyrolysis of FeCl3 salt with biomass (Chen et al. 2011). The impregnation of biochar with Mn oxides has been proven beneficial for the immobilization of potentially cost-effective adsorbents for the remediation of As in soils (Yu et al. 2017). It is well known that As species in most soils exist mainly as oxyanions and exhibit a high affinity for Fe oxides. In order to meet the requirements of As-contaminated soil remediation, combined mixture ZVI and biochar, Fe-modified biochar can be expected to play an important role in the near future. In addition, Fe-biochar composite had an apparently higher porosity in comparison with the powder of Fe(0) or Fe oxyhydroxides (Hanaoka and Okumura, 2014), which could thus minimize their negative effect on soil aggregate cementation.
Up to now, research on remediation of As-contaminated soils using Fe-modified biochar is very limited, though the ability of modified biochar on removing As in solution has been demonstrated by many facts (Zhang et al. 2016). The effect of modified biochar on As mobility and transfer in soil-plant system has not been completely recognized, and less attention has been paid to the corresponding technology adopted in agricultural production system. In this study, we manufactured Fe-modified biochar (MBC) originated from wheat straw and compared the immobilization effects among different amendments, including MBC, zero-valent iron (ZVI), BC, and their complexes, for remediation of As-contaminated subtropical red soils in southern China. The study was based on the hypothesis that BC can increase plant yield and improve soil quality, while ZVI is able to immobilize As in soils, which could also be realized by MBC alone or by MBC mixture with ZVI (Fe0). The immobilization efficiency of each treatment was measured in microcosm trials using an As-contaminated soil. The uptake of As by vegetables from different soils grown under field conditions was evaluated, and the ability of MBC and of MBC in combination with ZVI to reduce the plant uptake of As in soils was determined. The mechanisms underlying the immobilization and transformation effects of these amendments on As in agricultural soil were also investigated.
Materials and methods
Soil and amendments
Contaminated soil for this investigation was collected from mining spoil used for vegetable cultivation at an industrial and mining area in Chenzhou City, Hunan Province, China. Soil samples were homogenized, air-dried, crushed, and sieved to < 2 mm. The average soil As concentration was 95.6 mg kg−1, and the other main physical and chemical properties were as follows: pH = 7.05, organic matter content = 2.60%, total nitrogen (TN) = 1.77%, total phosphorous (P) = 0.45%, cation exchange capacity (CEC) = 14.9 cmol kg−1, silt content = 48.64%, sand content = 25.99%, and clay content = 25.47%. The amendments applied to the soil were ZVI (Fe > 99%, Mn ≤ 0.35%, silicon (Si) ≤ 0.10%, C ≤ 0.03%, S ≤ 0.02%, p ≤ 0.02). Biochar was produced from wheat straw at 500 °C for 4 h in a high-performance automatic furnace at Shangqiu City, Henan Province. The property of biochar was as follows: pH = 10.25, Brunauer–Emmett–Teller (BET) = 4.12 m2 g−1, and Fe2O3 = 1.03%, and that of MBC was as follows: pH = 7.92, BET value = 107.62 m2 g−1, and Fe2O3 = 28.43%. The MBC was produced according to the following procedure. The original BC was first treated with 200 mL of 1 M HCl for 12 h and then washed with distilled water continuously until the pH of leached liquid was neutral. After this procedure, about 100 g of the pretreated BC was added to 1000 mL of 0.5 M FeCl3 solution, with NaOH then added to adjust to pH = 7. The solution was stirred at room temperature, immersed in the FeCl3 solution for 24 h, and then vacuum-filtered and dried in an oven at 105 °C for over 8 h. This enabled Fe oxides (e.g., Fe2O3) to be loaded on the BC. The treated BC was then allowed to cool, broken into pieces, and rewashed with double deionized water until the water was clean and the pH of the leached liquid was neutral. It was then dried at 105 °C and finally placed in a vacuum drier for future use, with the final product being MBC.
Pot experiments
Six treatments were applied in pot experiments, with different ratios of the amendment and soil: control (no addition); 0.5% zero-valent iron, ZVI; 4% biochar, BC; 4% modified biochar, MBC; 0.5% ZVI + 4% BC, ZBC; and 0.5% ZVI + 4% modified biochar, ZMBC. Each treatment was repeated three times. Then, 1 kg of soil was added to each pot. Contaminated soil was manually mixed with BC (4% w/w), ZVI (0.5% w/w), MBC (4% w/w), ZVI (0.5% w/w) combined with BC (4% w/w) labeled as ZBC, and ZVI (0.5% w/w) combined with MBC (4% w/w) labeled as ZMBC using an end-over-end method in a sealed drum until a visually homogeneous incorporation was achieved. The mixture was left to equilibrate for 48 h in the dark and then placed into triplicated 1 L pots. Deionized water was added to each pot to reach 70% of the field moisture capacity, which was held constant throughout the entire experiment. Chemical fertilizer was added to all treatments (N:P2O5:K2O ratio 5:6:4), together with 0.15 g urea, 0.18 g KH2PO4, and 0.12 g KCl. Brassica rapa (campestris) L. was selected as the “green and green stem” cabbage cultivated by Beijing Jingyi Seedling Vegetable Research Centre, China. Ten seeds were planted in each pot, and when the cabbage grew to the third leaf, five seedlings were removed, and the remaining five seedlings were planted. Using the constant weight method, the soil moisture was determined to be 70% of the field moisture capacity throughout the entire procedure. The pot experiment was conducted in a greenhouse on the roof of the Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agriculture Sciences. The plants were harvested, and rhizosphere soil was collected after 45 days.
