Abstract
The current study aimed to evaluate the removal of a pesticide mixture composed of the insecticides chlorpyrifos (CP) and diazinon (DZ) from liquid medium, soil and a biobed biomixture by a Streptomyces mixed culture. Liquid medium contaminated with 100 mg L−1 CP plus DZ was inoculated with the Streptomyces mixed culture. Results indicated that microorganisms increased their biomass and that the inoculum was viable. The inoculum was able to remove the pesticide mixture with a removal rate of 0.036 and 0.015 h−1 and a half-life of 19 and 46 h−1 for CP and DZ, respectively. The sterilized soil and biobed biomixture inoculated with the mixed culture showed that Streptomyces was able to colonize the substrates, exhibiting an increase in population determined by quantitative polymerase chain reaction (q-PCR), enzymatic activity dehydrogenase (DHA) and acid phosphatase (APP). In both the soil and biomixture, limited CP removal was observed (6–14%), while DZ exhibited a removal rate of 0.024 and 0.060 day−1 and a half-life of 29 and 11 days, respectively. Removal of the organophosphorus pesticide (OP) mixture composed of CP and DZ from different environmental matrices by Streptomyces spp. is reported here for the first time. The decontamination strategy using a Streptomyces mixed culture could represent a promising alternative to eliminate CP and DZ residues from liquids as well as to eliminate DZ from soil and biobed biomixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural and livestock farming activities indiscriminately use organophosphorus pesticides (OPs) for pest and insect control. OPs are highly toxic compounds that inhibit acetylcholinesterase in the central nervous system synapses of insects and vertebrates, including humans. These compounds are considered food chain contaminants due to the presence of their residues in vegetables and fruits. As a result, OPs trigger several metabolic human health problems, such as type 2 diabetes (Lasram et al. 2014). OPs arrive in the environment by different routes, resulting in significant environmental pollution and human health risks. After pesticide application, it is estimated that less than 1% of all pesticides used in agriculture reach the crops, while the remaining amount contaminates the soil, air, water and food (Berberidou et al. 2016; Fosu-Mensah et al. 2016; Pozo et al. 2016; Yu et al. 2016). Wastewater from agricultural industries characterized by high pesticides loads or from industries related to manufacturing, packaging, transporting, storing and delivering and distributing pesticides are another source of pesticide pollution (Navaratna 2012; Karas et al. 2016) in addition to the production of forbidden and expired pesticides (Yañez-Ocampo et al. 2011).
As a way of controlling or preventing point and nonpoint pollution from pesticide use, several treatment systems are being studied. In the environment, pesticides can be degraded by microbial, chemical and photodegradation processes. However, the use of biological processes as pesticide decontamination tools is recognized as the primary mechanism of pesticide removal from soil and water (Chishti et al. 2013). Studies focused on microbial degradation are being extensively performed due to the urgent need to develop technologies that guarantee harmless, effective and inexpensive decontamination of pesticide residues from the environment (Cycón et al. 2017). Therefore, bacterial species belonging to a wide range of genera that are capable of degrading OPs from liquid samples and soils have been reported; they include Stenotrophomonas sp., Pseudomonas sp., Sphingobium sp., and Ralstonia sp. (Abo-Amer 2012; Cao et al. 2013; Deng et al. 2015; Wang and Liu 2016). In addition, Actinobacteria can degrade a wide range of pesticides (Alvarez et al. 2017). However, compared with gram-negative bacteria, these bacteria have not been studied extensively. The most representative genus of pesticide degrading Actinobacteria is Streptomyces sp., which has the capacity to grow on and degrade different classes of pesticides, including OPs (Briceño et al. 2013). Streptomyces spp. are considered to be a promising source of a wide range of enzymes; therefore, they are capable of performing microbial transformations of organic compounds (Ballav et al. 2012). Moreover, they may be particularly well-suited as inoculum for the treatment of pesticides because they exhibit a mycelial growth habit, relatively rapid growth rates, colonization of semi-selective substrates, susceptibility to genetic manipulation, surfactant production that may increase the bioavailability of toxic compounds, and formation of biofilms (Hopwood 2006; Flärdh and Buttner 2009; Schreiberová et al. 2012; Colin et al. 2016). These properties may increase their ability to contribute to degradation.
Biological removal of OPs in water, wastewater and soils is highly relevant to the development of bioremediation strategies as well as for the performance of pesticide biopurification systems. Biobed biomixtures were proposed to remove pesticides coming from improper disposal and accidental leakage (Castillo et al. 2008). Moreover, biomixtures have additionally been proposed as a component of biopurification systems for treatment of agricultural wastewater containing pesticides (Karas et al. 2016; Ruíz-Hidalgo et al. 2016). Accordingly, several studies have been performed to understand the capacity of biobed biomixtures to remove pesticides (Castillo et al. 2008; Diez et al. 2013; Tortella et al. 2013; Castro-Gutierrez et al. 2017), and bioaugmentation has been studied as a possibility to increase biobed performance (Campos et al. 2017) by introducing specific microorganisms.
The insecticides chlorpyrifos, CP, (O, O-diethyl O-[3,5,6-trichloro-2-pyridinyl] phosphorothioate) and diazinon, DZ, (O, O-diethyl-O-[2-isopropyl-4-methyl-6-pyrimidinyl] phosphorothioate) are commonly used in Chile for the treatment of pests present in vegetable and fruit farming and in the livestock industry, respectively (Afipa 2010; SAG 2012). Both CP and DZ are released into the environment solely by human activities, and the routes by which these compounds enter into the soil and water include extensive use, drift during and after pesticide application, run-off from agricultural areas, accidental releases, etc. In the environment, CP has reduced mobility in the soil due to low solubility (<2 mg L−1) in water and high Koc (4100–6100). Moreover, CP has short to moderate persistence with a half-life for hydrolysis of 16–73 days, an aerobic soil metabolism of 22–51 days and terrestrial field dissipation between 2 and 120 days (Solomon et al. 2014). Meanwhile, DZ is mobile and moderately persistent in the environment given by its high solubility in water (60 mg L−1), low Koc (40–432) depending on the type of soil, and a half-life for hydrolysis of approximately 12–138 days in water and 200 days in soil (Dębski et al. 2007; Aggarwal et al. 2013). For both CP and DZ, degradation can occur by a combination of processes, including chemical hydrolysis, photolysis and biodegradation, with 3,5,6-trichloro-2-pyridinol (TCP) and 2-isopropyl-6-methyl-4-pyrimidinol (IMHP) as main degradation by-products, respectively (Dębski et al. 2007; Solomon et al. 2014).
