Abstract
This study is the first report describing the occurrence of 15 phthalate acid esters (PAEs) in the three typical water sources of YiXing City, Taihu Upper-River Basin, East China. The fate of target PAEs in the Jiubin drinking water treatment plant (JTP) was also analyzed. The amounts of Σ15PAE in the Hengshan (HS), Youche (YC), and Xijiu (XJ) water sources were relatively moderate, with mean values of 360, 357, and 697 ng L−1, respectively. Bis(2-ethylhexyl) phthalate (DEHP) dominated the PAE concentration, making up 80% of the 15 total PAEs. The highest levels of Σ15PAE were found in HS, YC, and XJ in March 2015, January 2015, and July 2014, respectively. The occurrence and concentrations of these compounds were spatially dependent, and the mean concentrations of Σ15PAE in HS, YC, and XJ samples increased from the surface layer to the bottom layer with varied percentage increases. The removal efficiency of the PAEs in the finished water varied markedly, and the removal of PAEs by the JTP ranged from 12.8 to 64.5%. The potential ecosystem risk assessment indicated that the risk of PAEs was relatively low in these three water sources. However, risks posed by PAEs due to drinking water still exist; therefore, special attention should be paid to source control in the JTP, and advanced treatment processes for drinking water supplies should be implemented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution has become a critical worldwide issue in recent years, and organic compound pollutants from various sources released into the environment have caused wide public concern throughout the world (Tang et al. 2014). Endocrine disruptor chemicals (EDCs) are synthetic or natural chemical compounds present in the environment that interfere with normal endocrine function (Ponzo and Silvia 2013). Some phthalate acid esters (PAEs), which possess reproductive and developmental toxicity in animals and bio-accumulate in fats, have been shown to be carcinogenic and estrogenic (He et al. 2013; Keresztes et al. 2013). In addition, the alkyl chain length and the hydroxylation of PAEs significantly affect their ability to bind to estrogen receptors and peroxisome proliferator-activated receptors (Okamoto et al. 2011). PAEs are a main concern on the growing list of suspected EDCs, with several million tons of PAEs produced worldwide annually (Schecter et al. 2013). PAEs are common additives in paints, lubricants, adhesives, insecticides, packaging, and cosmetics, and are continually released into the environment (Peng et al. 2013). The global production of PAEs is growing rapidly: 1.8 million tons in 1975, 6.2 million tons in 2009, and 8 million tons in 2011 (Net et al. 2015a, b). There have been many reports that PAEs are ubiquitous in many types of environmental samples, such as air, water, soils, and sediments (Fierens et al. 2012; Kang et al. 2012; Net et al. 2015a, b; Peng et al. 2013; Stackelberg et al. 2004).
PAEs are used in a wide variety of consumer products, and human exposure to this class of compounds is widespread. Fourteen phthalate metabolites was found to occur in human urine samples collected from some Asian countries, and phthalate metabolites were found in all samples (Guo et al. 2011a, b; Fierens et al. 2012; Kang et al. 2012; Net et al. 2015a, b; Peng et al. 2013; Stackelberg et al. 2004). Extensive exposure to PAEs increases the risk to human reproductive development, and thus several phthalates have been listed as priority pollutants by many national and international regulatory organizations. Currently, dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP), butyl benzyl phthalate (BBP), bis(2-ethylhexyl) phthalate (DEHP), and di-n-octyl phthalate (DNOP) are listed as priority pollutants by the United States Environmental Protection Agency (Abdel Daiem et al. 2012). DEHP and DBP are the most widely used phthalates in many countries (Jin et al. 2015). In addition, of the phthalates produced in the European Union (EU), DEHP might account for ~30%, and in China, 80% (Meng et al. 2014). The Ministry of Environmental Protection in China has regulated phthalates as environmental pollutants. In addition, the standard in China regarding the analytical control of drinking water specifically identified PAEs as organic pollutants and required monitoring for PAEs in drinking water supplies in 2012 (Xue-Li et al. 2007). However, official data regarding the presence of these pollutants in the aquatic environment of some cities in China are not available.
Taihu Lake is the third largest freshwater lake in China, with an average depth of 1.9 m. The PAE pollution in several regions of the lake has been extensively investigated, and their possible sources and environmental risks discussed (Wang et al. 2003). YiXing City accounted for approximately half of the entire water and pollution load into Taihu Lake (Liu et al. 2012). However, few special reports are available about the PAE levels in YiXing City, which is the famous pottery capital and one of most powerful county-level cities in China.
The objectives of the present study were as follows: (1) to determine the occurrence of PAEs and clarify the fate and distribution of pollutants in water from different sources; (2) to ascertain the temporal and spatial variation of PAEs in surface water; (3) to examine the removal efficiencies of the PAEs by the Jiubin drinking water treatment plant (JTP); and (4) to evaluate the potential risk of PAEs on human health. Therefore, PAEs from three typical water sources were investigated to provide data regarding water contamination in YiXing City.
