Abstract
A study was conducted in fluoride-affected Bankura and Purulia districts of West Bengal to assess the potential health risk from fluoride exposure among children, teenagers, and adults due to consumption of rice, pulses, and vegetables in addition to drinking water and incidental ingestion of soil by children. Higher mean fluoride contents (13–63 mg/kg dry weight) were observed in radish, carrot, onion bulb, brinjal, potato tuber, cauliflower, cabbage, coriander, and pigeon pea. The combined influence of rice, pulses, and vegetables to cumulative estimated daily intake (EDI) of fluoride for the studied population was found to be 9.5–16%. Results also showed that intake of ivy gourd, broad beans, rice, turnip, fenugreek leaves, mustard, spinach, and amaranth grown in the study area is safe at least for time being. The cumulative EDI values of fluoride (0.06–0.19 mg/kg-day) among different age group of people of the study area were evaluated to be ~104 times higher than those living in the control area; the values for children (0.19 and 0.52 mg/kg-day for CTE and RME scenarios, respectively) were also greater than the “Tolerable Upper Intake Level” value of fluoride. The estimated hazard index (HI) for children (3.2 and 8.7 for CTE and RME scenarios, respectively) living in the two affected districts reveals that they are at high risk of developing dental fluorosis due to the consumption of fluoride-contaminated rice, pulses, and vegetables grown in the study area in addition to the consumption of contaminated drinking water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Consumption of fluoride-contaminated groundwater has endangered approximately 200 million people living in 29 different countries of the world by causing health-related risks to different degrees (WHO 2006; Bhattacharya and Chakrabarti 2011; Brindha and Elango 2011). In India, fluorosis is endemic in 20 out of total 29 states including 65% of rural habitations (UNICEF 1999; Kundu and Mandal 2009). More than 65 million Indians including 6 million children are at risk due to the fluoride pollution in groundwater (Andezhath et al. 1999; UNICEF 1999). According to Suthar et al. (2008) elevated presence of fluoride in groundwater is the resultant of weathering of minerals like fluorite, topaz, and apatite. Anthropogenic activities like overexploitation of groundwater, uses of phosphate containing agrochemicals, and contamination with sewage and effluent were also reported to be responsible for the enhanced fluoride pollution in groundwater (EPA 1997; Ramanaiah et al. 2006). Fluoride consumption within the permitted range of 0.5–1 mg/l was detected to be beneficial in production and maintenance of healthy teeth and bones in human beings (Wood 1974). But, consumption of fluoride beyond this range would cause mottling teeth, softening of bones, and neurological damage (Steinberg et al. 1955). Thus, the World Health Organization (WHO) has set 1.5 mg/l as the permissible limit of fluoride in drinking water (WHO 2006). Fluoride intake in excess of 2 mg/l causes severe dental and skeletal fluorosis (Chatterjee et al. 2008). Drinking water has long been considered to be the major contributor for fluorosis (Susheela 1999). However, complete mitigation of fluorosis by altering the source of drinking water or by reducing its fluoride content was unsuccessful (Changqing et al. 2005). The fractional intake of fluoride in a human body through water, food, and air needs to be assessed to understand the total amount of fluoride accumulation in body (Khandare and Rao 2006; Gupta and Banerjee 2011; Pandey and Pandey 2011).
Fluoride has been observed to accumulate in plants from aerosol, soil, and irrigation water at different phases (Fornasiero 2001; Kusa et al. 2004; Kalinic et al. 2005; Kozyrenko et al. 2007; Cressey et al. 2009; Mishra et al. 2009; Paul et al. 2011; Pal et al. 2012; Saini et al. 2013). Thus, consumption of vegetables and crops is now presumed to be another potent route for fluoride entry into human food chain along with the drinking water pathway. Plant roots by absorbing fluoride from soil transport it via xylem to different organs, mainly the leaves, and its accumulation results into different adverse effects (Davison and Weinstein 1998). Uptake of fluoride by plants is facilitated in acidic soils due to its enhanced solubility (Daines et al. 1952; Ruan et al. 2004). But the ability and extent of a plant’s fluoride absorption were established to be dependent on the plant species itself and on the nature of fluoride ionic species present in soil solution (Mezghani et al. 2005; Okibe et al. 2010). Excess accumulation of fluoride in vegetation leads to chlorosis (Mcnulty and Newman 1961), decreased plant growth, leaf tip burn, leaf necrosis (Elloumi et al. 2005; Zouari et al. 2014), damage to fruits, changes in the yield (Anil and Bhaskara 2008), inhibited germination, ultra structural malformations, reduced photosynthetic capacity, alteration in membrane permeability, reduced productivity (Gautam et al. 2010), and phytotoxicity (Liang et al. 1997; Clausen et al. 2015). Intake of highly fluoride-contaminated plants was found to induce chronic toxicity in grazing animals and humans including bone damage and tooth wear (Clark and Stewart 1983) while diets high in fat were confirmed to increase deposition of fluoride in bones and thus enhanced toxicity in humans (USDHHS 1991).