Residues of CP and DZ have been found in concentrations exceeding the maximum residue limits for pesticides in foods such as lettuce, cherimoya, lemons and mangos (ISP 2012). In a study performed by Muñoz-Quezada et al. (2012), a high association was found between urinary OP metabolites in schoolchildren and the consumption of fruits and vegetables with the presence of low doses of CP and phosmet. Studies showing the presence of CP or DZ residues in water or soil are non-existent, probably due to the lack of economic resources in Chile to perform environmental monitoring of these contaminants. However, recently, Pozo et al. (2016) reported the presence of CP in the Chilean atmosphere, specifically in the Araucania region, which is characterized by high agricultural activity.
In our previous study, DZ and CP removal was evaluated using different Streptomyces spp. Results showed that by using single strains, removal of DZ between 24 and 32% was observed, and these values increased to 50% when a mixture of four strains was studied (Briceño et al. 2016a). Similarly, between 67 and 74%, CP removal was observed by using single strains, and these values increased to 90% by using a mixed culture of Streptomyces strains (Briceño et al. 2016b). Moreover, our results indicated that a Streptomyces mixed culture might be useful for the efficient removal not only of single CP or DZ but also other OPs from liquids as well as from other environmental matrices. Therefore, the aim of this study was to evaluate the ability of a Streptomyces spp. mixed culture to simultaneously remove a pesticide mixture composed of CP and DZ from liquid medium, soil and a biobed biomixture under sterile conditions focusing on the culture’s capability to colonize the environmental matrices.
Materials and methods
Chemicals, Streptomyces mixed cultures and inoculum preparation
Analytical grade CP, DZ and its metabolites (TCP and IMHP) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other reagents and solvents were of analytical grade and obtained from standard manufacturers. CP and DZ methanol stock solutions (5000 mg L−1) were sterilized by filtration through 0.22 μm pore-size membranes and used for contamination of the liquid media.
A defined mixed culture of four Streptomyces strains was used in this study. These strains were previously isolated from agricultural soil (Briceño et al. 2012), and taxonomic identification of these strains were confirmed by amplification and partial sequencing of their 16S rDNA genes [GenBank IDs: JQ289350 (Streptomyces sp. AC5), JQ289353 (Streptomyces sp. AC9) (Briceño et al. 2012), KT271897 (Streptomyces sp. GA11) and 289355 (Streptomyces sp. ISP13) (Briceño et al. 2016a)]. These four strains were previously studied for DZ (Briceño et al. 2016a) and CP removal (Briceño et al. 2016b) and selected as inoculum because together they increase CP and DZ removal. The four strains produce the enzyme organophosphorus hydrolase related to OP degradation, emphasizing the AC5 strain for presenting a higher production of the enzyme and the ability to degrade the main CP metabolite (Briceño et al. 2012; Briceño et al. 2016b).
Streptomyces strains were grown in 100-mL flasks containing 30 mL of sterilized ISP-2 medium. After incubation in a rotary shaker (96 h at 28 °C and 120 rpm), cultures were harvested by centrifugation (8500×g for 10 min at 4 °C), and cell pellets were then washed with a sterile 0.85% NaCl solution. Finally, for the pesticide removal study, the inoculum was prepared by combining equal amounts of each strain.
Liquid medium OP removal
Removal of CP and DZ was evaluated in liquid minimal medium (MM) containing 0.5 g L-asparagine, 0.5 g K2HPO4, 0.20 g MgSO4*7H2O, 0.01 g FeSO4*7H2O, 1000 mL distilled water and pH 7.0.
The Streptomyces inoculum was added at a final concentration of 1% (w/v) wet weight in flasks containing 30 mL of MM with 50 mg L−1 CP and 50 mg L−1 DZ, in mixture. Cultures were incubated at 28 °C and 120 rpm for 240 h. Samples were collected at different times and centrifuged using the pellet to measure microbial growth after drying at 105 °C. Survival of the mixed culture was examined after 96 h of incubation; culture samples were obtained and processed for live/dead cells using confocal microscopy and flow cytometry as described by Briceño et al. (2016b). In addition, at day 15 and 30 of incubation, 1 mL of the liquid sample was taken, serially diluted and added to a plate with starch-casein agar to observe the presence or absence of the strains.
To determine the residual concentration of CP, DZ and their corresponding metabolites, TCP and IMHP, 10 mL of supernatant was removed and analyzed via HPLC. Removal was estimated by comparing concentrations in the samples and controls over time.
Removal of OPs in the soil and biomixture
Removal tests were conducted on soil samples (0–20 cm) collected from the Maquehue experimental station (Andisol Freire series; 38° 50′ S, 72° 41′ W) at La Frontera University; this site had no history of CP or DZ contamination. The soil was mainly composed of sand (30.7%), silt (41.8%), clay (27.4%), and organic matter (17%) and had a pH of 5.8. This soil was used to prepare the biomixture by mixing top soil, commercial peat (36.6% organic carbon) and winter wheat straw (34% organic carbon) in the volumetric proportion of 1:1:2 according to the procedure described by Tortella et al. (2013). After a period of maturation and stabilization of the biomixture (pH 5.0; 38% organic matter), the biomixture was used for the removal assay.
Ten grams of soil or biomixture sterilized three times at 121 °C was placed in a 125-mL Erlenmeyer flask. Next, each pesticide was added at a concentration of 50 mg kg−1. After homogenizing the pesticides in the soil and the biomixture, a solution containing the Streptomyces mixed culture was added at a final concentration of 5% (w/v) wet weight. Soil and biomixture without inoculum were considered as controls to verify that degradation was due mainly to the action of microorganisms rather than abiotic factors. Soil and biomixture moisture were adjusted by the addition of distilled water to 50 and 60% of its water holding capacity, respectively. Incubation was performed for 28 days in an incubator at 25 ± 1 °C with complete darkness. Throughout the incubation period, water losses were compensated by the addition of sterile distilled water. Samples of soil and biomixture were taken weekly for chemical analyses to determine the concentration of pesticides and for microbiological and molecular analyses.
Biochemical and molecular analyses in the soil and biomixture
Samples of soil and biomixture obtained at different times were used to determine dehydrogenase (DHA) and acid phosphatase (APP) activity according to Alef (1995) and Tabatabai and Bremmer (1969), respectively. DHA activity was expressed as μg triphenyl formazan (TPF) g−1 h−1 after quantification at 546 nm, and APP activity was expressed as μg p-nitrophenol g−1 h−1 after quantification of p-nitrophenol (p-NP) at 400 nm. To determine inoculum persistence at the end of the assay, 1 g of soil and biomixture were sampled to ascertain microbial growth, and soil extract was added to a plate with starch-casein agar.