The plant samples were rinsed with deionized water and then heated at 105 °C for 30 min, dried at 65 °C to achieve constant weight, and crushed for total As analysis. Rhizosphere soil samples were dried in the shade, crushed using an agate mortar, and then sieved through a 0.85-mm and 0.15-mm mesh, respectively. The soils sieved through the 0.85-mm mesh were used to determine As fractions, and the soils sieved through the 0.15-mm mesh were used for total As analysis.
Chemical analysis
Soil pH was measured potentiometrically at a 1:2.5 ratio of soil to H2O after 1 min of shaking. Water-soluble As (WSAs) was extracted with deionized water at a 1:10 ratio of soil to water (Casado et al. 2007). Soil-available As was extracted with 0.5 mol L−1 NaHCO3 (Woolson et al. 1971) and measured by hydride generation atomic fluorescence spectrometry (HG-AFS). The CEC was measured by the ammonium acetate (1 mol L−1, pH = 7.0) saturation method. Total organic carbon (TOC) and TN were measured in an automatic microanalyzer (EuroEA3000; Eurovector, Milan, Italy). Dissolved organic carbon (DOC) was measured in an automatic analyzer for liquid samples (TOC-V CSN Analyzer, Shimadzu, Tokyo, Japan), after nylon-membrane (0.45 μm) filtration. Water-soluble Fe was determined by an atomic absorption spectrophotometer (SHIMADZU 6800G/F). Total P was analyzed using the method recommended by Lu (1999).
Total As concentration
Pulverized tissue samples (0.5 □g) were slowly heated and digested in 10 mL of 68% HNO3, and 2 mL of HClO4 on a hot plate. When the mixture became clear, the volume was adjusted to 50 mL with ultrapure water. Then, 0.5 g of sieved soil (0.15 mm) was digested with 10 mL 68% HNO3 and 10 mL 30% H2O2 until the soil had turned to a gray white color and the supernatant became slightly yellowish. The digestion solution was filtered, and the volume was adjusted to 50 mL. The digested plant and soil samples were analyzed for total As concentration by HG-AFS (detection limit < 0.02 μg L−1 As, model 9120; Jitian Instrument Co., Beijing, China). All chemicals were of analytical grade or better. National standard plant reference materials (GBW10015, GBW-07404) were used for quality assurance and quality control (QA/QC).
As fraction
An improved sequential extraction method, proposed by Wenzel et al. (2001) was adopted for use in this study. First, 1 g of soil was placed in a 50 mL centrifuge tube. The As fractions were extracted according to the following five extraction steps: (1) 0.05 M (NH4)2SO4, 20 °C/4 h; (2) 0.05 M NH4H2PO4, 20 °C/16 h; (3) 0.2 M NH4+-oxalate buffer in the dark, pH 3.25, 20 °C/4 h; (4) 0.2 M NH4+-oxalate buffer + ascorbic acid, pH 3.25, 96 °C/0.5 h; and (5) HNO3/H2O2 microwave digestion. The five sequential extraction steps were assumed to correspond to these As fractions primarily associated with (1) non-specifically sorbed (F1); (2) specifically sorbed (F2); (3) amorphous and poorly crystalline hydrous oxides of Fe and Al (F3); (4) well-crystallized hydrous oxides of Fe and Al (F4); and (5) residual phases. Soil samples were digested with HCl and HNO3 (NY/T. 1121.11, 2006), and total As was measured by HG-AFS (detection limit < 0.02 □g L−1 As, model 9120; Jitian Instrument Co.).
Characterization
The composition of each sample was characterized by x-ray diffraction (XRD; D/max2550 VB + 18 kW). The surface composition of BC and MBC was determined by x-ray photoelectron spectroscopy (XPS), which was conducted using a Kalpha 1063 instrument (Thermo Fisher Scientific, Waltham, MA, USA) using the Al Ka x-ray as the excitation source. Morphological changes of the samples were observed by scanning electron microscopy (SEM) (Nova NanoSEM 230; Thermo Fisher Scientific). The specific surface area of the BC and MBC was measured with a Monosorb Autosorb instrument (Quantachrome, Boynton Beach, FL, USA) following the classic BET method.
Statistical analysis
An analysis of variance (ANOVA) was used to determine the effects of the different amendment treatments on water-soluble As, NaHCO3-extracted As, and plant tissue As. All statistical analyses were performed using SPSS for Windows software (ver. 14.0; SPSS Inc., Chicago, IL, USA).