Sixty blocks spread over 8 districts of West Bengal have moderate to high fluoride contamination (Chatterjee et al. 2008) which is affecting ~12% of total rural population of the state (PHED Report 2007). The present study was carried out in fluoride-contaminated lateritic Bankura and Purulia districts of West Bengal. Residents of the study area utilize groundwater for drinking as well as irrigation purposes. Incidence of endemic fluorosis in this area is increasing because of altered environmental conditions such as, decrease in rainfall, excessive usage of groundwater, and lowered groundwater level (Khandare and Rao 2006). In our previous investigation in this area, fluoride in the collected water and soil samples was estimated in the range of 0.01–1.6 mg/l and 55–399 mg/kg, respectively (Samal et al. 2015), and a high possibility of accumulation of fluoride from contaminated soil and irrigation water to cultivated crops and vegetables of the study area was hypothesized. The ability of plants to uptake and accumulate fluorine makes it a potential threat to human health through its entrance into the human food chain (Pal et al. 2012). A significant risk of dental fluorosis was cautioned by Bhattacharya (2016) in infants and children of Purulia District. But, no study on fluoride accumulation in crops and vegetables cultivated with fluoride-contaminated groundwater of the present study area has been reported yet. Thus, keeping an account of the importance of public health, the main objective of this survey was to investigate the risk of fluoride intake via the consumption of rice, pulses, and vegetables, cultivated in the study area. An attempt has also been made to examine the risk of developing fluorosis in people, especially children residing in the study area by quantifying all the possible pathways (rice, pulses, vegetables, soil, and drinking water) of fluoride exposure.
Materials and methods
Study area and sampling locations
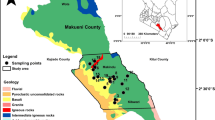
The Bankura District is geographically located between latitude 22° 38′–23° 38′ N and longitude 86° 36′–87° 46′ E, covering 6882 km2 area with a population of 3,596,674, and out of this nearly 91.7% live in rural areas (Census 2011). The location of Purulia District is in between latitude 22° 42′–23° 42′ N and longitude 85° 49′–86° 54′ E, having an area of 6259 km2. Out of the total population of 2,930,115 in Purulia District, nearly 87.3% live in rural areas (Census 2011). The average elevation of Bankura and Purulia districts are 78 and 228 m, respectively. The drainage pattern of the area is parallel to sub-parallel and geological structural elements primarily govern the patterns. The average slope of the study area varies between 0.4 and 10%. The presence of fluoride beyond the WHO recommended permissible limit in groundwater of some parts of the two districts was reported by the Public Health Engineering Department, Government of West Bengal (PHED Report 2007). The largely fluoride-affected ten blocks of Bankura District (Chhatna, Shaltora, Gangajal Ghati, Bankura II, Indpur, Hirbundh, Khatra, Sarenga, Raipur, and Simlapal) and six blocks of Purulia District (Hura, Kashipur, Raghunathpur I, Santuri, Puncha, and Manbazar I) have been chosen for the present study (Fig. 1). The major occupation of the residents of these two districts is farming, utilizing groundwater irrigation through shallow and deep tube well pumps. Rice is the main crop of both the districts. Besides rice, the major cultivated crops are potato, mustard, and seasonal vegetables. In addition to these two fluoride-affected districts, Nadia District (West Bengal) has been chosen as the control study area without groundwater fluoride contamination.
Sample collection, processing, and preservation
The principal cereal crop rice (Oryza sativa L.) and the edible parts of commonly grown pulses and vegetables of the study area as well as the control area such as mustard seed (Brassica juncea), potato tuber (Solanum tuberosum), tomato (Lycopersicon esculentum), cauliflower (Brassica oleracea var. Botrytis), cabbage (B. oleracea var. Capitata), carrot roots (Daucus carota), radish (Raphanus sativus), beetroot (Beta vulgaris), spinach leaves (Spinacea oleracea), Shim/broad beans (Vicia faba), Lal saag/red amaranth leaves (Amaranthus sp.), Lau saag/bottle gourd leaves (Lagenaria siceraria), Methi saag/fenugreek leaves (Trigonella foenum-graecum), coriander (Coriandrum sativum), pigeon pea (Cajanus cajan), Kundri/ivy gourd (Coccinia grandis), brinjal (Solanum melongena), Olkopi/turnip (Brassica rapa subsp. rapa), and onion bulb (Allium cepa) were collected from the agricultural fields during their respective growing seasons in the years 2015–2016. The number of samples of each vegetable at each sampling site varied generally between 2 and 5. The collected samples were stored in plastic zipper bags with proper labeling for further analysis. The sampling sites were mapped using the global positioning system. The sampling was performed at a spatial distance of around ~2 km away from each other sampling points. A part of the collected samples was kept for the moisture content determination; the rest of the samples were thoroughly washed, chopped into small pieces, air dried for 2 days and then oven dried at 105 °C. The dried samples were then milled to pass through 70 mesh sieve to get homogenized representative powder sample and kept for fluoride determination. Soil samples from the respective rice, pulse and vegetable fields were randomly collected in triplicate from a depth of 0–45 cm by composite sampling technique and stored in plastic zipper bags with proper labeling. The soil samples were immediately sun dried and later dried in a hot air oven at 105 °C for 72 h. The dried soil samples were then grinded by cautiously disaggregating in a mortar and screened through 70 mesh sieve to get homogenized representative powder sample. Finally the samples were stored in airtight polyethylene bags at room temperature. Proper care was taken at each step to minimize any contamination.
Determination of total fluoride
The total fluoride content in rice, pulse, vegetable, and soil samples were estimated through the NaOH fusion method (McQuaker and Gurney 1977). About 0.5 g of the homogenized powdered sample was transferred to a 100-ml nickel crucible and moistened with small amount of Milli-Q water (ultrapure water of “Type 1”). Then 6 ml NaOH (16.75 N) was added and the crucible was placed in an oven at 150 °C for ~1 h. After removal of the crucible from oven time was given to solidify the NaOH. Then the crucible was placed in a muffle furnace at 300 °C, further raised to 600 °C and kept for 30 min to fuse the sample. Thereafter, the crucible was allowed to cool; 10 ml Milli-Q water was added and heated slightly to facilitate the dissolution of the NaOH fusion cake. Then with constants stirring ~8 ml concentrated HCl was slowly added to the content to adjust the pH at 8–9. Finally, the sample solution was transferred to a 50-ml polyethylene volumetric flask; volume was made up with Milli-Q water, and filtered through a Whatman No. 40 filter paper.