On days 1, 7, 14, 21 and 28, DNA from the soil and biomixture were extracted using the commercial NucleoSpin® Soil kit (Macherey-Nagel) following the manufacturer’s instructions. Quantitative PCR (q-PCR) for the analysis of the Streptomyces population was performed using an Applied Biosystems, Step One Plus Real-Time PCRT System. 16S rRNA gene amplification reactions were performed with the Actino235/Eub518 set of primers (Fierer et al. 2005). The combination of both primers generates a PCR fragment of approximately 200 bp suitable for subsequent q-PCR analysis. For the reaction, 4 μL of buffer (HOT FIRE Pol® EvaGreen® q-PCR Mix Plus, Solis BioDyne Data Sheet), 0.6 μL of forward primer (10 μM), 0.6 μL of reverse primer (10 μM), 5 μL ADN (1.0 ng/μL) and 9.8 μL of ionized water were used. For the PCR program, initial denaturation was 15 min at 95 °C, 40 cycles for 15 s at 95 °C with hybridization for 20 s. Primer extension was conducted at 72 °C for 20 s. For the analysis of Streptomyces populations, PCR products were analyzed using Step One Software V2.2.2. To quantify PCR products, a standard curve (pure Streptomyces sp.) was used for the analysis of accumulated DNA. Results were expressed as copied numbers of the ribosomal region.
Pesticide analysis
Samples of supernatants from centrifuged cultures were taken and extracted with dichloromethane and ethyl acetate according to Briceño et al. (2016b). For pesticide and metabolite determination from the soil and biomixture, samples (10 g) were extracted with 25 mL of acetone on a vertical shaker for 1 h. Later, samples were sonicated for 30 m and centrifuged at 8500g. Supernatants were concentrated and re-suspended in 5 mL acetonitrile and stored at −20 °C until further analysis for CP, DZ, TCP and IMHP determination by HPLC.
Analyses were performed using a Shimadzu LC-20AT liquid chromatograph equipped with a diode array. For separation, a Purospher Star RP-18e column (Merck®, film thickness 5 μm, 150 × 4.6 mm) was used. The chromatographic condition was as follows: oven temperature of 35 °C with the mobile phase at 25% 0.1% phosphoric acid-75% acetonitrile with a flow rate of 1 mL min−1. Retention times of CP and TCP were 8.10 (λ = 289) and 2.50 (λ = 298) min, respectively, and 6.10 (λ = 247) and 1.96 (λ = 267) min for DZ and IMHP, respectively. Calibration was performed using a standard for each compound, with a linear curve ranging from 0.01 to 10 mg L−1.
Kinetics and statistical analysis
Removal of CP and DZ from the liquid medium, soil and biomixture was fitted to the first-order kinetic model ln Ct/C0 = e-kt, where C0 is the amount of contaminant in the liquid medium at time zero, Ct is the amount of contaminant at time t, and k and t are the rate constant and degradation time in hours, respectively. Time in which CP and TCP concentrations in the liquid medium were reduced by 50% (T1/2) was calculated using the equation T1/2 = ln(2)/k.
Data were statistically analyzed by an analysis of variance (ANOVA), and three replicates were compared using Tukey’s minimum significant differences test (p ≤ 0.05). Statistical analyses were performed using SPSS statistical software version 17.
Results
Growth and OP removal in the liquid medium
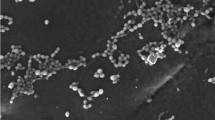
Results for microbial growth monitored by dry weight at 105 °C and removal of CP and DZ in liquid culture by a mixed culture of Streptomyces spp. strains AC5, AC9, GA11 and ISP13 are shown in Fig. 1. Results showed an increase of biomass during the first 24 h in the medium supplemented with the pesticide mixture. Specific growth rate increased from 0.008 observed in the control treatment to 0.021 in the medium with the pesticide mixture, requiring 86 and 33 h−1, respectively, for the mixed culture to double its biomass (Table 1). After 48 h, biomasses were similar in both treatments; this trend was observed until the study concluded. In addition to studying microbial growth of the Streptomyces mixed culture exposed to 100 mg L−1 CP plus DZ, culture survival was analyzed at 96 h by live/death cell analysis. Figure 2 shows confocal images of a sample acquired from the liquid culture contaminated with the pesticide mixture. As seen in the figure, most cells were alive (green), and very few cells were dead (red). The analysis performed by flow cytometry showed 84.7% live cells and 15.3% dead cells. This survival was greater than that observed in the treatment without pesticides, where approximately 78.5 and 21.5% were alive and dead, respectively. At the end of the assay and by plate count, we qualitatively observed the existence of the four strains in the inoculum, which were differentiated by form and color of the prevailing colonies (data not shown) among all strains of the Streptomyces sp. strain AC9.
Confocal laser-scanning fluorescence microscopy analysis of the Streptomyces mixed culture exposed in liquid medium to 100 mg L−1 chlorpyrifos (CP) plus diazinon (DZ). Samples were stained with SYTO 9 and propidium iodide. The red and the green colors indicate dead and living mycelium or spores, respectively
HPLC analysis showed that the Streptomyces mixed culture was able to remove CP and DZ from the liquid medium (Fig. 1). After 24 h, removal of approximately 45 and 90% DZ and CP, respectively, was seen. For CP, residual concentration decreased slowly until no residues were detected at day 10 of incubation. For DZ, residual concentration disappeared as time progressed; 50, 75 and 85% was removed after 48, 96 and 168 h, respectively. At the end of the assay, the final DZ concentration was approximately 1% of the initial concentration. With these results, kinetics data were determined showing that DZ removal was characterized by a rate constant of 0.015 h−1 and T1/2 of 45 h−1, while CP had a rate constant of 0.036 h−1 and T1/2 19 h−1 in the liquid medium contaminated with the pesticide mixture (Table 1). In the same table, the rate of TCP and IMHP production is presented. According to our results, TCP and IMHP production was 0.003 and 0.008 mg L−1 h−1, respectively, in the liquid culture; therefore, approximately 0.213 ± 0.005 and 0.302 ± 0.005 of TCP and IMHP were produced at the end of the assay.