Results
Influence of soil amendments on plant growth and As uptake
Figure 1 shows that the application of the different materials directly to the soil resulted in a variety of yield changes. The largest biomass, of 4.06 g pot−1, was found in the BC treatment, which was significantly higher than that of the control, with the biomass in the MBC treatment also increasing to some extent. No significant difference was found between the control and the other treatments (ZVI, MBC, ZBC, and ZMBC), although the yield was reduced to some extent (< 5%) in the ZVI and ZMBC treatments. Therefore, for all treatments, any negative effect of the soil amendments on the growth of B. campestris L. could be ignored.
The biomass of Brassica campestris L. grown for 45 days in arsenic (As)-contaminated soil with different soil amendments. Results are provided as the fresh weight mean ± standard error of the mean, n = 3 (n = 5 for the untreated control). Different letters indicate significant differences between treatments at p < 0.05 [zero-valent iron (ZVI), 0.5% by soil weight, BC (4%), a mixture of ZVI (0.5%) + BC (4%) (ZBC), modified biochar (MBC, 4%), or ZVI (0.5%) + MBC (4%) (ZMBC)]
Among the different treatments, the concentration of As in shoots and roots of B. campestris L. was effectively reduced by the ZVI, MBC, ZBC, and ZMBC treatments (Fig. 2). In the ZVI, ZBC, MBC, and ZMBC treatments, the concentration of As was 3.74, 3.23, 2.66, and 1.20 mg kg−1 in shoots and 49.03, 35.76, 28.44, and 14.66 mg kg−1 in roots, respectively. Compared to the control, the As concentration in the shoots was reduced by 22.02% in the ZVI treatment, 44.55% in the MBC treatment, 32.62% in the ZBC treatment, and 74.92% in the ZMBC treatment, while in the BC treatment, it increased by 72.50%. Meanwhile, the MBC treatment decreased As concentrations in roots by 45.40%, and root uptake of As in ZVI, ZBC, and ZMBC treatments was also reduced by 5.80%, 31.30%, and 71.80%, respectively, compared with the control without amendments (Fig. 2). When BC was applied to the soil, the As content in the shoots and roots increased significantly compared to the control. The lowest As concentration in shoots was observed in the ZMBC treatment, with the value being significantly lower than that of control soil; and there was a similar result for the root As concentration. Thus, the composites of ZVI and MBC significantly cut down the bioavailability of As with the best immobilization effect, whose stabilizing effect was much better than that of single MBC or single ZVI. Moreover, the combined amendment of ZVI and biochar showed the combined effect of biochar and ZVI that immobilizes As.
Transfer of As was evaluated using transfer factor (TF): TF = As concentration in shoot or root/total As concentration in soil. From Table 1, it can be seen that less than 8.60% of soil As was transferred from soil to shoot. The lowest TF occurred in the ZMBC treatment for both shoots and roots, while BC had the highest one. The TF for the different treatments followed the order of BC > Control > ZVI > ZBC > MBC > ZMBC. Soil amendments containing Fe significantly decreased the TF value by 0.0109–0.0375 in shoot As of B. campestris L., and the most drastic effect was detected in the ZMBC treatment which had a TF of 0.0126, 74.85% lower than the control. Similarly, the TF value of shoot As in MBC was 0.0281, 43.91% reduction than the control treatment. Therefore, the application of ZMBC and MBC into soils significantly reduced the TF value of B. campestris L., resulting in decreased mobilization to edible parts of plants from soil, which inevitably decreased the health risk in the route of As entry into the human food chain.
Influence of amendments on soil mobile As and fertility
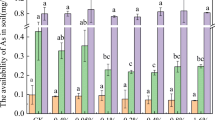
The basic characteristics of soils with the different treatments are presented in Table 2. The soil contained tiny amount of water-soluble As (WSAs), 0.39 mg kg−1, 0.42% of the total As in soil. Despite its low relative abundance, WSAs is considered to be one of the more labile fractions of the total As-pool. After the pot experiment was completed, WSAs in soils treated with different amendments followed the decreasing order of BC (0.41 mg kg−1) > control (0.39 mg kg−1) > ZBC (0.23 mg kg−1) > ZVI (0.15 mg kg−1) > MBC (0.04 mg kg−1) > ZMBC (0.03 mg kg−1). When the amendments were added to the soil, the WSAs concentration declined substantially, with the reductions relative to the control being 92.30% in the ZMBC treatment, 89.74% in the MBC treatment, 61.54% in the ZVI treatment, and 40.03% in the ZBC treatment. Available As, defined as the As that can be extracted by NaHCO3 treatment, represents both soluble and exchangeable As. Available As levels are 10 times higher than water-soluble As. With the exception of BC treatment, amendment with ZVI, MBC, ZBC, and ZMBC decreased soil available As concentration by 9.20–34.38%. ZMBC treatment in particular resulted in the deepest reduction of available As, 34.38% lower than the control.