The fluoride concentration of the digested samples were measured electrochemically using the Thermo Scientific VSTAR40A- Orion Versa Star pH/Ion Selective Electrode bench top meter by an approved American Society for Testing and Materials (ASTM) standard test method (ASTM D 1179). The detection limit of this method is 0.005 mg/l. The standard curve was obtained by using the 0.1, 1, and 10 mg/l standard fluoride solutions. All samples were analyzed after adding TISAB-III solution (10:1 composition) to attain the final pH of 5.2. The experiments were carried out in triplicate and the results were found reproducible with ±2% error. The average recoveries based on the spiked samples at two different levels of fluoride were 94 ± 5–99 ± 4%.
Determination of bioconcentration factor
The bioconcentration factor (BCF) was determined to estimate the rate of flow of fluoride from agricultural soil to rice, pulses and vegetables using the equation:
Study of the nutrition pattern
One hundred and twelve families comprising of total 503 individuals (294 male and 209 female subjects—all permanent residents and born and raised in the study area) in different age groups residing in the 16 blocks of the study area were randomly selected and administered a survey to evaluate the daily diets, frequency, and quantity of eating rice, pulses, and seasonal vegetables. The indicated intake frequency was just an assumption near the mean values. The population was categorized into three age groups: children (3–6 years), teenagers (7–18 years), and adults (19–70 years). The inquiry was conducted on the basis of a set of questionnaires following the guidelines developed by the National Institute of Nutrition, India (Thimmayamma and Rau 1987). In studying human subjects’ medicines, injections or chemicals were not employed to assess fluoride toxicity symptoms. This study satisfies all the criteria of ethical treatment of human subjects and the identity of the studied human subjects has been kept classified.
Determination of moisture content
To determine the average consumption of rice, pulses, and vegetables by the studied group of population on dry weight basis, the moisture content of the collected plant samples was analyzed. The freshly collected plant samples of about 50 g were shredded; air dried for 2 days and then dried in a hot air oven at 70 °C for 3 days till a constant weight was attained. The moisture content was computed using the equation:
Here W 1 is the fresh weight of the plant sample and W 2 is the weight of the plant sample after drying at 70 °C.
Assessment of the dose of fluoride exposures and risk characterization from fluoride exposure
The generic equation given by the US Environmental Protection Agency (USEPA 1992) was used to calculate the dose of fluoride exposures in terms of the estimated daily intake (EDI):
where EDI is the estimated daily intake (milligrams per kilogram-day), C is the concentration of fluoride in rice/ pulses/vegetables/soil/ drinking water (milligrams per liter or milligrams per kilogram), IR is the ingestion or intake rate (milligrams per day), EF is the exposure frequency, i.e., how often the exposure occurs (days per year), ED is the exposure duration, i.e., the length of the time that the exposure occurs (years), AF is the absorption factor (unit less), CF is the conversion factor (10−6 kg per milligram), BW is the body weight (kilograms), and AT is the averaging time (days) (USEPA 1992).
Using the above equation, the EDI was calculated for the three age groups (children, teenagers, and adults). Two values for each exposure parameter were used in characterizing potential exposures: one value to represent an average or central tendency exposure (CTE) and another value (90th percentile value of the observed fluoride concentration) for the high-end or reasonable maximum exposure (RME), which was intended to represent a plausible worst-case exposure scenario (USEPA 1989; Erdal and Buchanan 2005). Fluoride is promptly absorbed from the gastro-intestinal tract with 75–100% efficiency (ATSDR 2001). Thus, in the present study to assess the EDI for CTE and RME scenarios, the AF was taken as 75% (i.e., AF = 0.75) and 100% (i.e., AF = 1), respectively, to ensure that risks are not underestimated, which is particularly important for children. In absence of any national statistics on body weight data in India, the average body weights of children, teenagers, and adults were taken as per USEPA’s the “Exposure Factor Handbook” presented 19, 52, and 80 kg, respectively, (USEPA 2011) for this study. The AT is equal to ED times 365 days/year. For complete risk characterization, intake of fluoride through drinking water and incidental ingestion of soil (by only children), were considered along with the consumption of rice, pulses, and vegetables by all the receptors of the study area in the calculation of cumulative EDI using the equation:
The hazard index (HI) was estimated to assess the risk due to fluoride exposure in various age groups of people living in the area (USEPA 1993):
The reference dose (RfD) is an approximation of the daily fluoride exposure to human beings, which in all probability is the ‘no observed adverse effect level’ (NOAEL) during their lifetime (USEPA 1993). The RfD was derived from a well conducted epidemiological study in children (Hodge 1950) for which the uncertainty factor (UF) and the modifying factor (MF) were both assumed to be unity, i.e.,
The USEPA recommended RfD for fluoride is 0.06 mg/kg-day (USEPA 2003a). The scientific basis and rationale of the fluoride RfD can be found in the Integrated Risk Information System (IRIS) published by the USEPA (USEPA 1987).
The cumulative noncancerous lifetime risk (ages, 3–70 years) of the population of the study area due to lifelong fluoride exposure was evaluated to project fluoride vulnerability for the entire life (USEPA 2003a; Gržetić and Ghariani 2008):
Statistical analyses
Means of the replicates, standard deviation, and Pearson’s correlation coefficient (r) on the experimental data were evaluated using SPSS, version 15.0 for windows (SPSS Inc., Chicago, USA). The sampling location map was prepared using Golden surfer, version 8.0 (Golden Software Inc., CO, USA).