OP removal from the soil and biomixture
We studied enzyme activity, quantitative PCR and CP and DZ removal in the Streptomyces mixed culture for 4 weeks in both the sterilized soil and biomixture. Similar experiments were performed without inoculum as controls. Results obtained in this study showed that sterility conditions were maintained throughout the study; therefore, DHA and APP activities were negligible. For the contaminated soil and biomixture, DHA enzyme activity values increased to 4.64 and 7.35 μg TPF g−1 h−1, respectively, when the mixed culture of Streptomyces spp. was inoculated. However, in the soil, activity was constantly maintained and no significant differences were observed (P ≤ 0.05) throughout the study. A different response was observed in the inoculated biomixture, which showed an increase in activity at day 7 with a value of 12.49 μg TPF g−1 h−1. After this time, activity was variable but without significant differences as observed during the first days of the study. APP activity for the inoculated soil and biomixture showed significant differences (P ≤ 0.05) over time. In the sterilized soil, activity after inoculation increased from 0 to 8.70 μg p-NP g−1 h−1, maintaining this trend at day 7. After that, there was a slight decrease and then another increase at days 21 and 28 showing APP activity over 11 μg p-NP g−1 h−1. In the biomixture, the Streptomyces inoculation increased initial APP activity from 0 to 2.07 μg p-NP g−1 h−1; this trend continued over the next weeks where values between 16.60–39.76 μg p-NP g−1 h−1 were observed (Table 2).
16S rRNA quantified from DNA extracted from the inoculated soil and biomixture ranged from 6.29 × 106 (± 8.63 × 104) at day 1 to 1.09 × 108 (± 1.54 × 103) copy number per gram of dry soil at day 7 after inoculation. Subsequently, copy number values decreased, fluctuating from 4.31 × 107 to 7.09 × 107. In the biomixture, the copy number increased over time; after 1 day of inoculation, a value of 1.46 × 108 copy number per gram of dry biomixture was observed, while a 4.05 × 108 copy number was observed after 28 days in the Streptomyces spp. inoculated biomixture (Fig. 3). For both the soil and biomixture at the end of the assay, all four strains of the inoculum were present in a plate with starch casein agar, with strain AC9 as the dominant strain present while strain ISP13 was practically non-existent.
Pesticide residues were analyzed in both the soil and biomixture. Figure 4 shows CP and DZ residues at day 7, 14, 21 and 28 after the Streptomyces spp. mixed culture was added. As indicated by the data, CP and DZ were not removed under uninoculated conditions. Therefore, sterile conditions were maintained, and abiotic removal was absent. In the inoculated soil (Fig. 4a) and biomixture (Fig. 4b), CP was scarcely removed. It was observed that approximately 14% of the applied dose in the soil and 6% of the applied dose in the biomixture disappeared after 28 days. A different response was observed for DZ: approximately 22% of DZ removal was observed in the inoculated soil after 1 week, and DZ removal increased slightly in the following 2 weeks reaching an elimination of approximately 30%. During the last week, residual concentration of DZ decreased further, and approximately 50% remained in the soil by day 28. In the inoculated biomixture during the first 2 weeks, DZ concentration declined rapidly showing 55% removal at day 14. After this time, removal was very slow; it was observed that approximately 60% of the DZ was removed when the study was completed. Finally, as indicated by the data analysis, only DZ removal followed the model utilized and was characterized by a rate constant of 0.024 ± 0.004 and 0.060 ± 0.002 day−1 in the inoculated soil and biomixture, resulting in T1/2 values equal to 29 and 11 days, respectively (Table 3).
Discussion
In this study, we evaluated the simultaneous removal of CP and DZ from three different environmental matrices by a previously defined Streptomyces spp. mixed culture with a proven ability to remove pesticides (Briceño et al. 2016a, b). Previous studies performed by us have shown that both pure and mixed cultures of Streptomyces spp. grow in the presence of OPs, and this trend is maintained in a liquid culture contaminated with a CP and DZ mixture applied in a total concentration of 100 mg L−1. According to these results, the mixed culture used the insecticides as carbon and nitrogen sources to grow, and the pesticide mixture promoted a decrease in the biomass duplication time previously reported for DZ (168 h−1) (Briceño et al. 2016a) and CP (161 h−1) (Briceño et al. 2016b). Using confocal microscopy, the mixed culture survival compared with the control allows us to confirm the absence of possible antagonistic effects of pollutants on the bioinoculum.
Removal of OPs from water is of high significance due to the toxicity of OPs, especially in the production of drinking water. Therefore, several studies have been performed to investigate the simultaneous removal of OPs by both single bacteria (Shimazu et al. 2001; Deng et al. 2015) and bacteria consortia (Pino and Peñuela 2011; Abraham et al. 2014) as alternative treatments. In this study, both CP and DZ were simultaneously removed by the Streptomyces mixed culture, and a reduction in the T1/2 for each compound was observed compared to previous results where the pesticides were treated as single contaminants (Briceño et al. 2016a, b). This result may be explained due to a complementary metabolic effect among members of the mixed culture making the most efficient use of these insecticides as nutrient sources (Fuentes et al. 2013) and by the structural similarity of both molecules in which P-O cleavage bonds are present (Deng et al. 2015). In previous studies, Pino and Peñuela (2011) observed that the effect of mixing pesticides was not positive for the simultaneous degradation of methyl parathion and CP by a consortium formed by Acinetobacter sp., P. putida, Bacillus sp., among others. However, Fuentes et al. (2013) revealed that Streptomyces strains were able to grow and simultaneously remove a mixture of pentachlorophenol and CP; therefore, Streptomyces strains were proposed as potential tools in the treatment of multiple pesticides. Similarly, our results showed the ability of Streptomyces spp. to efficiently remove a pesticide mixture formed by CP and DZ from a liquid matrix, which is relevant when there is evidence of aquatic contamination by a combination of these two pesticides (Walker 2016).