When the pot experiment was finished, some changes in the soil physicochemical properties, reflecting soil fertility, were apparent from the soil quality analysis. The TOC, TN, and TP contents in soils amended with the BC, MBC, ZBC, and ZMBC treatments were all substantially improved, indicating enhanced soil quality, with a greater nutrient supply. For example, the value of TN increased from 1.77 g kg−1 in control to 1.81 g kg−1 in MBC, to 1.92 g kg−1 in ZMBC. Content of TP was enhanced from 0.45 g kg−1 in control to 0.68 g kg−1 in MBC, to 0.59 g kg-1 in ZMBC, which is similar to TOC. Thus, the addition of MBC and ZMBC greatly reduced WSAs and NaHCO3 extracted, and also improved the soil fertility.
Influence of different amendments on As fractionation in soils
A sequential extraction was used to investigate the distribution of As, the total content (sum) of all As fractions and the mobility factor (MF) as the ratio of the sum of the F1 + F2 content to that of the F1 + F2 + F3 + F4 + F5 content among different soil pools (Table 3). The data showed that the most labile fractions, i.e., the first and second fractions of the sequential extraction, were greatly reduced due to the addition of amendments, except for the BC and ZBC treatments. When the different soil amendments were applied, the As content of F1 declined from 0.92 mg kg−1 in the control to 0.64 mg kg−1 in the ZVI treatment, 0.49 mg kg−1 in the MBC treatment, and 0.32 mg kg−1 in the ZMBC treatment; while the As content of the F5 increased from 22.45 mg kg−1 in the control to 29.43 mg kg−1 in the ZVI treatment, 30.36 mg kg−1 in the MBC treatment, and 32.37 mg kg−1 in the ZMBC treatment, indicating the shift of As distribution from the F1 and F2 fractions to F5. The decrease in the labile As fraction was most notable in soil amended with the ZMBC treatment, in which the relative content of non-specifically absorbed As decreased by 65.22%, while the As content in the F1 fraction in the MBC treatment declined by 46.74% compared to the control. Furthermore, the As content of the F1 fraction in the different treatments followed the order of BC > ZBC > Control > ZVI > MBC > ZMBC, indicating that MBC and ZMBC treatments were most effective for reducing the mobility of As in soils. With the reduction of F1 and F2 fractions, the residual As (F5) increased substantially in the ZVI, MBC, and ZMBC treatments. The content of F5 fraction increased by 44.19% and 35.23%, respectively, in the ZMBC and MBC treatments compared to the control. If compared to the ZVI treatment, there was also 3.16% and 6.83% increase for F5 fraction in the MBC and ZMBC treatments, respectively, and F1 fraction in the MBC and ZMBC treatments was reduced by 23.44% and 50.00%, separately.
The mobility of As and heavy metals in soil can be assessed on the basis of the absolute and relative content of the fractions that are weakly bound to soil components (Table 3). Initially, the ratio of the labile fraction (F1 + F2) to the sum of all fractions (i.e., the MF) was 23.04%. After amending with the different materials, the MF values decreased to 20.74%, 19.15%, 20.75% and 16.88% for the ZVI, MBC, ZBC, and ZMBC treatments, respectively. However, with the addition of BC into contaminated soil, a notable enhancement of the MF was observed, indicating that the labile As increased in soils, and thus BC amendment could increase the mobility of As in soils. The application of MBC and ZMBC had the advantage of stabilizing the available As in soils. For Fe-filling amendments, the MF value also declined from 20.74 (ZVI) to 19.15 (MBC) and 16.88 (ZMBC), with the corresponding percentage reduction being 7.67% and 18.61%, respectively. Therefore, both the MBC and ZMBC treatments substantially reduced the availability of As, which indicates their potential utility in the remediation of As-contaminated soil.
Relationship between parameters related to plant As uptake
Following the application of soil amendments to the As-contaminated soil, many soil parameters including the WSAs, available As, water-soluble iron (WSFe), and DOC content changed, which may indirectly affect plant As uptake (PAs; mg kg−1). From Table 2, it can be seen that the lowest WSAs and DOC content in soils occurred with the ZMBC treatment, while the highest WSFe was also observed with the ZMBC treatment; this indicates that As is more easily fixed with the addition of Fe-containing materials to soils. A significant negative correlation between WSAs and WSFe was presented in Fig. 3, and a significant positive relationship between WSAs and DOC was also shown. The highest DOC and WSAs content appeared in BC treatment, indicating that BC application enhanced As mobilization in soils perhaps due to the release of greater amounts of DOC.
There was a direct relationship between PAs and some other parameters, as shown in Fig. 4. The figure illustrated that the PAs was greatly influenced by WSFe, DOC, and WSAs. The relationship between PAs and the above three parameters can be described by Eq. (1) using regression analysis. The WSAs, DOC, and WSFe in soils (mg kg−1) were all obtained from a water extraction,
and DOC, WSAs, and WSFe all significantly influenced PAs. The positive relationship indicates that the higher contents of WSAs and DOC lead to the higher content of PAs. On the contrary, the higher WSFe content leads to a lower content of PAs, which is good for controlling uptake by the plant. In detail, the ZMBC treatment played a key role in stabilizing the available As due to its large specific surface area of MBC and higher Fe content. ZMBC had the greatest potential to immobilize As in As-contaminated farmland and ensure the safety of crops to the greatest extent.