Results and discussion
Accumulation of fluoride in soil
Geological weathering of minerals like fluorite, mica, and apatite generally supplies fluoride to soil. Different anthropogenic activities including irrigation and application of fertilizer for agriculture lead to high fluoride content in soil (Brindha et al. 2011). The aquifers receive fluoride from soil through natural leaching process. The fluoride concentrations in 79 agricultural field soils of the study area and that of 18 sites in the control area are presented in Table 1. The maximum and minimum content of fluoride in sampled soils was 399 mg/kg (Raghunathpur I block, Purulia) and 51 mg/kg (Raipur block, Bankura), respectively. The observed range was detected to be higher than that are reported for various tropical areas of the world (Hall and Cain 1972) and much higher than the average concentration of fluoride recorded in the control area (0.72 ± 0.16 mg/kg). In our previous investigation in the study area, a significant correlation was detected between the fluoride content in groundwater and that in agricultural field soils (Samal et al. 2015). The established major factors that influence the mobility of fluoride in soil are soil pH, formation of stable aluminum and calcium complexes, rate of deposition, and climatic conditions of the area (Pickering 1985). The presence of significant positive correlation between soil fluoride content and soil pH of the study area was previously reported by Samal et al. (2015). High pH condition in soil was found to increase fluoride concentration in soil solution (Barrow and Ellis 1986), and thus, more soluble fluoride becomes available for plant uptake (Jha et al. 2011).
Uptake of fluoride by rice, pulses, and vegetables
The fluoride contents in edible parts of the collected rice, pulse, and vegetable samples of the study area and that of the control area were described in Table 2. It is evident from the results that the accumulation of fluoride in rice, pulse, and vegetable samples of the study area is in much higher order than their respective accumulation in the control area. The total fluoride concentrations in crops and vegetables of the study area varied between <0.005 and 86 mg/kg dry weights. The maximum mean concentration of fluoride (63 ± 7.8 mg/kg dry weight) was detected in radish samples collected from Bankura District (52 ± 4.5 mg/kg dry weight in samples collected from Purulia District). The other higher mean fluoride contents (mg/kg dry weight) were observed in carrot (62 ± 12 in Purulia and 49 ± 8.1 in Bankura), onion bulb (23 ± 4.7 in Bankura and 23 ± 6 in Purulia), brinjal (19 ± 2.6 in Bankura and 17 ± 3.8 in Purulia), potato tuber (17 ± 4.8 in Purulia and 10 ± 6.3 in Bankura), cauliflower (17 ± 5.9 in Purulia and 12 ± 1.5 in Bankura), cabbage (17 ± 6.2 in Bankura and 16 ± 6.7 in Purulia), coriander (16 ± 5 in Purulia and 15 ± 3.4 in Bankura), and pigeon pea (15 ± 4.2 in Purulia and 13 ± 5.8 in Bankura), which were all many folds higher than the observed fluoride contents in samples collected from the control area. Comparatively lower mean fluoride concentrations (mg/kg dry weight) could be seen in fenugreek leaves (2.1 ± 0.56 in Bankura and 0.94 ± 0.2 in Purulia), turnip (1.2 ± 0.27 in Purulia and 0.89 ± 0.35 in Bankura), rice (0.83 ± 0.19 in Purulia and 0.56 ± 0.14 in Bankura), broad beans (0.71 ± 0.29 in Bankura and 0.55 ± 0.23 in Purulia), and the lowest content was detected in ivy gourd (0.37 ± 0.11 in Purulia).
According to Khandare and Rao (2006), all vegetables do not accumulate fluoride to the same extent and variations among them are significantly high. Considerably, high amount fluoride accumulation in different crops, pulse, and vegetables was previously reported by Paul et al. (2011) and Saini et al. (2013). Moreover, the combined influence of rice and vegetables to total fluoride consumption by humans were established to be as high as 56% (Gupta and Banerjee 2011). The richness of fluoride concentration in radish, carrot, onion, brinjal, potato, cauliflower, and cabbage of the study area is concurrent with the previous findings by Susheela (1999) and Gautam et al. (2010), and this accumulation can significantly contribute to the total fluoride intake in human food chain. But, this richness can also be utilized to lower the fluoride content of soil by economical and sustainable phytoremediation technique. In some studies, spinach is described as a good accumulator of fluoride, especially in areas adjacent to industries (Haidouti et al. 1993; Saini et al. 2013), but the fluoride contents measured in spinach of the present study were found to be lower in comparison to those reported values. This may be due to the absence of gaseous fluoride in the present experimental site as in the case of other non-industrial sites (Khandare and Rao 2006). It can be concluded from the results that consumption of ivy gourd, broad beans, rice, turnip, fenugreek leaves, mustard, spinach, and amaranth grown in the study area is unlikely to contribute appreciably to the total fluoride intake.