Simultaneous removal of CP and DZ from the soil and biobed biomixture was evaluated using biochemical and molecular aspects to determine the effect of Streptomyces inoculation in both substrates. DHA activity, which provides a determination of overall living cell activity of microorganism, and APP, which, when produced by microorganisms, is capable of hydrolyzing phosphate ester bonds, were measured throughout the study. The Streptomyces mixed culture inoculated in both the sterilized soil and biomixture was active during the assay, and Streptomyces activity was higher in the biomixture due to the rich biomixture composition where straw was used by Streptomyces through cellulose and lignin degradation (Saini et al. 2015). Ligninolytic activity was verified for the studied strains through qualitative screening (data not shown). The above could explain the increased activity and colonization over time, the latter evaluated by q-PCR, this quantification of the amplified 16S rRNA products was assumed to give an estimation of the size in this case of the Streptomyces population (Accinelli et al. 2012). Mycelial growth of these Actinobacteria allowed efficient colonization of the biomixture, such as was observed by Campos et al. (2017) in a biomixture contaminated with the fungicide iprodione. In the inoculated soil, decreased DHA activity was in accordance with the abundance measured by q-PCR, which could be evidence of a slight impact of pesticides as has been reported in inoculated soil with applications of CP and DZ (Cycoń et al. 2009a; Jastrzebska 2011). However, DHA activity values were similarly observed in the same soil without pesticide application (Arriagada et al. 2012). Therefore, we suggest that application of OPs in the soil did not favor the abundance of Streptomyces mixed culture, but the application of this compound could enhance APP activity, which is involved in mineralization of organic P in the soil. Moreover, APP (positive by studied Streptomyces, data not shown) could be involved in the removal of OPs because APPs are enzymes able to catalyze the hydrolysis of O-P bonds present in CP and DZ (Singh et al. 2004; Singh et al. 2006; Thengodkar and Sivakami 2010).
Although inoculated microorganisms showed activity and the capacity to colonize in both the soil and biomixtures, it was impossible to observe simultaneous removal of both contaminants as observed in the liquid culture. CP was not efficiently removed compared with DZ probably due to the affinity of the adsorbed molecule in the soil particles of the biomixture and would not be available for uptake by the Streptomyces inoculum. Bioavailability of pesticides is one major factor that determines the bioremediation of pesticides in contaminated soil (Odukkathil and Vasudevan 2013) or biomixtures with high organic matter, especially when the compounds have high Koc values (Singh et al. 2006). Low water solubility of CP (<2.0 mg L−1) and high Koc (6862) (Njoroge et al. 2016) enables CP to have a large potential to adsorb in the soil (Solomon et al. 2014) with a strong dependence on the amount of organic matter. Therefore, it would not be surprising that in soil with 17% organic matter, only 14% CP was removed and only 6% CP was removed after 4 weeks in a biomixture with 38% organic matter (this interference is absent in liquid cultures). Another factor that could have influenced CP removal is the pH value, which is a parameter of vital importance in pesticide bioremediation, especially when bio-inoculation is performed (Odukkathil and Vasudevan 2013; Singh et al. 2006). In a natural and sterilized soil inoculated with Enterobacter sp., Singh et al. (2006) reported increased CP removal while the soil acidity decreased; therefore, the soil and biomixture with a pH value of 5.8 and 5.0, respectively, could somehow be restricting CP removal and favoring DZ hydrolysis under acidic conditions (Cycoń et al. 2009b; Briceño et al. 2016a). Availability and overall dissipation of a pesticide in the soil varies considerably from one pesticide to another and depends upon the soil type (Cycón et al. 2013; Fuentes et al. 2017). In this study, we used Chilean Andisols characterized by its high organic content matter, low pH and high superficial reactivity, which determine the availability of nutrients and pesticides (Palma et al. 2015). This soil was chosen, first because it is representative of the agricultural soils of southern Chile and second because it has been used as a constituent of biobed biomixtures (Tortella et al. 2013). Therefore, our results may be representative of these two systems, assuming that there are a number of factors between the soil textures that could be negatively or positively influencing the performance of the Streptomyces inoculum in CP and DZ removal. A study by Cycón et al. (2013) showed that CP removal was different in three soil types inoculated with Serratia marcescens, where fast removal in a sterilized and non-sterilized silty soil was observed. On the other hand, Fuentes et al. (2017) showed that the removal of three organochlorine pesticides in soils inoculated with a mixed culture of Streptomyces spp. was observed in the following order: clay silty loam soil > sandy soil > loam soil. These results were associated with soil texture where the presence of clay and silt particles could provide more sites for bacteria to attach and therefore pesticide utilization. In this study, the authors showed that Actinobacteria are well adapted to proliferate in different contaminated soils and colonize these matrices, as was observed for the silty loam soil and biomixture used in our study.
Various studies have demonstrated that OPs-contaminated soils can be decontaminated by inoculation with microorganisms (Chishti et al. 2013, Cycón et al. 2017). For example, Achromobacter xylosoxidans (JCp4) and Ochrobactrum sp. (FCp1) were able to degrade 93–100% of the input concentration of 200 mg kg−1 CP within 42 days in sterilized as well as non-sterilized soils (Akbar and Sultan 2016). Inoculation with Cupriavidus sp. DT-1 (106 cells g−1 of soil), resulted in a degradation of CP and TCP of 100 and 94.3%, respectively; as compared to a degradation of 28.2 and 19.9% in un-inoculated soil after 30 days of incubation (Lu et al. 2013). Bioremediation of CP-contaminated soil and inoculated with P. fluorescens, Brucella melitensis, Bacillus Subtilis and P. aeruginosa individually resulted in CP degradation within a range of 85–92% as compared to 34% in the control soil after 30 days. In that study, survival of the inoculum was of 60–70%, and TCP was produced by P. aeruginosa (Lakshmi et al. 2008). In relation to DZ degradation, the inoculation of soil with S. marcescens DI101 (106 cells g−1 of soil) in a soil treated with 100 mg kg−1 of DZ resulted in a faster degradation within 14 and 16 days in sterilized and non-sterilized soil, with degradation rates of 0.275 and 0.238 day−1, respectively (Abo-Amer 2011). Finally, bioremediation of DZ-contaminated soil (100 mg kg−1) and soil inoculated with S. liquefaciens, S. marcescens and Pseudomonas sp. individually and the consortium showed a T1/2 within a range of 11.5–24.5 days. The consortium was more efficient in DZ degradation (Cycoń et al. 2009b). Most studies have been focused on single pesticide degradation. Nevertheless, bacteria that can degrade various OPs have also been reported (Cycón et al. 2013; Pino and Peñuela 2011). According to our results, DZ removal from soil was in accordance with the removal showed by S. liquefaciens as previously was report by Cycoń et al. (2009b).
Both single bacteria and bacteria consortia of are able to efficiently degrade OPs. In previous studies performed with liquid medium, we observed that the mixed culture consisting of four Streptomyces strains degraded CP and DZ more efficiently than the individual strains or mixed cultures consisting of two or three strains (Briceño et al. 2016a, b). For the effective use of microorganisms in soil inoculation, it is important to determine the microbial potential by performing studies under optimal conditions in liquid medium (Cycón et al. 2017). However, the degradative ability of strains in liquid cultures and natural soils does not always coincide (Odukkathil and Vasudevan 2016), and this was the case observed for CP removal in our study. Streptomyces mixed cultures have shown adequate removal of organochlorine compounds such as lindane (Fuentes et al. 2011, Saez et al. 2015). According with our results, CP removal by the defined Streptomyces mixed culture could be strongly influenced by intrinsic properties of the soil and the biomixture; therefore, more advanced studies are needed in order to ensure the successful decontamination of environmental matrices with pesticides (Odukkathil and Vasudevan 2016).