Discussion
Stabilization of available As in soils by ZMBC
Iron oxides (the most stable species of Fe in aerated soils) can reduce metalloid solubility. For example, Fe-based amendments have been reported to significantly reduce As, Cd, and copper (Cu) concentrations in pore water compared to untreated mine spoil (Moreno et al. 2017). When activated carbon is modified by Fe, it can effectively remove As in polished water, indicating that the Fe content, surface area, and charge distribution play key roles (Aricibar et al. 2014). In a previous study, the combination of Fe and BC enabled the growth of sunflowers and reduced As leaching (Sneath et al. 2013), while the application of BC has also been shown to increase the As concentration in pore water and reduce uptake in tomato plants (Solanum lycopersicum L.) (Beesley et al. 2013). In this study, MBC and ZMBC both have significantly reduced the shoot As and root As of B. campestris L. successfully, showing great potential of remediating As-contaminated cultivation land, especially for ZMBC treatment. Of course, the combined mixture of ZVI and biochar also reflected immobilization effect of reducing As bioavailability, which is better than single ZVI. The presence of carbon might accelerate the corrosion of ZVI and result in the rapid formation of iron oxides (Dou et al. 2010). The possible mechanism of the acceleration in corrosion of ZVI in the presence of biochar can be reasonably assigned to electron acceleration between ZVI and biochar due to biochar’s electrical conductivity (Klüpfel et al. 2014). Thus, it can be proposed that after contacting the surface of biochar, ZVI might be corroded quickly due to the possible catalytic effect of biochar surface on the corrosion reactions, resulting in the formation of iron oxides, which can enhance the immobilization of As.
As for an Fe2O3-coated BC, more sorption sites are formed, and more available As can be adsorbed on the coating through precipitation and coprecipitation due to the addition of MBC into soils, resulting in a reduction of WSAs and lowering of the bioavailability of As. When the biochar was modified with Fe, the BET surface area was increased from 4.12 m2 g−1 (BC) to 107.62 m2 g−1 (MBC), and the Fe2O3 content increased from 1.03% (BC) to 28.43% (MBC); more mobile As was absorbed and deposited on the Fe2O3-coated biochar forming Fe(As)-oxyhydroxides, and available As was reduced greatly by stabilization, which indirectly led to a decrease in plant uptake. Totally, ZMBC showed the best stabilization effect due to the strengthened fixation of ZVI since ZVI provides electrons for reduction of As5+ to As3+ and then As3t is coprecipitated with Fe3t on the related biochar surfaces (Bakshi et al. 2018). In the meanwhile, exposure to water and O2 promoted oxidation of ZVI to FeOOH which was retained by As through coprecipitation of Fe(As)OOH followed by intra-particle diffusion (Dixit and Hering 2003), and coprecipitation of As3+ with Fe3+ during the neo-formation of Fe(As)OOH mineral phases may occur when As is present in solution as ZVI corrodes, and intra-particle diffusion was suggested as the primary adsorption process (Ciopec et al. 2014). Unlike MBC and ZMBC, the original BC contained more organic matter, which may have resulted in the ready desorption and dissolution of As from soils, and therefore greater As bioavailability and plant uptake was observed (Debiec et al. 2017).
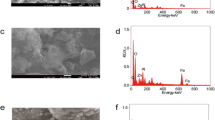
The XRD patterns of BC, MBC, and Fe2O3 were shown in Fig. 5, indicating that the BC contained a strong coating of Fe2O3, which was also evident from the SEM images (Fig. 6). In many previous studies, ZVI has been shown to be a good chemical stabilizer that can be used to remediate As-contaminated sites. When Fe(0) oxidizes within the soil, it can be transformed to poorly crystalized Fe oxides and hydroxides that bind As (Kumpiene et al. 2008). Though, BC has been reported to improve plant yields (Rillig et al. 2010) and increase soil N and P (Taghizadeh et al. 2012), However, most research has proved that BC can increase bioavailability of As (Yin et al. 2017). Through the modification of BC, i.e., the coating of Fe2O3 particles on the surface of BC, many of the original advantages of BC have been maintained, and stabilization of Fe2O3 coated on biochar in soils has also been achieved. The treatment of 4% MBC improved the plant biomass and significantly reduced As uptake. The combined treatment of Fe(0) and MBC resulted in the lowest As concentration in the shoot and root in B. campestris, indicating the potential for use in the remediation of As-contaminated farmland. In addition, the MBC treatment was inexpensive, strengthened the immobilization of As by Fe(0), and improved soil fertility and crop production.