BCF of fluoride in rice, pulses, and vegetables
The BCF in studied plant samples was evaluated to estimate the chemical concentration of fluoride in a plant’s tissue with respect to its equilibrium concentration in soil. The mean BCF values of fluoride in rice, pulses, and vegetables at Bankura, Purulia, and Nadia districts are shown in Fig. 2. No definite pattern of fluoride distribution in edible part of plants can be concluded from the figure. On an average <1 BCF values for all the collected plant samples indicate that none of them are hyperaccumulator of fluoride in the study area. The highest mean BCF value of fluoride (0.48) was detected in radish samples collected from Bankura district (0.29 in Purulia), followed by in carrots (0.37 in Bankura and 0.34 in Purulia). Thus, in the analyzed samples of the study area, radish and carrot have the maximum affinity in accumulating fluoride from soil which is also reflected in Table 2. The highest fluoride accumulation and much higher mean BCF value (1.4) in radish were also reported by Pal et al. (2012). BCF was reported to vary in plants as it is controlled by different soil properties (like pH, % of clay, organic matter, and fluoride contents) as well as by plant factors (like plant type and its growth rate) (Swartjes et al. 2007). Higher metabolic rates had been argued to be responsible for enhanced fluoride translocation in plants (Ribang et al. 1975). The bioconcentration of fluoride was found to be modest in grain-yielding crops (mustard and pigeon pea), fruiting vegetables (tomato, brinjal, and broad beans) and tubers (potato). On contrary to the findings by Gupta and Banerjee (2011) and Pal et al. (2012), the leafy vegetables (leaves of bottle gourd, fenugreek, and amaranth, and spinach) of the study area also indicated much lower ability in translocating fluoride from soil. The BCF values of rice, pulses, and vegetables cultivated in the control area depicted comparatively lower affinity in transferring fluoride.
Estimation of nutrition pattern
The mean intake frequency and exposure frequency of rice, pulses and vegetables by the residents of the study area were estimated during our enquiry (Table 3). Rice and vegetables are the main consumed food of the rural people living in the studied area. They consume rice with vegetables thrice a day (during breakfast, lunch, and dinner). Availability of seasonal vegetables was found to be responsible for the variation of consumption pattern in the studied population. The daily mean fresh weights of consumption of rice, pulses, and vegetables were evaluated and expressed in gram fresh weight/day (gfwt/day). Table 4 demonstrates the mean daily intakes of rice, pulses and vegetables by children, teenagers, and adults on dry weight basis (gdwt/day) which were estimated incorporating their respective moisture contents.
Assessment of the dose of fluoride exposures
The quantitative health risk assessment was evaluated by determining the doses of fluoride exposures due to the consumption of rice, pulses, and various seasonal vegetables. To perform the risk analysis, a risk range has been assessed, one focusing on the central tendency exposure (CTE) scenario while the other on the reasonable maximum exposure (RME) scenario by using the 90th percentile value of the observed fluoride concentration in rice, pulses, and vegetables. The summary of exposure parameters used in the calculation of estimated daily intakes (EDI) of fluoride for the CTE and RME scenarios is given in Table 5. A specific pathway of fluoride exposure to children–incidental ingestion of soil was also included in Table 5. Children inadvertently ingest soil through normal hand-to-mouth behavior and thus expose themselves towards enhanced fluoride vulnerability (Erdal and Buchanan 2005). The mean (233 mg/kg) and the 90th percentile value (300 mg/kg) of observed fluoride concentration in soils of the study area, and the USEPA prescribed intake rates (0.1 and 0.4 g/day) (USEPA 2002) were used for the calculation of the EDISoil for CTE and RME scenarios, respectively. The major pathway of fluoride exposure, i.e., drinking water for all the receptors of the study area was also considered (Table 5) by using our previously reported values −1.1 mg/l for children and 0.55 mg/l for teenagers and adults for the CTE scenario and 2.9 and 1.4 mg/l for RME scenario, respectively (Samal et al. 2015).
The mean EDI of fluoride from rice, pulses, and vegetables for the CTE and RME scenarios among different age groups of people living in the Bankura, Purulia, and Nadia districts are shown in Tables 6 and 7, respectively. The cumulative EDIs of fluoride by considering all the possible pathways of fluoride exposure, i.e., rice, pulses, vegetables, soil (by only children), and drinking water for the CTE and RME scenarios among different age groups of people of the study area and that of the control area are presented in Tables 8 and 9, respectively. The results depict that for the CTE scenario the cumulative EDI for children, teenagers, and adults were 0.19, 0.09, and 0.06 mg/kg-day, respectively, in both the studied districts (Table 8). For the RME scenario, the cumulative EDIs for the three different age groups were found to be 0.52, 0.24, and 0.15 mg/kg-day, respectively, again identical for the two districts (Table 9). It is evident from Table 8 that the cumulative EDIs of fluoride among different age groups of people of the Bankura and Purulia districts are ~104 times higher than their counterparts living in the control area. Moreover, the cumulative EDI of fluoride for children was observed to be higher as compared to the EDIs for teenagers and adults. The combined influence of rice, pulses, and vegetables to cumulative EDI of fluoride for the studied population was found to be 9.5–16% in the CTE scenario and 5–12% in the RME scenario, respectively. It was also established that the EDI of fluoride from vegetables in all the three age groups in all the three districts was more than the EDIs of rice or pulses. Further, the Institute of Medicine (IOM, US) Standing Committee on the “Scientific evaluation of dietary reference intakes” has recommended the “Tolerable Upper Intake Level” (UL) value of fluoride as 0.1 mg/kg/day for infants, toddlers and children up to 8 years, and 0.15–0.2 mg/kg/day for children and adults >8 years (IOM 1997). Thus, the cumulative EDI of fluoride corresponding to the children of the study area was detected to be higher than the UL value of fluoride for the CTE scenario while the cumulative EDIs for all the age groups were observed to be higher than the UL values for the RME scenario.