Materials and methods
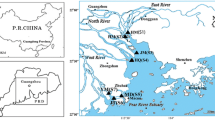
Description of sampling area and sample collection
The study region and the details of the sampling sites are presented in Fig. 1 and Table S1, respectively. The Hengshan Reservoir (HS) and the Youche Reservoir (YC) have become the most important resources of drinking water for YiXing City in recent years. Xijiu Reservoir (XJ) was the original water source, but it is now a backup source due to serious water pollution. The JTP has a daily production of 300,000 m3 of drinking water, and depends completely on the water supply from HS and YC. A total of 24 sampling points were selected among the three water sources, and surface water samples were collected monthly from July 2014 to June 2015. Among these, a total of six sampling sites were defined in the HS water source, a total of five sampling sites in YC, a total of seven sampling sites in XJ, and a total of six sites were located on the tributaries of XJ. For the sampling points denoted S4 (HS), S11 (YC), and S12 (XJ), samples were taken at six depths: surface (0.5 m below the water surface), 0.2H, 0.4H, 0.6H, 0.8H, and bottom (1.0 m above the bed), Here, H represents the water depth during the sampling, and corresponds to values of 14, 13.5, and 5.5 m for HS, YC, and XJ, respectively. That is, the appropriate depths of the samples were 0.5, 3.1, 5.7, 8.3, 10.9, and 13 m (for HS); 0.5, 2.6, 4.4, 6.6, 8.8, and 11 m (for YC); and 0.5, 1.3, 2.1, 2.9, 3.7, and 4.5 m (for XJ). To avoid the water between the different target layers, a specific-depth water sampler was used. This device flips up and down automatically to open and close, thereby acquiring water samples at the desired depth. Moreover, two sets of water samples were collected from the JTP at raw and drinking water sampling points each month. At each sampling point, water samples were collected in triplicate (in full 5.0 L polyethylene bottles) and then kept at 4 °C prior to extraction (within 2 days). All containers in contact with the samples were sequentially washed with deionized water, and then river water, prior to sampling to eliminate disturbances due to other organic materials.
Chemicals
Six PAEs listed as priority pollutants by the United States Environmental Protection Agency (EPA), including DMP, DEP, diisobutyl phthalate (DIBP), DBP, DEHP, and DNOP; and another nine prevalent PAE congeners, bis(4-methyl-2-pentyl) phthalate (BMPP), bis(2-methoxyethyl) phthalate (DMEP), bis(2-ethoxyethyl) phthalate (DEEP), dipentyl phthalate (DPP), dihexyl phthalate (DHXP), BBP, di(2-n-butoxyethyl) phthalate (DBEP), dicyclohexyl phthalate (DCHP), and dinoctyl phthalate (DNP), were selected as target compounds in this study. A mixture solution of 1000 μg/mL of the above-mentioned 15 standard PAEs and a mixture solution of 500 μg/mL of each of three surrogate standards (diisophenyl phthalate, di-n-phenyl phthalate, and di-n-benzyl phthalate) were supplied by AccuStandard Inc. The internal standard, benzyl benzoate, was also purchased from AccuStandard Inc. All solvents (acetone, hexane, and dichloromethane) used were HPLC-grade and were purchased from J. T. Baker Co. (USA). Anhydrous sodium sulfate (Tianjin Chengguang Chemical Reagent Co., China) was baked at 600 °C for 6 h and then kept in a desiccator for later use.
Sample preparation and chemical analysis
In a vacuum, water samples were filtered through glass fiber filters (0.7 μm pore sizes). Prior to extraction, each sample was spiked with 50 ng L−1 of the surrogate standards. The water samples were extracted using a classical liquid phase extraction method (USEPA, Method 8061) with slight modifications. Briefly, the 1-L water sample was placed in a separatory funnel with 150 mL of dichloromethane, and extracted with mechanical shaking. Next, it was mixed with sodium sulfate (approximately 20 g), and filtered. The organic extracts were concentrated using a rotary evaporator. Solvent exchange was performed by replacing dichloromethane with hexane. Finally, the remains were reduced to 0.5 mL under gentle nitrogen flow. The 0.05 ng internal standard was added to the sample prior to instrumental analysis.
Quantification of the PAEs was performed on an Agilent 6890N GC and an Agilent 5975 mass spectrometer detector (GC–MSD). The mass spectra were recorded at a selected ion mode (SIM) covering the range 50–500 mass units for PAEs in water samples. These were operated in electron impact mode (70 eV). Separation was carried out using a 30 m × 0.25 mm × 0.25 μm HP-5 MS capillary column (Agilent Co., USA). The instrumental conditions were as follows: injector temperature, 280 °C and ion source temperature, 230 °C. The temperature program was as follows: the column started initially at 100 °C and was held for 3 min, increased to 200 °C at a rate of 15 °C min−1, and then increased to 280 °C at a rate of 5 °C min−1 and was held for 5 min. The carrier gas was helium at a constant flow rate of 1.0 mL min−1. One microliter of sample was injected in split-less mode.