Influence of As fraction, pH, and other soil quality parameters on bioavailability
In this investigation, it was shown that soil amendments, expect for the BC treatment, resulted in a rapid decrease in the F1 and F2 fractions; therefore, the direct toxicity of As was reduced, especially for the ZMBC treatment. This phenomenon may be attributed to the effective formation of ferric arsenate from the unstable phase, which was ascertained by the increase in the F5 fraction from 24.31% to 35.06% with the ZMBC treatment. In a correlation analysis, the As fraction in soil was shown to be closely related to PAs. From Table 4, it can be seen that there was a significant positive correlation between the As content in shoots and the F1, F2, and F4 fractions in soils. The F5 fraction was negatively correlated with As uptake of shoot, indicating that a larger F5 fraction resulted in less PAs, and the shoot uptake of As was dependent on the As content in the F1, F2, and F4 fractions in soils. It would be beneficial to control the amount of As in the F1, F2, and F4 fractions in soils, or to transform the F1, F2, and F4 fractions to F5 to secure the safety of vegetables with less PAs.
Generally speaking, the mobility and availability of heavy metal in soils is often controlled by pH (Manninen and Pantsar-Kallio 1997). In this study, when the experiment was completed, the pH of the soils was 8.09 in the control, 7.94 in the ZVI treatment, 7.91 in the ZMBC treatment, 7.89 in the ZBC treatment, 7.78 in the MBC treatment, and 7.76 in the BC treatment conditions. No obvious correlation was obtained between the As concentration in plants and pH, although the decrease in pH may have led to the reduced mobility of As (Moreno et al. 2012); this indicates that pH was not the only important factor, and other parameters such as DOC and WSFe also influenced the As availability. In this study, the highest WSFe concentrations in soils corresponded to the lowest WSAs content and the least uptake by roots and shoots, which is similar to Yin et al. (2017) verifying that application of Fe-biochar into soils caused DCB-extracted Fe concentrations increased while soluble As concentration reduced due to higher As retention in soils with Fe-biochar. As more WSFe was released, As uptake by plants was reduced significantly (R2 = 0.3944, p < 0.01) in this works. According to previous research, biochar amendment increased the leaching of DOC from sediment (Smebye et al. 2016) and contributed approximately 10–13% and 87–90% of As(V) reduction under abiotic and biotic conditions, and promoted As(III) release in soils (Chen et al. 2016), other researchers obtained similar results (Beesley et al. 2013; Wang et al. 2017). This was confirmed in this study by the significant positive relationship between DOC and WAs, and BC application to soils improved the available As and thus enhanced PAs while transforming residual As (F5) to available As (F1, F2) although BC increased crop yield. Thus, in order to use BC to improve soil fertility and remediate As-contaminated red soils, ZVI can be used to immobilize the available As in soils to counteract its negative effects. The application of both ZVI and ZMBC can decrease the WSAs and NaHCO3−extracted As content and also reduce the As uptake of B. campestris L. tissue.
Conclusion
A novel iron-impregnated BC composite combining the advantages of iron oxide and BC was successfully synthesized and effectively utilized in As immobilization of farmland. MBC and ZMBC were shown to effectively reduce the concentration of free As originating from a contaminated soil system and substantially decrease As uptake in the shoot and root of B. campestris L. Similar to the influence of BC, MBC utilization improved soil quality, nutrient supply, and plant yield. When MBC was applied to soil, the available As was significantly reduced, and the As fraction was transformed from non-specifically sorbed As and specifically sorbed As to residual As. MBC alone, and in combination with ZVI, can be used as amendments to stabilize soil As, which would enable the safety of crops cultivated in As-contaminated soil to be secured.
References
Agrafioti E, Kalderis D, Diamadopoulos E et al (2014) Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions. J Environ Manag 146:444–450
Aredes S, Klein B, Pawlik M (2012) The removal of arsenic from water using natural iron oxide minerals. J Clean Prod 29:208–213
Argos M, Pierce BL, van Geen A et al (2010) Arsenic exposure from drinking water and mortality in Bangladesh Reply. LANCET 376(9753):1642
Aricibar OJA, Josue DBJ, Rios HJC et al (2014) Influence of iron content, surface area and charge distribution in the arsenic removal by activated carbons. Chem Eng J 249:201–209
Bakshi S, Banik C, Rathke SJ, Laird DA (2018) Arsenic sorption on zero-valent iron-biochar complexes. Water Res 137:153–163
Beesley L, Marmiroli M, Pagano L et al (2013) Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci Total Environ 454–455:598–603