Assessment of risk from fluoride exposure
The HI considers all the exposure pathways applicable for a given exposure group. For human health risk assessment HI <1 is considered to be acceptable while definite risk management measures are required to be taken when HI becomes >1 (Canada Health Act Annual Report 2004). Thus, in the present study, the HI was estimated for the three age groups of the Bankura and Purulia districts and that of the control area (Fig. 3). In India, there are no specified guidelines yet for the HI; thus, the USEPA recommended RfD of 0.06 mg/kg-day was employed in the calculation of HI; this RfD value includes dietary intake of fluoride (0.01 mg/kg-day) as well as fluoride consumed through drinking water (0.05 mg/kg-day) (USEPA 1987). The HI in case of children, teenagers and adults for the two contaminated districts were observed to be 3.2, 1.5, and 1 for the CTE scenario and 8.7, 4, and 2.5 for the RME scenario, respectively. Hence, children and teenagers of the study area are found to be under severe risk (HI > 1) from fluoride exposure through the intake of rice, pulses, vegetables, soil (by only children), and drinking water pathways. The HI values for children residing in the study area reveal that they are receiving fluoride in excess of those “likely to be without appreciable deleterious effects” (USEPA 2003b; Erdal and Buchanan 2005) and have high potential for developing dental fluorosis. This is an alarming value for toxicologists since it indicates that the health of children of the study area is endangered. The cumulative noncancerous lifetime hazard (HICumulative) in the studied population (ages, 3–70 years) due to fluoride exposure was evaluated for the CTE and RME scenarios and was found to be 5.7 and 15.2, respectively (Tables 8 and 9), and thus of deep concern. Moreover, the cumulative lifetime risk of the people residing in the study area from fluoride exposure was established to be ~104 times than the risk of the people living in the control area.
Conclusions
The present status of fluoride contamination in rice, pulses, and vegetables of the Bankura and Purulia districts was investigated in this study. The range of fluoride in agricultural field soils was estimated to be 51–399 mg/kg with average value of 132 ± 38 mg/kg in Bankura and 185 ± 50 mg/kg in Purulia districts, respectively. Transfer of fluoride from contaminated soil and groundwater to plants was found to enhance the severity of fluoride toxicity in residents. The accumulation of fluoride (<0.005—86 mg/kg dry weight) in rice, pulse, and vegetable samples of the study area was detected to be in higher order than their respective accumulation in the control area. But <1 BCF values for all the collected plant samples indicate that none of them are hyperaccumulator of fluoride in the study area. The cumulative EDI of fluoride due to consumption of rice, pulses, vegetables, soil (by only children), and drinking water among children, teenagers, and adults living in the Bankura and Purulia districts were observed to be higher than their counterparts living in the control area. Moreover, the cumulative EDI values for the children were detected to be higher than the IOM (US) recommended UL values for fluoride. Hence, efficient fluorosis risk management for every potential exposure pathways are required to be addressed immediately for the betterment of public health of Bankura and Purulia districts. A master plan on fluoride mitigation by mapping the fluoride-affected areas of the state and subsequently supplying of drinking water as well as irrigation water from centralized defluoridation treatment facilities in the concerned areas is to be implemented in an urgent basis. Recharging of groundwater of the region by rain water harvesting is also recommended to improve the scenario of fluoride pollution on long-term basis.
References
Andezhath SK, Susheela AK, Ghosh G (1999) Fluorosis management in India: the impact due to networking between health and rural drinking water supply agencies. Interdiscip Perspect Drink Water Risk Assess Manag 260:159–165
Anil KK, Bhaskara RAV (2008) Physiological responses to fluoride in two cultivars of mulberry. World J Agric Sci 4(4):463–466
ATSDR (2001) Toxicological profile for fluorides, hydrogen fluoride, and fluorine. Draft for public comment, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA
Barrow NJ, Ellis AS (1986) Testing a mechanistic model III: the effect of pH on fluoride retention by a soil. J Soil Sci 37:287–293
Bhattacharya P (2016) Analysis of fluoride distribution and community health risk in Purulia district of West Bengal, India. In Proceedings of the 9th National Level Science Symposium organized by the Christ college 3:88−92
Bhattacharya HN, Chakrabarti S (2011) Incidence of fluoride in the groundwater of Purulia district, West Bengal: a geo-environmental appraisal. Curr Sci 101(2):152–155
Brindha K, Elango L (2011) Fluoride in groundwater: causes, implications and mitigation measures. In: Monroy SD (ed) Fluoride properties, applications and environmental management. Nova Science Publishing, pp 111–136
Brindha K, Rajesh R, Murugan R, Elango L (2011) Fluoride contamination in groundwater in parts of Nalgonda District, Andhra Pradesh, India. Environ Monit Assess 172:481–492
Canada Health Act Annual Report (2004). http://www.hc-sc.gc.ca/hcs-sss/pubs/cha-lcs/2003-cha-lcs-ar-ra/index-eng.php. Accessed 17 May 2016