The inoculation of biomixtures has been studied as a strategy for optimization of biobed performance (Karas et al. 2016). Therefore, diverse fungi, such as Lentinula edodes EL1, and Trametes versicolor (Ruiz-Hidalgo et al. 2014; Pinto et al. 2016), and bacteria, such as Sphingomonas haloaromaticamans, P. putida and Arthrobacter strain C1 (Karas et al. 2016; Campos et al. 2017), have been used as inoculum in biomixtures showing very good results in the removal of thiabendazole, terbuthylazine, iprodione and carbofuran. This report is the first in which Streptomyces strains are used as the inoculum in the biobed biomixture. According to our results, we suggest that Streptomyces mixed cultures have the ability to act in a very complex system, such as a biobed biomixture, in which DZ required only 11 days to be reduced by 50%. Removal of DZ was substantially faster in the biomixture compared to soil. The increased ability of biomixture to reduce the T1/2 of DZ in about 63% compared to soil is because a biomixture which is mainly straw guarantees a continuous supply of nutrients and high microbial activity (Pinto et al. 2016). Hence, this mixture shows superiority over soil regarding pesticide dissipation.
Bioaugmentation of a biobed biomixture with S. haloaromaticamans, P. putida and a consortium comprised of different proteobacteria showed complete dissipation of thiabendazole in bioaugmented biobed compared to a significantly lower dissipation (86.7%) in the corresponding non-bioaugmented biobed (Karas et al. 2016). A biobed packing material inoculated with an iprodione-degrading Arthrobacter strain C1 resulted in an accelerated dissipation of iprodione compared to the non-bioaugmented biomixture (Campos et al. 2017).
Finally, a study by Diez et al. (2013) showed that at least 50% DZ is removed in different non-bioaugmented biomixtures after 40 and 90 days depending on the applied concentration. Therefore, the Streptomyces mixed culture inoculation could be a tool or strategy for ameliorating the removal of pesticides in wastewater containing DZ.
This type of study can provide a closer insight into microorganisms and their growth requirements before any in situ treatment for decontamination could be performed (Fuentes et al. 2017). Our results display a first report regarding the feasibility to treat OPs in soil and biobed biomixtures using Actinobacteria; inoculation in the soil and biomixture could favor removal of DZ from environmental matrices. However, future assays should be performed using non-sterilized soil and biomixtures to fully understand the interaction and ability of the Streptomyces spp. to survey and compete with indigenous microorganisms present in the environmental matrices before this inoculum could be used in the field for DZ decontamination.
Conclusions
Use of a Streptomyces spp. mixed culture appears to be a good option to remove pesticide mixtures composed of CP and DZ. After 10 days, complete depletion was observed for CP, while DZ was almost completely removed from the liquid medium, and the primary metabolites, TCP and IMHP, were observed. The Streptomyces mixed culture colonized properly in both the soil and biobed biomixture, which was shown as an increase in population and microbial activity. APP activity was the most representative enzymatic test. When the mixed culture was inoculated into the soil and biomixture, only 14 and 6% CP were removed, respectively. The majority of the DZ was removed in the biomixture with a T1/2 of 11 days. Both the soil and biomixture were studied in sterile conditions; therefore, microbiological interferences could be discarded. Consequently, assays conducted in more realistic conditions should be performed in the future. However, results obtained in this work indicate that the decontamination strategy using a Streptomyces mixed culture could represent a promising alternative for eliminating CP and DZ residues from liquids and DZ from soil and biobed biomixtures.
References
Abo-Amer AE (2011) Biodegradation of diazinon by Serratia marcescens DI101 and its use in bioremediation of contaminated environment. J Microbiol Biotechnol 21:71–80
Abo-Amer AE (2012) Characterization of a strain of Pseudomonas putida isolated from agricultural soil that degrades cadusafos (an organophosphorus pesticide). World J Microbiol Biotechnol 28:805–814
Abraham J, Silambarasan S, Logeswari P (2014) Simultaneous degradation of organophosphorus and organochlorine pesticides by bacterial consortium. J Taiwan Inst Chem Eng 45:2590–2596
Accinelli C, Saccà ML, Mencarelli M, Vicari A (2012) Application of bioplastic moving bed biofilm carriers for the removal of synthetic pollutants from wastewater. Bioresour Technol 120:180–186
Afipa, Asociacion Nacional de Fabricantes e Importadores de Productos Fitosanitarios Agrícolas AG (2010) Manual fitosaniatrio 2009–2010. Facultad de Agronomía e Ingeniería Forestal, Pontificia Universidad Católica de Chile, Chile
Aggarwal V, Deng X, Tuli A, Goh KS (2013) Diazinon—chemistry and environmental fate: a California perspective. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology. Springer, New York. doi:10.1007/978-1-4614-5577-6_5
Akbar S, Sultan S (2016) Soil bacteria showing a potential of chlorpyrifos degradation and plant growth enhancement. Braz J Microbiol 47:263–570
Alef K (1995) Dehydrogenase activity. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London
Alvarez A, Saez JM, Davila JS, Colin VL, Fuentes MS, Cuozzo SA, Benimeli CS, Polti MA, Amoroso MJ (2017) Actinobacteria: current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 166:41–62
Arriagada C, Manquel D, Cornejo P, Soto J, Sampedro I, Ocampo J (2012) Effects of the co-inoculation with saprobe and mycorrhizal fungi on Vaccinium corymbosum growth and some soil enzymatic activities. J Soil Sci Plant Nutr 12:283–294
Ballav S, Dastager SG, Kerkar S (2012) Biotechnological significance of Actinobacterial research in India. Recent Res Sci Technol 4:31–39