Quality assurance and quality control
Because phthalates are ubiquitous laboratory contaminants, a strict regime of quality control must be provided in such experiments. Procedural blanks are processed simultaneously in each batch of samples in exactly the way that the samples are prepared. An appropriate concentration of standard solution was spiked into each sample to estimate the recovery and the performance of the methods. The concentrations of the blanks were subtracted from the sample concentrations of detectable compounds. The instruments were calibrated daily with calibration standards. In addition, the mean surrogate recoveries of diisophenyl phthalate, di-n-phenyl phthalate, and di-n-benzyl phthalate for water samples were 52.8, 84.7, and 82.1%, respectively. The recoveries from the mixture solution of 15 PAEs (each 50 ng L−1) ranged from 75.4 to 105.6% in the spiked water. Moreover, the chromatograms of 10 μg L−1 of the mixture solutions of 15 standard PAEs compounds were provided in Fig. S1. The inner marker method was applied to carry out quantitative analysis, and the Method Determination Limit (MDL) of PAEs ranged from 0.03 to 0.49 ng L−1. All the results were corrected for the blanks and recoveries. Each extract was analyzed in duplicate form, and relative standard deviations were less than 25%.
Results and discussion
PAEs levels in the water resources
The PAEs in the three typical water sources from the sampling sites were investigated, and the results of relative contributions of the 15 PAE congeners are presented in Table 1 and Fig. 2. The total PAE concentrations ranged from 293 to 497 ng L−1 (a geometric mean of 360 ng L−1), from 307 to 455 ng L−1 (a geometric mean of 357 ng L−1), and from 421 to 814 ng L−1 (a geometric mean of 697 ng L−1) for HS, YC, and XJ, respectively. Among the 15 PAEs detected in the three water sources, DMP, DEP, DIBP, DBP, and DEHP were detected in all the samples at average concentrations of 30.5, 6.1, 38.6, 45.5, and 411 ng L−1, respectively. Considering the individual PAE congeners in this area, the results showed that DEHP was the most abundant in the water samples, contributing from 60.0 to 80.2% for HS, 67 to 75.1% for YC, and 57.3 to 85.5% for XJ. The next dominant PAE was DIBP, contributing from 2.7 to 15.9%, followed by DBP, contributing from 6.7 to 13.9% of the Σ15PAE concentrations. This result is consistent with previous findings that DEHP and DnBP/DIBP are the most commonly produced PAEs (Blanchard et al. 2013; Penalver et al. 2000; Vethaak et al. 2005). Prevailing DEHP values have been reported in Malaysia, Canada, and European countries. Moreover, the three PAE congeners are important and popular additives in many industrial products, including flexible polyvinyl chloride (PVC) materials and household products, suggesting that these uses are the main source of PAE contaminants in water (Peijnenburg and Struijs 2006). Although DNP and DNOP had relatively low concentrations that ranged from below the detection limit to 2.9 ng L−1, they have also attracted much attention as priority pollutants from the China National Environmental Monitoring Center. In contrast, the concentrations and the detectable frequencies of DMEP, BMPP, DEEP, DPP, DHXP, BBP, DBEP, DCHP, and DNP were substantially lower, illustrating their minimal pollution levels in the three water sources of YiXing City.
There was a slight variation in the total concentration of the 15 PAEs in HS and YC; whereas fluctuation was observed in the total concentrations of 15 PAEs in surface water from XJ. Higher concentrations of the 15 PAEs were measured at site S6 of HS, site S10 of YC, and site S13 of XJ. The Σ15PAE of HS and YC were relatively low, which may be because they are located far from urban and suburban regions. The XJ water source is surrounded by dense restaurants and commercial streets and receives a large amount of industrial and domestic PAEs from YiXing City. The correlations in the concentrations of DEHP, DBP, and DIBP with the concentrations of total PAEs in the water samples are shown in Fig. 3. Significant correlations existed between DEHP, DBP, and DIBP, and Σ15PAE, (correlation coefficients r = 0.973, 0.812, and 0.738, p < 0.01, respectively), suggesting the importance of the three substances on the total concentrations of PAEs in the three water sources.
Although research on the distribution of DMP, DEP, DIBP, BBP, and DEHP have been reported (as shown in Table 2), the data on all 15 PAEs are limited. Therefore, DMP, DEP, DIBP, BBP, and DEHP were selected among the contaminants in the surface water from the three water sources in YiXing City, for comparison with the contaminant levels in other areas in the world; the results are presented in Table 2. The median concentration of DEHP found in the present study was approximately 1–2 orders of magnitude lower than those determined in the Klang River Basin of Malaysia and Taiwan River of China (16.6 and ND-18.5 μg L−1, respectively). The median concentrations of DEHP, DMP, DEP, and DIBP had the same order of magnitude as the levels of PAEs measured in the Ogun River of Nigeria and on the Dutch coast of The Netherlands. In comparison, the median concentration of DEHP in our study was approximately ten times higher than the concentration levels reported for the Gomti River of India. BBP, DEP, and DMP were detected at relatively low concentrations in all studies, and the concentrations measured in the present study varied by a factor of ~10 in comparison to other studies. The concentrations of DIBP measured in the three water sources were lower than those determined in the Klang River Basin of Malaysia, the Rieti District of Italy, and Taihu Lake of China. On the other hand, the concentrations of DIBP were approximately 1 order of magnitude higher than found in False Creek Harbour of Canada.