Census (2011) Bankura district: census 2011 data, Government of India http://www.census2011.co.in/census/district/13-bankura.html. Accessed 5 December 2015
Changqing H, Hongwei X, Hongyan T, Xiuli Z, Changdou M, Chenghai W et al (2005) Epidemiology and clinical study of endemic fluorosis in a village that has improved water for 40 years. Chin J Endemiol 24:557–560
Chatterjee A, Roy RK, Ghosh UC, Pramanik T, Kabi SP, Biswas K (2008) Fluoride in water in parts of Raniganj Coalfield, West Bengal. Curr Sci 94(3):309–311
Clark RG, Stewart DJ (1983) Fluorine. In: Grace NE (ed) The mineral requirements of grazing ruminants. New Zealand Society of Animal Production, Occasional Publication 9:129–134
Clausen LPW, Karlson UG, Trapp S (2015) Phytotoxicity of sodium fluoride and uptake of fluoride in willow trees. Int J Phytorem 17(4):369–376
Cressey P, Gaw S, Love J (2009) Estimated dietary fluoride intake for New Zealanders. Institute of Environmental Science and Research Limited, Christchurch. www.esr.cri.nz. Accessed 23 March 2016
Daines RM, Leone I, Brennane E (1952) The effect of fluoride on plants as determined by soil. Nutrition and Fumigation Studies, McGraw-Hill, New York
Davison A, Weinstein LW (1998) The effects of fluorides on plants. Earth Isl 13:257–264
Elloumi N, Abdallah FB, Mezghani I, Rhouma A, Boukhris M (2005) Effect of fluoride on almond seedlings in culture solution. Fluoride 38:193–198
EPA (1997) Public health global for fluoride in drinking water. Pesticide and Environmental Toxicology, Section Office of Environmental Health Hazard Assessment, Environmental Protection Agency, California
Erdal S, Buchanan SN (2005) A quantitative look at fluorosis, fluoride exposure, and intake in children using a health risk assessment approach. Environ Health Perspect 113(1):111–117
Fornasiero RB (2001) Phytotoxic effects of fluoride. Plant Sci 161:979–985
Gautam R, Bhardwaj N, Saini Y (2010) Fluoride accumulation by vegetables and crops grown in Nawa Tehsil of Nagaur District (Rajasthan, India). J Phytol 2(2):80–85
Gržetić I, Ghariani RHA (2008) Potential health risk assessment for soil heavy metal contamination in the central zone of Belgrade (Serbia). J Serb Chem Soc 73(8–9):923–934
Gupta S, Banerjee S (2011) Fluoride accumulation in crops and vegetables and dietary intake in a fluoride-endemic area of West Bengal. Fluoride 44(3):153–157
Haidouti C, Chronopoulou A, Chronopoulos J (1993) Effects of fluoride emissions from industry on the fluoride concentration of soils and vegetation. Biochem Syst Ecol 21(2):195–208
Hall RJ, Cain RG (1972) Organic fluorine in tropical soils. New Phytol 71:839–853
Hodge HC (1950) The concentration of fluorides in drinking water to give the point of minimum caries with maximum safety. J Am Dent Assoc 40:436–449
IOM (1997) Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Institute of Medicine (US), National Academies Press, Washington DC
Jha SK, Nayak AK, Sharma YK (2011) Site specific toxicological risk from fluoride exposure through ingestion of vegetables and cereal crops in Unnao District, Uttar Pradesh, India. Ecotoxicol Environ Saf 74:940–946
Kalinic N, Hrsak J, Vadjic V, Lambasa-Belak Z, Mihelcic V, Perkovic B (2005) Fluoride content in soil and vegetation. Bull Environ Contam Toxicol 75:157–162
Khandare AL, Rao GS (2006) Uptake of fluoride, aluminum and molybdenum by some vegetables from irrigation water. J Hum Ecol 19(4):283–288
Kozyrenko MM, Artyukovo EV, Shmakov VN, Konstantinov YM (2007) Effect of fluoride pollution on genetic variability of Larix gmelinii (Poaceae) in East Siberia. J For Res 12:388–392
Kundu MC, Mandal B (2009) Agricultural activities influence nitrate and fluoride contamination in drinking groundwater of an intensively cultivated district in India. Water Air Soil Pollut 198:243–252
Kusa Z, Wardas W, Sochacka J, Pawlowska-Góral (2004) Fluoride accumulation in selected vegetables during their vegetation. Pol J Environ Stud 13(1):55–58
Liang CK, Ji R, Cao SR (1997) Epidemiological analysis of endemic fluorosis in China. Environ Carcinog Ecotoxicol Rev 15(2):123–138
Mcnulty IB, Newman DW (1961) Mechanism(s) of fluoride induced chlorosis. Plant Physiol 36:385–388
McQuaker NR, Gurney M (1977) Determination of total fluoride in soil and vegetation using an alkali fusion selective ion electrode technique. Anal Chem 49(1):53–56
Mezghani I, Elloumi N, Abdallah FB, Chaieb M, Boukhris M (2005) Fluoride accumulation by vegetation in the vicinity of a phosphate fertilizer plant in Tunisia. Fluoride 38(1):69–75
Mishra PC, Meher K, Bhosagar D, Pradhan K (2009) Fluoride distribution in different environmental segment at Hirakud Orissa (India). Afr J Environ Sci Technol 3(9):260–264
Okibe FG, Ekanem EJ, Paul ED, Shallangwa GA, Ekwumemgbo PA, Sallau MS, Abanka OC (2010) Fluoride content of soil and vegetables from irrigation farms on the bank of river Galma, Zaria, Nigeria. Aust J Basic Appl Sci 4(5):779–784
Pal KC, Mondal NK, Bhaumik R, Banerjee A, Datta JK (2012) Incorporation of fluoride in vegetation and associated biochemical changes due to fluoride contamination in water and soil: a comparative field study. Ann Environ Sci 6:123–139
Pandey J, Pandey U (2011) Fluoride contamination and fluorosis in rural community in the vicinity of a phosphate fertilizer factory in India. Bull Environ Contam Toxicol 87:245–249
Paul ED, Gimba CE, Kagbu JA, Ndukwe GI, Okibe FG (2011) Spectrometric determination of fluoride in water, soil and vegetables from the precinct of river Basawa, Zaria, Nigeria. J Basic Appl Chem 1(6):33–38