Casado M, Anawar HM, Carcia-sanchez A et al (2007) Arsenic bioavailability in polluted mining soils and uptake by tolerant plants (EI Cabaco mine, Spain). Bull Environ Contam Toxicol 79(1):29
Chen BL, Chen ZM, Lv SF (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol 102(2):716–723
Chen Z, Wang YP, Xia D, Jiang X, Fu D, Shen L, Wang H, Li QB (2016) Enhanced bioreduction of iron and arsenic in sediment by biochar amendment influencing microbial community composition and dissolved organic matter content and composition. J Hazard Mater 311:20–29
Ciopec M, Negrea A, Lupa L, Davidescu C, Negrea P (2014) Studies regarding As(V) adsorption from underground water by Fe-XAD-DEHPA impregnated resin. Equilibrium sorption and fixed-bed column tests. Molecules 19:16082–16101
Clemente R, Pardo T, Madejón P, Madejón E, Bernal MP (2015) Food byproducts as amendments in trace elements contaminated soils. Food Res Int 73:176–189
Debiec K, Rzepa G, Bajda T, Zych L, Krzysztoforski J, Sklodowska A, Drewniak L (2017) The influence of thermal treatment on bioweathering and arsenic sorption capacity of a natural iron (oxyhydr) oxide-based adsorbent. Chemosphere 188:99–109
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152(1):1–31
Dixit S, Hering JG (2003) Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37(18):4182–4189
Dou XM, Li R, Zhao B, Liang W (2010) Arsenate removal from water by zero valent iron/activated carbon galvanic couples. J Hazard Mater 182:108–114
Duan XH, Zhang CC, Srinivasakannan C, Wang X (2017) Waste walnut shell valorization to iron loaded biochar and its application to arsenic removal. Resource-Efficient Technol 3:29–36
Farrow EM, Wang J, Burken JG, Shi H, Yan W, Yang J, Hua B, Deng B (2015) Reducing arsenic accumulation in rice grain through iron oxide amendment. Ecotoxicol Environ Saf 118:55–61
Hale JR, Foos A, Zubrow JS, Cook J (1997) Better characterization of arsenic and chromium in soils: a field-scale example. J Soil Contam 6:371–389
Hanaoka, Okumura (2014) Effect of metal content on CO2 gasification behavior of K- and Fe-loaded bio-chars. J THERM SCI TECH-JPN 9(2):6
Hartley W, Lepp NW (2008) Remediation of arsenic contaminated soils by iron-oxide application, evaluated in terms of plant productivity, arsenic and phytotoxic metal uptake. Sci Total Environ 390:35–44
He RZ, Peng ZY, Huang H et al (2018) Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci Total Environ 612:1177–1186
Klüpfel L, Keiluweit M, Kleber M, Sander M (2014) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48:5601–5611
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides—a review. Environ Pollut 172:9–22
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments - a review. Waste Manag 28:215–225
Laird D, Fleming P, Wang B, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158:436–442
Lee SH, Kim EY, Park H, Yun J, Kim JG (2011) In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 161:1–7
Li LF, Zeng XB, Bai LY et al (2009) Cadmium accumulation in vegetable plantation land soils under protected cultivation: a case study. Commun Soil Sci Plan 40:2169–2184
Li L-F, Zeng X-B, Bai L-Y et al (2010) Soil arsenic content and its health risk assessment for agricultural products in the region surrounding Shimen arsenic sulphide mine. Chin Appl Ecol 21(11):2946–2951 (in Chinese)
Li LF, Zeng XB, Su SM, Wu CX, Wang YL (2014) Arsenic content and the bioavailability in farmland soils affected by mining activities of a realgar ore, South China. Adv Mater Res 955-959:3645–3654
Li YL, Zhang BG, Cheng M et al (2016) Spontaneous arsenic (III) oxidation with bioelectricity generation insingle-chamber microbial fuel cells. J Hazard MaterJ Hazard Mater 306:8–12
Li JS, Bei YJZ, Tsang DCW et al (2017a) Arsenic-containing soil from geogenic source in Hong Kong: leaching characteristics and stabilization/solidification. Chemosphere 182:31–39
Li H, Dong X, Silva D et al (2017b) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478
Liao XY, Chen TB, Xie H, Liu YR (2005) Soil As contamination and its risk assessment in areas near the industrial districts of Chenzhou City, Southern China. Environ Int 31(6):791–798
Liu HY, Probst A, Liao BH (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166
Liu ZY, Yang W, Xu W et al (2018) Removal of elemental mercury by bio-chars derived from seaweed impregnated with potassium iodine. Chem Eng J 339:468–478
Lu RK (1999) The analysis method of soil agricultural chemistry. China agricultural science and Technology Press, Beijing
Madeira AC, Varennes AD, Abreu MM et al (2012) Tomato and parsley growth, arsenic uptake and translocation in a contaminated amended soil. J Geochem Explor 123:114–121
Manninen PKG, Pantsar-Kallio M (1997) Speciation of mobile arsenic in soil samples as a function of Ph. Sci Total Environ 204:193–200
Miretzky P, Cirelli AF (2010) Remediation of arsenic-contaminated soils by iron amendments: a review. Crit Rev Env Sci Tec 40(2):93–115
Moreno JE, Esteban E, Penalosa JM (2012) The fate of arsenic in the soil–plant system. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology. Springer, USA