Temporal and spatial variation of PAEs
The distributions of the 15 PAEs (Σ15PAE) studied and the 6 US EPA priority PAEs (Σ6PAE, including DMP, DEP, DIBP, DBP, DEHP, and DNOP) in HS, YC, and XJ were determined each month from July 2014 to June 2015, and are shown in Fig. 4. The annual Σ15PAE concentrations in HS, YC, and XJ ranged from 427 to 931, 546 to 873, and 902 to 1744 ng L−1, respectively, with mean values of 718, 716, and 1333 ng L−1. The Σ6PAE concentrations in the surface water from HS, YC, and XJ ranged from 389 to 841, 510 to 795, and 869 to 1674 ng L−1 with mean values of 651, 666, and 1258 ng L−1, respectively. There was marked variation in the total Σ15PAE concentrations of the three water sources during the study period, and the distribution spectra of Σ6PAE, which was observed for all the water sources, were similar to Σ15PAE. In addition, Fig. 4 shows that Σ6PAE made up the vast majority of the total Σ15PAE, and that the other nine PAEs corresponded to a small proportion of the total Σ15PAE. The highest levels of Σ15PAE and Σ6PAE contamination in HS were observed in March 2015 at 932 and 843 ng L−1, respectively. Some heavily polluted periods included June, September, and October, 2014, during which mean contamination values of Σ15PAE and Σ6PAE were 876 and 792, 860 and 729, and 842 and 762 ng L−1, respectively. In contrast, the lowest concentrations of Σ15PAE and Σ6PAE in HS were measured in January 2015 (428 and 390 ng L−1, respectively). For the YC, the highest levels of Σ15PAE and Σ6PAE were detected in January 2015 (873 and 796 ng L−1, respectively), and the lowest levels were in February 2015 (546 and 502 ng L−1). Other heavily polluted periods were July, September, and August 2014, and the PAE pollution levels of YC were similar to those in HS. However, the higher PAE pollution levels were 2–3-fold higher in XJ than in HS and YC. The highest levels of Σ15PAE and Σ6PAE contamination of XJ were in July 2014 (1743 and 1674 ng L−1, respectively). The next most heavily polluted periods were January and May 2014 and October 2015, with mean Σ15PAE and Σ6PAE contamination values of 1693 and 1598, 1627 and 1597, and 1606 and 1468 ng L−1, respectively, indicating that these periods were important times of PAE contamination. The levels of contamination varied widely in the water samples examined, especially those from the XJ. In general, it should be noted that PAE levels may be related to the input of local waste, such as sewage water, food packaging, and scrap material near the sampling points, all of which were observed during the sampling period. The degree of PAEs pollution may also have depended on the rainfall season, in that the mean concentrations of PAEs during the high water period were lower than during the low water period. In addition, no big differences were found in the proportions of individual PAEs to the total PAEs during the two periods, indicating a possible similarity in the sources of PAEs. The results of this study coincide with other findings because the HS and YC are located far from urban areas and are mainly surrounded by farmland, whereas the regions of the XJ reservoir include important channels of river transportation and were subject to automotive exhausts, industrial emissions, and sewage discharge, all of which enhance PAE contaminant levels. Moreover, distributed around XJ are several petrochemical facilities, chemical factories, and printing and dyeing enterprises, which may contribute to the water pollution in this area.
Spatial variations in the annual concentrations of individual PAEs in the three water sources in YiXing City are shown in Fig. 5. Because DMP, DEP, DIBP, DBP, DEHP, and DNOP were the main PAE contaminants in the three water sources, and because DPP and DBEP were also frequently detected, the present study focused on these eight PAE contaminants in the spatial variation studies. The average content of DEHP, which is the major component of PAEs, indicated sustained serious pollution in the YiXing area. DEP, DPP, DBEP, and DNOP were the minor components among PAEs, with mean concentrations of 6.1, 2.1, 1.9, and 1.8 ng L−1, respectively, at all the sampling sites, and DMP, DEP, and DBP had comparatively high concentrations, with mean values of 32.9, 36.3, and 40.6 ng L−1, respectively. Moreover, DEHP was high in the XJ, reaching 736 ng L−1, and low in the HS. Runoff and pollution source distribution were the main causes of the spatial variance. Runoff is an important factor influencing the temporal distribution of PAEs and may suggest a variety of original sources of the PAEs. Moreover, major sources of PAE pollution are distributed differently in the three water sources, which may cause spatial variation of PAE concentrations in the different water sources. Previous research has shown that there is a positive correlation between the concentrations of PAEs with DMP and DEP, and agricultural runoff. The solubility of DMP and DEP indicate the reason for their potential abundance in water (dissolution phase) and that their daily production and consumption were extremely low (He et al. 2013). DEHP dominated the domestic plasticizers, and the trend of the DEHP distribution was closely associated with the locations of industries and urbanization. The high level of DEHP in XJ could be related to contributions from industrial sources and residential activities. In general, the results showed that the spatial distributions of PAEs in the three water sources were site-specific. Moreover, the vertical distributions of the mean concentrations of the total 15 PAEs (Σ15PAE) and the 6 US EPA priority PAEs in the three water sources are shown in Fig. S2.