Berberidou C, Kitsiou V, Lambropoulou DA, Antoniadis A, Ntonou E, Zalidis GC, Poulios I (2016) Evaluation of an alternative method for wastewater treatment containing pesticides using solar photocatalytic oxidation and constructed wetlands. J Environ Manag 195:133–139
Briceño G, Fuentes MS, Palma G, Jorquera MA, Amoroso MJ, Diez MC (2012) Chlorpyrifos biodegradation and 3,5,6-trichloro-2-pyridinol production by actinobacteria isolated from soil. Int Biodeter Biodegr 73:1–7
Briceño G, Pizzul L, Diez MC (2013) Biodegradation of pesticides by actinobacteria and their possible application in biobed systems. In: Amoroso MJ, Benimeli CS, Cuozzo S (eds) Actinobacteria: application in bioremediation and production of industrial enzymes. Press, CRC, 286 pp
Briceño G, Schalchli H, Rubilar O, Tortella GR, Mutis A, Benimeli CS, Palma G, Diez MC (2016a) Increased diazinon hydrolysis to 2-isopropyl-6-methyl-4-pyrimidinol in liquid medium by a specific Streptomyces mixed culture. Chemosphere 156:195–203
Briceño G, Schalchli H, Mutis A, Benimeli CS, Palma G, Tortella GR, Diez MC (2016b) Use of pure and mixed culture of diazinon-degrading Streptomyces to remove other organophosphorus pesticides. Int Biodeter Biodegr 114:193–201
Campos M, Perruchon C, Karas PA, Karavasilis D, Diez MC, Karpouzas DG (2017) Bioaugmentation and rhizosphere-assisted biodegradation as strategies for optimization of the dissipation capacity of biobeds. J Environ Manag 187:103–110
Cao X, Yang C, Liu R, Li Q, Zhang W, Liu J, Song C, Qiao C, Mulchandani A (2013) Simultaneous degradation of organophosphate and organochlorine pesticides by Sphingobium japonicum UT26 with surface-displayed organophosphorus hydrolase. Biodegradation 24:295–303
Castillo MDP, Torstensson L, Stenstrom J (2008) Biobeds for environmental protection from pesticide use—a review. J Agric Food Chem 56:6206–6219
Castro-Gutierrez V, Masís-Mora M, Diez MC, Tortella GR, Rodríguez-Rodríguez CE (2017) Aging of biomixtures: effects on carbofuran removal and microbial community structure. Chemosphere 168:418–425
Chishti Z, Hussain S, Arshad KR, Khalid A, Arshad M (2013) Microbial degradation of chlorpyrifos in liquid media and soil. J Environ Manag 114:372–380
Colin VL, Cortes AA, Aparicio JD, Amoroso MJ (2016) Potential application of a bioemulsifier-producing actinobacterium for treatment of vinasse. Chemosphere 144:842–847
Cycoń M, Piotrowska-Seget Z, Kozdrój J (2009a) Microbial characteristics of sandy soils exposed to diazinon under laboratory conditions. World J Microbiol Biotechnol 26:409–418
Cycoń M, Wójcik M, Piotrowska-Seget Z (2009b) Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere 76:494–501
Cycón M, Zmijowska A, Wójcik M, Piotrowska-Seget Z (2013) Biodegradation and bioremediation potential of diazinon-degrading Serratia marcescens to remove other organophosphorus pesticides from soils. J Environ Manag 117:7–16
Cycón M, Mrozik A, Piotrowska-Seget Z (2017) Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: a review. Chemosphere 172:52–71
Dębski B, Kania BF, Kuryl T (2007) Transformations of diazinon, an organophosphate compound in the environment and poisoning by this compound. Ekológia (Bratislava) 26:68–82
Deng S, Chen Y, Wang D, Shi T, Wu X, Ma X, Li X, Hua R, Tang X, Li QX (2015) Rapid biodegradation of organophosphorus pesticides by Stenotrophomonas sp. G1. J Hazard Mater 297:17–24
Diez MC, Levio M, Briceño G, Rubilar O, Tortella G, Gallardo F (2013) Biochar as a partial replacement of peat in pesticide-degrading biomixtures formulated with different soil types. J Biobased Mater Bioenergy 7:1–7
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120
Flärdh K, Buttner MJ (2009) Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 7:36–49
Fosu-Mensah B, Dartey E, Darko E, Gordon C (2016) Organophosphorus pesticide residues in soils and drinking water sources from cocoa producing areas in Ghana. Environ Syst Res 2016:5–10
Fuentes MS, Saez MJ, Benimeli CS, Amoroso MJ (2011) Lindane biodegradation by defined consortia of indigenous Streptomyces strains. Water Air Soil Pollut 222:217–231
Fuentes MS, Briceño G, Saez JM, Benimeli CS, Diez MC, Amoroso MJ (2013) Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. Biomed Res Int 2013:1–9
Fuentes MS, Raimondo EE, Amoroso MJ, Benimeli CS (2017) Removal of a mixture of pesticides by a Streptomyces consortium: influence of different soil systems. Chemosphere 173:359–367
Hopwood DA (2006) Soil to genomics: the Streptomyces chromosome. Annu Rev Genet 40:1–23
ISP, Instituto de Salud Pública (2012) Informe de resultados de vigilancia de laboratorio. Plan nacional de vigilancia de residuos de plaguicidas en alimentos 2012. http://www.ispch.cl/sites/default/files/documento_tecnico/2013/12/Informe%20Plaguicidas%202012-2013.pdf. Accessed 10 november 2016
Jastrzebska E (2011) The effect of chlorpyrifos and teflubenzuron on the enzymatic activity of soil. Polish J Environ Stud 20:209–210
Karas PA, Perruchon C, Karanasios E, Papadopoulou ES, Manthou E, Sitra E, Ehaliotis C, Karpouzas DG (2016) Integrated biodepuration of pesticide-contaminated wastewaters from the fruit-packaging industry using biobeds: bioaugmentation, risk assessment and optimized management. J Hazard Mater 320:635–644
Lakshmi CV, Kumar M, Khanna S (2008) Biotransformation of chlorpyrifos and bioremediation of contaminated soil. Int Biodeterior Biodegr 62:204–209
Lasram MM, Dhouib IB, Annabi A, El Fazaa S, Gharbi N (2014) A review on the molecular mechanisms involved in insulin resistance induced by organophosphorus pesticides. Toxicology 322:1–13
Lu P, Li Q, Liu H, Feng Z, Yan X, Hong Q, Li S (2013) Biodegradation of chlorpyrifos and 3,5,6-trichloro-2-pyridinol by Cupriavidus Sp. DT-1. Bioresour Technol 127:337–342
Muñoz-Quezada MT, Iglesias V, Lucero B, Steenland K, Boyd Barr D, Levy K, Ryan B, Alvarado S, Concha C (2012) Predictors of exposure to organophosphate pesticides in schoolchildren in the province of Talca, Chile. Environ Int 47:28–36