PHED Report (2007) Activities and achievements in rural drinking water supply and other areas. http://www.wbphed.gov.in/applications/im/uploads/000643.pdf. Accessed 8 March 2016
Pickering WF (1985) The mobility of soluble fluoride in soils. Environ Pollut B9:281–308
Ramanaiah SV, Venkatamohan S, Rajkumar B, Sarma PN (2006) Monitoring of fluoride concentration in groundwater of Prakasham district in India: correlation with physico-chemical parameters. J Environ Sci Eng 48:129–134
Ribang L, Jianan T, Lizhen W, Daxian Z, Wuyi W (1975) The fluoride content in cultivated soil under different geographical conditions in China and its relation to endemic fluorosis. Geogr Res 4:30
Ruan J, Ma L, Shi Y, Han W (2004) The impact of pH and calcium on the uptake of fluoride by tea plants (Camellia sinensis L.) Ann Bot 93(1):97–105
Saini P, Khan S, Baunthiyal M, Sharma V (2013) Mapping of fluoride endemic area assessment of F-1 accumulation in soil and vegetation. Environ Monit Assess 185(2):2001–2008
Samal AC, Bhattacharya P, Mallick A, Ali MM, Pyne J, Santra SC (2015) A study to investigate fluoride contamination and fluoride exposure dose assessment in lateritic zones of West Bengal, India. Environ Sci Pollut Res 22(8):6220–6229. doi:10.1007/s11356-014-3817-4
Steinberg CL, Gardner DE, Smith FA, Hofge HC (1955) Comparison of rheumatoid (ankylosing) spondilitis and crippling fluorosis. Ann Rheum Dis 14:378–384
Susheela AK (1999) Fluorosis management programme in India. Curr Sci 77:1250–1256
Suthar S, Garg VK, Jangir S, Kaur S, Goswami N, Singh S (2008) Fluoride contamination in drinking water in rural habitations of Northern Rajasthan, India. Environ Monit Asses 145:1–6
Swartjes FA, Dirven-Van Breemen EM, Otte PF, Van Beelen P, Rikken MGJ, Uinstra J, Spijker J, Lijzen JPA (2007) Human health risks due to consumption of vegetables from contaminated sites: towards a protocol for site-specific assessment. RIVM Report 711701040, Bilthoven, the Netherlands
Thimmayamma BVS, Rau P (1987) Diet survey methods. In: Thimayamma BVS (ed) A handbook of schedule and guidelines in socio-economic and diet survey. National Institute of Nutrition, Indian Council of Medical Research, India, pp 1–8
UNICEF (1999) States of the art report on the extent of fluoride in drinking water and the resulting endemicity in India. Report by fluorosis and rural development foundation for UNICEF, New Delhi
United States Department of Health and Human Services (USDHHS) (1991) Review of fluoride: benefits and risks. Report of the ad-hoc committee on fluorides. Committee to Coordinate Environmental Health and Related Programs, Department of Health and Human Services, USA
USEPA (1987) US Environmental Protection Agency, National Center for Environmental Assessment, Integrated Risk Information System (IRIS), Chemical Assessment Summary of fluorine. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0053_summary.pdf. Accessed 17 September 2016
USEPA (1989) Risk assessment guidance for superfund, vol 1: Human health evaluation manual (part A). EPA/540/1-890002. US Environmental Protection Agency, Office of Emergency Response, Washington DC
USEPA (1992) Guidelines for exposure assessment. Federal Register Notice 57, Environmental Protection Agency, pp 22887–22938
USEPA (1993) Reference dose (RfD): description and use in health risk assessments. Background document 1A, office of Health and Environmental Assessment, Washington DC. https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments#main-content. Accessed 14 March 2016
USEPA (2002) Child specific exposure factors handbook. Risk Assessment guidance for superfund, vol 1: human health evaluation manual (part A). EPA/540/1-890002. US Environmental Protection Agency, Office of Emergency Response, Washington, DC
USEPA (2003a) Framework for cumulative risk assessment. Risk Assessment Forum, US Environmental Protection Agency, Washington, DC 20460. https://www.epa.gov/sites/production/files/2014-11/documents/frmwrk_cum_risk_assmnt.pdf. Accessed 8 May 2017
USEPA (2003b) Integrated risk information system. Cincinnati, OH: US. Environmental Protection Agency, Environmental Criteria and Assessment Office. http://www.epa.gov/iris. Accessed 17 June 2016
USEPA (2011) Exposure factors handbook: 2011 edition. National Center for Environmental Assessment Office of Research and Development, US Environmental Protection Agency, Washington, DC 20460. https://www.epa.gov/sites/production/files/2015-09/documents/efh-frontmatter.pdf. Accessed 8 May 2017
WHO (2006) Fluoride in drinking water. IWA publishing, London, p 144
Wood JM (1974) Biological cycles for toxic elements in the environment. Science 183:1049–1052
Zouari M, Ahmed CB, Fourati R, Delmail D, Rouina BB, Labrousse P, Abdallah FB (2014) Soil fluoride spiking effects on olive trees (Olea europaea L. cv. Chemlali). Ecotoxicol Environ Saf 108:78–83
Acknowledgements
Piyal Bhattacharya is thankful to the DST, Government of India, for funding this investigation (Sanction Number: YSS/2015/000454).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bhattacharya, P., Samal, A.C., Banerjee, S. et al. Assessment of potential health risk of fluoride consumption through rice, pulses, and vegetables in addition to consumption of fluoride-contaminated drinking water of West Bengal, India. Environ Sci Pollut Res 24, 20300–20314 (2017). https://doi.org/10.1007/s11356-017-9649-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9649-2