PAEs profiles and sources
The varying PAE patterns may indicate different sources of PAEs and measurements of the individual PAE compositions are helpful in tracking contaminant sources and in demonstrating the transport and fate of PAEs in water (Liu et al. 2013; Wang et al. 2015). The relative contributions of the eight detectable PAE congeners (the six US EPA priority PAEs, including DMP, DEP, DIBP, DBP, DEHP, and DNOP, and two other PAEs, DPP and DBEP) were determined frequently, and other components of the Σ15PAE concentrations in HS, YC, and XJ are presented in Fig. 6. It is clear that DEHP was most abundant in the water sources, with contributions of 77.8, 73.3, and 76.1% to total PAEs loads in the water for HS, YC, and XJ, respectively. Moreover, DEHP was also the main PAE contaminant of the six tributaries of the XJ, with contributions ranging from 54.5 to 83.3% at sites 19 and 22, respectively. DBP and DIBP in the water sources and the six tributaries accounted for 6.3 and 9.4, 10.3 and 6.4, and 7.6 and 4.9% of the Σ15PAE for HS, YC, and XJ, respectively. For S23, DBP and DIBP accounted for 18.3 and 4.3%. In addition, the content of DMP ranged from 2.0 to 17.3% for all water sources, and the highest level was found in the tributaries corresponding to sample S19. Together, DEP, DNOP, DBEP, and DPP accounted for 2.26% in water sources, and the other nine PAEs accounted for 2.63% of the Σ15PAE, as shown. These patterns of PAE congeners could reflect several factors, including source compositions, sedimentary dispersion/accumulation patterns, and environmental degradation. PAEs of low molecular weights, such as DMP and DEP, are widely used in cosmetics and personal care products. DEHP has wide applications as a plasticizer in the polymer industry (Zeng et al. 2008). DIBP, BBP, and DEHP, which are longer and/or branch alkyl PAEs, more easily undergo sorption in sediment and are resistant to degradation. The results indicate different patterns of plastic contaminant input during the sampling periods. A chemical factory lies near the XJ water source; similarly, DIBP and DBP are used in epoxy resins and special adhesive formulations, which are important indications that the PAEs at the sampling locations are from industrial pollution.
PAEs levels in drinking water treatment plants
The JTP operation includes coagulation, sedimentation, and filtration treatment processes, which are typical treatments for drinking water. The schematic diagram of the waterworks is shown in Fig. S3. The JTP draws water from the HS and the YC and was investigated to assess the fate of PAEs during the drinking water treatment process. The measured concentrations in the raw and final water samples taken from the JTP are shown in Fig. 7. A total of 15 PAEs were identified in the treated water samples collected from the storage tank of the JTP. Eight out of 15 PAEs were detected in the final samples from the JTP, including DMP, DEP, DIBP, DBP, DPP, DBEP, DEHP, and DNOP. The other phthalates investigated were of minor importance and their concentrations were all below the limit of detection. As shown in Fig. 7, the DEP, DPP, DBHP, and DNOP detected were at extremely low levels in the drinking water, with variable degrees of removal. The removal efficiency for the PAEs measured in the finished water varied markedly; the removal of PAEs by the JTP waterworks ranged from 12.8 to 64.5%, indicating unstable removal efficiencies. The most important compound detected in the finished water was DEHP, with a mean concentration of 150 ng L−1 and a removal rate of 58.7%, suggesting that DEHP makes up the highest relative composition of total PAE concentrations in the drinking water. The mean concentrations of DMP, DIBP, and DBP in the finished water were 13.9, 24.3, and 17.2 ng L−1, with removal of 15, 24.3, and 64.5%, respectively. Given that the bulk organic parameter in water generally reflects the content of many organic pollutants, the calculated removal values of the parameters were measured. As shown in the Table S2, the removals of TOC, UV254, and CODMn were 34.7, 56.6, and 54.5%, respectively, for the raw HS water source and 25.6, 45.7, and 57.1%, respectively, for the raw YC water source. However, the removal efficiency of total PAEs was lower than the efficiency of the bulk organic parameters, indicating that traditional drinking water treatment does not perform well in eliminating these micro-pollutants, regardless of the water source. Traditional drinking water treatment focuses on particles and colloids in terms of physical processes. Previous studies have shown that oxidation and microbial action are the principal mechanisms for removing PAEs from aquatic systems (Fromme et al. 2002; Yuan et al. 2008; Yunrui et al. 2007). Therefore, the treatment process should include a combination of these key techniques for removing PAEs from water, and further research on advanced drinking water treatments for PAE removal is required.