Navaratna D (2012) Reducing herbicide discharge to sensitive environments using membrane bioreactors. Submitted in partial fulfillment of the requirements for the degree of Doctor of Phylosophy (Engineering). Deakin University. 374 pp
Njoroge SM, Munyao TM, Osano O (2016) Modeling relationship between organic carbon partition coefficient and pesticides solubility of pesticides used along the shore of lake Naivasha, Kenya. Am J Environ Eng 6:32–37
Odukkathil G, Vasudevan N (2013) Toxicity and bioremediation of pesticides in agricultural soil. Rev Environ Sci Biotechnol 12:421–444
Odukkathil G, Vasudevan N (2016) Residues of endosulfan in surface and subsurface agricultural soil and its bioremediation. J Environ Manag 165:72–80
Palma G, Demanet R, Jorquera M, Mora ML, Briceño G, Violante V (2015) Effect of pH on sorption kinetic process of acidic herbicides in a volcanic soil. J Soil Sci Plant Nutr 15:549–560
Pino N, Peñuela G (2011) Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site. Int Biodeter Biodegr 65:827–831
Pinto AP, Rodrigues SC, Caldeira AT, Teixeira DM (2016) Exploring the potential of novel biomixtures and Lentinula edodes fungus for the degradation of selected pesticides. Evaluation for use in biobed systems. Sci Total Environ 541:1372–1381
Pozo K, Llanos Y, Estellano VH, Cortés S, Jorquera H, Gerli L, Pozo K, Encina F, Palma R, Focardi S (2016) Occurrence of chlorpyrifos in the atmosphere of the Araucanía region in Chile using polyurethane foam-based passive air samplers. Atmos Pollut Res 7:706–710
Ruiz-Hidalgo K, Chin-Pampillo JS, Masís-Mora M, Carazo E, Rodríguez-Rodríguez CE (2014) Degradation of carbofuran by Trametes versicolor in rice husk as a potential lignocellulosic substrate for biomixtures: from mineralization to toxicity reduction. Process Biochem 49:2266–2271
Ruíz-Hidalgo K, Masís-Mora M, Barbieri E, Carazo-Rojas E, Rodríguez-Rodríguez CE (2016) Ecotoxicological analysis during the removal of carbofuran in fungal bioaugmented matrices. Chemosphere 144:864–871
Saez JM, Aparicio JD, Amoroso MJ, Benimeli CS (2015) Effect of the acclimation of a Streptomyces consortium on lindane biodegradation by free and immobilized cells. Process Biochem 50:1923–1933
SAG, Servicio Agrícola y Ganadero (2012) Informe de venta de plaguicidas de uso agrícola en Chile. Año 2012. http://www.sag.cl/sites/default/files/declaracion_de_venta_de_plaguicidas_ano_2012.pdf. Accessed 10 december 2016
Saini A, Aggarwal NK, Sharma A, Yadav A (2015) Actynomicetes: a source of lignocellulolityc enzymes. Enzyme Res 2015:1–15
Schreiberová O, Hedbávná P, Cejková A, Jirku V, Masák J (2012) Effect of surfactants on the biofilm of Rhodococcus erythropolis, a potent degrader of aromatic pollutants. New Biotechnol 30:1
Shimazu M, Mulchandani A, Chen W (2001) Simultaneous degradation of organophosphorus pesticides and p-nitrophenol by a genetically engineered Moxarella sp. with surface expressed organophosphorus hydrolase. Biotechnol Bioeng 76:318–324
Singh BK, Walker A, Morgan JAW, Wright DJ (2004) Biodegradation of chlorpyrifos by Enterobacter strain B-14 and its use in bioremediation of contaminated soils. Appl Environ Microbiol 70:4855–4863
Singh BK, Walker A, Wright DJ (2006) Bioremedial potential of fenamiphos and chlorpyrifos degrading isolates: influence of different environmental conditions. Soil Biol Biochem 38:2682–2693
Solomon KR, Williams WM, Mackay D, Purdy J, Giddings JM, Giesy JP (2014) Properties and uses of chlorpyrifos in the United States. In: Giesy JP, Solomon KR (ed) Ecological risk assessment for chlorpyrifos in terrestrial and aquatic systems in the United States, reviews of environmental contamination and toxicology 231. doi:10.1007/978-3-319-03865-0_2
Tabatabai MA, Bremmer JM (1969) Use of p-nitrophenylphosphate to assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Thengodkar RRM, Sivakami S (2010) Degradation of chlorpyrifos by an alkaline phosphatase from the cyanobacterium Spirulina platensis. Biodegradation 21:637–644
Tortella GR, Mella-Herrera RA, Sousa DZ, Rubilar O, Acuña JJ, Briceño G, Diez MC (2013) Atrazine dissipation and its impact on the microbial communities and community level physiological profiles in a microcosm simulating the biomixture of on-farm biopurification system. J Hazard Mater 260:459–467
Walker L (2016) Management of chlorpyrifos and diazinon discharges to the sacramento and feather rivers and the Sacramento-San Joaquin Delta: 2015 TMDL Compliance Monitoring Report. http://www.svwqc.org/wp-content/uploads/2016/05/chlorpyrifos_diazinon_TMDL_Report_2015.pdf. Accessed 28 December 2016
Wang G, Liu Y (2016) Diazinon degradation by a novel strain Ralstonia sp. DI-3 and X-ray crystal structure determination of the metabolite of diazinon. J Biosci 41:359–366
Yañez-Ocampo G, Sánchez-Salinas, Ortiz-Hernández ML (2011) Removal of methyl parathion and tetrachlorvinphos by a bacterial consortium immobilized on tezontle-packed up-flow reactor. Biodegradation 22:1203–1213
Yu R, Liu Q, Liu J, Wang Q, Wang Y (2016) Concentrations of organophosphorus pesticides in fresh vegetables and related human health risk assessment in Changchun, Northeast China. Food Control 60:353–360
Acknowledgements
The authors acknowledge the financial support from the National Fund for Scientific and Technological Development, FONDECYT Project N° 11130716 and FONDECYT Project N° 1161481; and the National Commission for Scientific and Technological Research (CONICYT)—Ministry of Science, Technology and Productive Innovation (MINCYT), Programme of Scientific International Cooperation (Chile-Argentina) PCCI 140056.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Briceño, G., Vergara, K., Schalchli, H. et al. Organophosphorus pesticide mixture removal from environmental matrices by a soil Streptomyces mixed culture. Environ Sci Pollut Res 25, 21296–21307 (2018). https://doi.org/10.1007/s11356-017-9790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9790-y