Moreno JE, Sepǘlveda R, Esteban E et al (2017) Efficiency of organic and mineral based amendments to reduce metal[loid]mobility and uptake (Lolium perenne) from a pyrite-waste contaminated soil. J Geochem Explor 174:46–52
Niazi NK, Burton ED (2016) Arsenic sorption to nanoparticulate mackinawite (FeS): an examination of phosphate competition. Environ Pollut 218:111–117
Niazi NK, Bibi I, Shahid M, Ok YS, Burton ED, Wang H, Shaheen SM, Rinklebe J, Lüttge A (2018a) Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: an integrated spectroscopic and microscopic examination. Environ Pollut 232:31–41
Niazi NK, Bibi I, Shahid M, Ok YS, Shaheen SM, Rinklebe J, Wang H, Murtaza B, Islam E, Farrakh Nawaz M, Lüttge A (2018b) Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci Total Environ 621:1642–1651
Rillig MC, Wagner M, Salem M, Antunes PM, George C, Ramke HG, Titirici MM, Antonietti M (2010) Material derived from hydrothermal carbonization: effects on plant growth and arbuscular mycorrhiza. Appl Soil Ecol 45:238–242
Shakoor MB, Niazi NK, Bibi I, Murtaza G, Kunhikrishnan A, Seshadri B, Shahid M, Ali S, Bolan NS, Ok YS, Abid M, Ali F (2016) Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit Rev Environ Sci Technol 46:467–499
Shi WY, Shao HB, Li H, Shao MA, du S (2009) Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J Hazard Mater 170:1–6
Smebye A, Alling V, Vogt RD, Gadmar TC, Mulder J, Cornelissen G, Hale SE (2016) Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 142:100–105
Sneath HE, Hutchings TR, Leij F (2013) Assessment of biochar and iron filing amendments for the remediation of a metal, arsenic and phenanthrene co-contaminated soil. Environ Pollut 178:361–366
Sun Y, Lei C, Khan E, Chen SS, Tsang DCW, Ok YS, Lin D, Feng Y, Li XD (2017) Nanoscale zero valent iron for metal/metalloid removal from model hydraulic fracturing wastewater. Chemosphere 176:315–323
Taghizadeh TA, Clough TJ, Sherlock RR et al (2012) Biochar adsorbed ammonia is bioavailable. Plant Soil 350:57–69
Trakal L, Michálková Z, Beesley L, Vítková M, Ouředníček P, Barceló AP, Ettler V, Číhalová S, Komárek M (2018) AMOchar: amorphous manganese oxide coating of biochar improves its efficiency at removing metal(loid)s from aqueous solutions. Sci Total Environ 625:71–78
Venegas A, Rigol A, Vidal M (2015) Viability of organic wastes and biochars as amendments for the remediation of heavy metal-contaminated soils. Chemosphere 119:190–198
Wang N, Xue XM, Juhasz AL, Chang ZZ, Li HB (2017) Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic. Environ Pollut 220:514–522
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–332
Woolson E A,et al. 1971. Correlation between available soil arsenic, estimated by six methods and response of corn[J]. Soil Sci Soc Am Proc 35:101–105
Xiu W, Guo HM, Liu Q, Liu Z, Zou Y’, Zhang B (2015) Arsenic removal and transformation by Pseudomonas sp. Strain GE-1-induced ferrihydrite: co-precipitation versus adsorption. Water Air Soil Pollut 226:167
Yin DX, Wang X, Peng B et al (2017) Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system. Chemosphere 186:928–937
Yu ZH, Qiu WW, Wang F, Lei M, Wang D, Song Z (2017) Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere 168:341–349
Zeng X-B, Li L-F, Bai L-Y et al (2007) Arsenic accumulation in different agricultural soils in Shouguang of Shandong Province. Chin Appl Ecol 18(2):310–316 (in Chinese)
Zhang M, Gao B, Varnoosfaderani S, Hebard A, Yao Y, Inyang M (2013) Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol 130:457–462
Zhang F, Wang X, Xionghui J, Ma L (2016) Efficient arsenate removal by magnetite-modified water hyacinth biochar. Environ Pollut 216:575–583
Zhao YY, Fang XL, Mu YH, Cheng Y, Ma Q, Nian H, Yang C (2014) Metal pollution (Cd, Pb, Zn, and As) in agricultural soils and soybean, glycine max, in southern China. Bull Environ Contam Toxicol 92:427–432
Zhu YG, Williams PN, Meharg AA (2008) Exposure to inorganic arsenic from rice: a global health issue? Environ Pollut 154:169–171
Funding
This study received support from the National Twelve-Five Year Science and Technology Support Program of China (2015BAD05B01) and the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (2016–2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Highlight
1. This study revealed that the inhibition of As uptake by Brassica campestris L. after amended with modified biochar singly and the complex with zero-valent iron.
2. The available As and water-soluble As in soils all decreased in MBC and ZMBC, while BC induced the improvement of As availability.
3. Modified biochar was effective at reducing As bioavailability, and immobilization effect of As in MBC can be strengthened by Fe(0), indicating potential for remediation of As-contaminated soil.
Rights and permissions
About this article
Cite this article
Li, L., Zhu, C., Liu, X. et al. Biochar amendment immobilizes arsenic in farmland and reduces its bioavailability. Environ Sci Pollut Res 25, 34091–34102 (2018). https://doi.org/10.1007/s11356-018-3021-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3021-z