Toxicological risks of PAEs in YiXing City
To assess the potential ecosystem risks of PAEs, quality guidelines for surface water and drinking water, and tolerable daily intake (TDI) were considered. The mean concentrations of DBP and DEHP detected in the present study were well below the reference dose (RfD: 3 and 8 μg L−1, respectively) regarded as unsafe by China for surface water (Environmental Quality Standard for Surface Water of China, GB3838-2002)(Gu 2003). According to the results, the concentration of DEHP in the drinking water was lower than the recommended limit (i.e., the level present in public water supplies must not exceed the drinking water standard: 6000 and 8000 ng L−1 for EPA and China, respectively) (Liu et al. 2013).
PAEs that are detected in drinking water are constantly ingested in daily life, indicating that drinking water is an important source of human exposure to PAE contaminants. Assuming a daily water consumption rate of 2 L and an average body weight of 60 kg for adults, the average daily intake of DEHP, DBP, and DIBP via drinking water from the JTP was estimated to be 12.8, 1.5, and 1.8 μg kg−1 bw day−1, respectively. In this study, the estimated daily intake levels of DEHP from the drinking water were much lower than the RfD of 20,000 ng/kg/day suggested by the EPA. Reference values of TDI were specified for DBP (10 μg kg−1 bw day−1) and DEHP (50 μg kg−1 bw day−1) by European Food Safety. RfD values have also been suggested for DEP (800 μg kg−1 bw day−1), DBP (100 μg kg−1 bw day−1), BBP (200 μg kg−1 bw day−1), and DEHP (20 μg kg−1 bw day−1) by the US EPA. A major source of human exposure to phthalates is diet (Schettler 2006). Studies have reported human dietary exposure to phthalates in collections from Harbin and Shanghai, China, in 2011 (Guo et al. 2012; Guo and Kannan 2011). These studies reported that the daily dietary intake of phthalates (EDIdiet) from DMP, DEP, DIBP, DBP, and DEHP (based on mean concentrations) were 0.092, 0.051, 0.505, 0.703, 0.022, and 1.60 μg/kg-bw/day, respectively, for Chinese adults, depending on the type of PAEs. All the values estimated in this study were far below the recommended limits, and thus do not represent a significant risk to human health. Moreover, the estimates were also below the overall dietary intake (DI) range of 0.08–69.6 μg kg−1 bw day−1 assessed when many exposure pathways were taken into account (Gavala et al. 2004; Net et al. 2015a, b). According to the results, the phthalates found in the water sources of YiXing City and drinking water from the JTP do not pose ecosystem risks for humans. However, PAEs are partially metabolized by organisms, and future experiments will be focused on determining the potential effects of the metabolites (Guo et al. 2011a, b).
Conclusions
This study provides the first detailed data on the contamination status of 15 PAEs from three typical water sources in YiXing City (Taihu Upper-River Basin, East China). Collecting more information on PAE contamination from typical water sources in YiXing City is particularly important to assure safe drinking water and to control water pollution in the Taihu Basin. The results showed that the levels of PAEs were relatively low in the three water sources compared with other rivers in the world. The total amount of PAE contamination for the three water sources decreased in the order XJ > HS > YC. The concentrations of Σ15PAE in the HS, YC and XJ ranged from 293 to 497, 307 to 455, and 412 to 814 ng L−1, respectively. The occurrence and distribution of PAEs for different sampling sites in the water sources varied largely. These results imply that no urgent remediation measures are required with respect to the PAEs in the water sources; however, the conventional water treatment processes at JTP were not efficient at removing PAEs from the drinking water supplies. Moreover, chlorine may react with trace organic compounds, including PAEs, to generate harmful by-products during chlorine disinfection of drinking water (Jiang et al. 2017; Skilhagen et al. 2008). In addition, the monitoring of the PAEs in the JTP revealed that PAEs were present in the drinking water, and that the ecological and health effects of these substances consumed in drinking water at such low concentrations require further notice in light of their possible biological manifestations. Hence, source control and advanced processes in drinking water treatment plants should be topics of future studies.
References
Abdel Daiem MM, Rivera-Utrilla J, Ocampo-Pérez R, Méndez-Díaz JD, Sánchez-Polo M (2012) Environmental impact of phthalic acid esters and their removal from water and sediments by different technologies—a review. J Environ Manag 109:164–178
Blanchard M, Moreau-Guigon E, Dargnat C, Alliot F, Bourges C, Desportes A, Chevreuil M (2013) Phthalate fate in the hydrographic network of the river Seine Basin (France) under contrasted hydrological conditions. Water Air Soil Pollut 224:1–14
Fierens T, Servaes K, Van Holderbeke M, Geerts L, De Henauw S, Sioen I, Vanermen G (2012) Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem Toxicol 50:2575–2583
Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A (2002) Occurrence of phthalates and bisphenol A and F in the environment. Water Res 36:1429–1438
Gavala HN, Yenal U, Ahring BK (2004) Thermal and enzymatic pretreatment of sludge containing phthalate esters prior to mesophilic anaerobic digestion. Biotechnol Bioeng 85:561–567
Gu Y (2003) Approach on the development of environmental quality standards for surface water. Shanghai Environ Sci
Guo Y, Kannan K (2011) Comparative assessment of human exposure to phthalate esters from house dust in China and the United States. Environ Sci Technol 45:3788–3794
Guo Y, Alomirah H, Cho H, Minh TB, Mohd MA, Nakata H, Kannan K (2011a) Occurrence of phthalate metabolites in human urine from several Asian countries. Environ Sci Technol 45:3138–3144
Guo Y, Wu Q, Kannan K (2011b) Phthalate metabolites in urine from China, and implications for human exposures. Environ Int 37:893–898
Guo Y, Zhang Z, Liu L, Li Y, Ren N, Kannan K (2012) Occurrence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J Agric Food Chem 60:6913–6919
He W, Qin N, Kong X, Liu W, He Q, Ouyang H, Yang C, Jiang Y, Wang Q, Yang B, Xu F (2013) Spatio-temporal distributions and the ecological and health risks of phthalate esters (PAEs) in the surface water of a large, shallow Chinese lake. Sci Total Environ 461–462:672–680
Jiang J, Zhang X, Zhu X, Li Y (2017) Removal of intermediate aromatic halogenated DBPs by activated carbon adsorption: a new approach to controlling halogenated DBPs in chlorinated drinking water. Environ Sci Technol 51(6):3435–3444
Jin D, Kong X, Li Y, Bai Z, Zhuang G, Zhuang X, Deng Y (2015) Biodegradation of di-n-butyl phthalate by Achromobacter sp. isolated from rural domestic wastewater. Int J Environ Res Public Health 12:13510–13522
Kang Y, Man YB, Cheung KC, Wong MH (2012) Risk assessment of human exposure to bioaccessible phthalate esters via indoor dust around the Pearl River Delta. Environ Sci Technol 46:8422–8430
Keresztes S, Tatár E, Czégény Z, Záray G, Mihucz VG (2013) Study on the leaching of phthalates from polyethylene terephthalate bottles into mineral water. Sci Total Environ 458–460:451–458
Li J, Wang G, Aggarwal SG, Huang Y, Ren Y, Zhou B, Singh K, Gupta PK, Cao J, Zhang R (2014) Comparison of abundances, compositions and sources of elements, inorganic ions and organic compounds in atmospheric aerosols from Xi’an and New Delhi, two megacities in China and India. Sci Total Environ 476-477:485
Liu H, Cai Y, Yu MQ, Gong LT, An SQ (2012) Assessment of river habitat quality in Yixing district of Taihu lake basin. Chin J Ecol 31:1288–1295
Liu Y, Chen Z, Shen J (2013) Occurrence and removal characteristics of phthalate esters from typical water sources in Northeast China. J Anal Methods Chem 2013:419349
Mackintosh CE, Maldonado JA, Ikonomou MG, Gobas FAPC (2006) Sorption of phthalate esters and PCBs in a marine ecosystem. Environ Sci Technol 40:3481–3488
Meng X, Wang Y, Xiang N, Chen L, Liu Z, Wu B, Dai X, Zhang Y, Xie Z, Ebinghaus R (2014) Flow of sewage sludge-borne phthalate esters (PAEs) from human release to human intake: implication for risk assessment of sludge applied to soil. Sci Total Environ 476–477:242–249
Net S, Rabodonirina S, Sghaier RB, Dumoulin D, Chbib C, Tlili I, Ouddane B (2015a) Distribution of phthalates, pesticides and drug residues in the dissolved, particulate and sedimentary phases from transboundary rivers (France–Belgium). Sci Total Environ 521–522:152–159
Net S, Delmont A, Sempéré R, Paluselli A, Ouddane B (2015b) Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): a review. Sci Total Environ 515–516:162–180
Okamoto Y, Ueda K, Kojima N (2011) Potential risks of phthalate esters acquisition of endocrine-disrupting activity during environmental and metabolic processing. J Health Sci 57:497–503
Peijnenburg WJGM, Struijs J (2006) Occurrence of phthalate esters in the environment of the Netherlands. Ecotoxicol Environ Saf 63:204–215
Penalver A, Pocurull E, Borrull F, Marce RM (2000) Determination of phthalate esters in water samples by solid-phase microextraction and gas chromatography with mass spectrometric detection. J Chromatogr A 872:191–201
Peng X, Feng L, Li X (2013) Pathway of diethyl phthalate photolysis in sea-water determined by gas chromatography–mass spectrometry and compound-specific isotope analysis. Chemosphere 90:220–226
Ponzo OJ, Silvia C (2013) Evidence of reproductive disruption associated with neuroendocrine changes induced by UV–B filters, phtalates and nonylphenol during sexual maturation in rats of both gender. Toxicology 311:41–51
Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D, Colacino JA, Birnbaum LS (2013) Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect 121:473–479
Schettler T (2006) Human exposure to phthalates via consumer products. Int J Androl 29:134–139
Sha Y, Xia X, Yang Z, Huang GH (2007) Distribution of PAEs in the middle and lower reaches of the Yellow River, China. Environ Monit Assess 124:277–287
Skilhagen SE, Dugstad JE, Aaberg RJ (2008) Osmotic power—power production based on the osmotic pressure difference between waters with varying salt gradients. Desalination 220:476–482
Srivastava A, Sharma VP, Tripathi R, Kumar R, Patel DK, Mathur PK (2010) Occurrence of phthalic acid esters in Gomti River sediment, India. Environ Monit Assess 169:397–406
Stackelberg PE, Furlong ET, Meyer MT, Zaugg SD, Henderson AK, Reissman DB (2004) Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci Total Environ 329:99–113
Tan GH (1995) Residue levels of phthalate esters in water and sediment samples from the Klang River basin. Bull Environ Contam Toxicol 54:171–176
Tang W, Zeng G, Gong J, Liang J, Xu P, Zhang C, Huang B (2014) Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials a review. Sci Total Environ 468–469:1014–1027
Thurén A (1986) Determination of phthalates in aquatic environments. Bull Environ Contam Toxicol 36:33–40
Vethaak AD, Lahr J, Schrap SM, Belfroid AC, Rijs GBJ, Gerritsen A, de Boer J, Bulder AS, Grinwis GCM, Kuiper RV, Legler J, Murk TAJ, Peijnenburg W, Verhaar HJM, de Voogt P (2005) An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of The Netherlands. Chemosphere 59:511–524
Vitali M, Guidotti M, Macilenti G, Cremisini C (1997) Phthalate esters in freshwaters as markers of contamination sources—a site study in Italy. Environ Int 23:337–347
Wang H, Wang C, Wu W, Mo Z, Wang Z (2003) Persistent organic pollutants in water and surface sediments of Taihu Lake, China and risk assessment. Chemosphere 50:557–562
Wang W, Wu Q, Wang C, He T, Hu H (2015) Health risk assessment of phthalate esters (PAEs) in drinking water sources of China. Environ Sci Pollut Res 22:3620–3630
Xue-Li E, Chen CJ, Zhang L (2007) Study on standard for drinking water quality in China. Carcinog Teratog Mutagene 19:168–170
Yuan SY, Liu C, Liao CS, Chang BV (2002) Occurrence and microbial degradation of phthalate esters in Taiwan river sediments. Chemosphere 49:1295–1299
Yuan B, Li X, Graham N (2008) Aqueous oxidation of dimethyl phthalate in a Fe(VI)-TiO2-UV reaction system. Water Res 42:1413–1420
Yunrui Z, Wanpeng Z, Fudong L, Jianbing W, Shaoxia Y (2007) Catalytic activity of Ru/Al2O3 for ozonation of dimethyl phthalate in aqueous solution. Chemosphere 66:145–150
Zeng F, Cui K, Xie Z, Liu M, Li Y, Lin Y, Zeng Z, Li F (2008) Occurrence of phthalate esters in water and sediment of urban lakes in a subtropical city, Guangzhou, South China. Environ Int 34:372–380
Zhang L, Liang D, Ren L, Shi S, Li Z, Zhang T, Huang Y (2012) Concentration and source identification of polycyclic aromatic hydrocarbons and phthalic acid esters in the surface water of the Yangtze River Delta, China. J Environ Sci 24:335–342
Zheng X, Zhang BT, Teng Y (2014) Distribution of phthalate acid esters in lakes of Beijing and its relationship with anthropogenic activities. Sci Total Environ 476-477:107
Acknowledgements
This study was supported by the Major Science and Technology Program for Water Pollution Control and Treatment (No. 2014ZX07405002) and the National Natural Science Foundation of China (No. 51608148)
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
ESM 1
(DOC 804 kb)
Rights and permissions
About this article
Cite this article
Kong, Y., Shen, J., Chen, Z. et al. Profiles and risk assessment of phthalate acid esters (PAEs) in drinking water sources and treatment plants, East China. Environ Sci Pollut Res 24, 23646–23657 (2017). https://doi.org/10.1007/s11356-017-9783-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9783-x