Abstract
We studied chronic fluoride intoxication in 10 villages of Udaipur receiving F emissions from phosphate fertilizer factories. Although fluoride remained below permissible limit in most of the drinking water samples, the incidence of fluorosis in adults as well as in children was surprisingly high. Khemli appeared to be the most affected village (with >48% cases) where, about 93% of 2 h air samples contained fluoride above 2.0 μg m−3 and crops and vegetable F ranged from 27.5 to 143.4 μg g−1. Concentrations of fluoride and inorganic P in urine showed asynchrony and were well linked with prevalence of fluorosis. The study indicated that air-borne fluoride was the major factor for higher prevalence of fluorosis in these rural areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
For developing countries such as ours, where majority of population live in rural areas with poor infrastructure and sanitation, availability of safe drinking water has always been a critical issue. Particularly in dry regions of our country, per capita availability of safe drinking water has become a matter of serious concern. The Rajasthan state of India, which witness high frequency of drought and faces water paucity at many parts, has also been identified as one of the endemic States for fluorosis (Susheela 1999). This disease is endemic in many parts of our country where F content is high in drinking waters. Recent years however, have witnessed high concentrations of this toxicant in air and in food stuffs especially around pollution emitting sources. Lakdawala and Punchar (1973) have made extensive study on prevalence of fluorosis and total fluoride intake through drinking water and dietary intake in Bombay (India).
Fluoride is ubiquitous and its concentration varies widely, reaching sometimes as high as 18,000 μg L−1 in hot springs (Madhavan and Subramanian 2001). Certain rock types contain fluoride ranging from 180 μg g−1 in sandstone and greywaeke to 800 μg g−1 in granite (Madhavan and Subramanian 2001). Rock phosphate, phosphatic nodules and phosphorites contain high concentrations of fluoride. Agricultural application of pesticides and fertilizers coupled with industrial activities most often contaminates ground water, surface waters and agro-ecosystems with fluoride (Samal 1988). Air-borne fluoride in the vicinity of aluminium factory, glass fibre factory and phosphate fertilizer factory often contaminate edible crops and dietary vegetables along with surface water bodies (Samal 1988; Pandey 2005a). Thus, in addition to drinking water, dietary intake and inhalation of air-borne fluoride could significantly enhance human exposure to fluoride. In a recent field trial, it was observed that, despite low level of fluoride in drinking water, a number of persons including school going children suffer from dental fluorosis (Pandey 2005a). It seemed that the total fluoride intake from different components of environment including those from air-borne sources need to be considered for explicitly addressing source-link relationships and fluoride associated human health issues. This has particular concern in the vicinity of pollution emitting sources. A number of reports focusing on endemic fluorosis are available for many parts of our country (Desai et al. 1993; Susheela 1999; Choubisa 2001; Susheela et al. 2010). However, so far no reports are available explicitly addressing the role of air-borne fluoride on human health in our country. This systematic study was an attempt to examine the cases of dental fluorosis in school going children and adult population in some rural areas of Udaipur exposed to air-borne fluoride emitted from phosphate fertilizer factories.
The present study was conducted in some rural areas of NE Udaipur (24° 35′ N lat and 72° 86′ E long; 530 m above msl), Rajasthan during 2004–2006. The studied area is not listed under endemic fluorosis. The climate of the region is tropical with three distinct seasons, a hot and dry summer (April to June), a warm and wet rainy (July to September) and a cool and dry winter (November to February). October and March constitute transitional months. The day time summer temperature ranged from 33.0 to 44.5°C. During winter, temperature varied between 8.6 and 23.5°C and night temperature some time dropped below freezing. The annual rainfall averaged 615 mm and relative humidity ranged between 14% and 97%. Wind direction shifts from pre-dominantly westerly and north-westerly in October to April and easterly and south-westerly in the remaining months.
Materials and Methods
For the present study, ten villages were selected and based on population size, 80–286 cases of adults in age group 21–65 (both men and women) were examined for dental fluorosis. Children of ten schools (115–364 students in age group 5–15) were also examined. Teeth of children and adults of both sexes were examined for dental fluorosis using grade II mottling as a criterion (WHO 1970). Drinking water sources and food habits of the villagers were also enquired. The main occupation of the residents is agriculture. Borewells and well waters are used for drinking, cooking and irrigation purposes. Since the area receives air emissions from two phosphate fertilizer units (Udaipur Phosphate Fertilizer Factory and Rama Phosphate Fertilizer Factory), in order to asses total fluoride intake we also analyzed locally grown crops and vegetables along with drinking water samples. Common agricultural crops and vegetables include Maize (Zea mays L.), Wheat (Triticum aestivum L.), Pearl millet (Pennisetum typhoides L.), Chick pea (Cicer arietinum L.), Tomato (Lycopersicon esculentum Mill.), Brinjal (Solanum melongena L.), Cauliflower (Brassica oleracea L.), Lady’s finger (Abelmoschus esculentus L.), Radish (Raphanus sativus L.) and Spinach (Spinacea oleracea L.). Samples of crops and vegetables were analyzed for fluoride concentration following Bellack (1972). Drinking water samples, collected in pre-sterilized bottles from all the sources were analyzed for fluoride concentration using a fluoride ion electrode (Model 90–91, Orion Research, USA; detection limit 0.019 ppm). Citrate was included in the total ionic strength adjustment buffer (TISAB) as a chelating agent in order to avoid interference by fluoride binding metal ions. Fluoride in ambient air was measured using High Volume Samplers (Envirotech APM-415) following Pandey (2005b). Extracted fluoride in air and urinary fluoride was analyzed using fluoride ion electrode. Inorganic phosphate in urine was analyzed using an automatic analyzer system. Quality control measures were taken to assess contamination and reliability of data. For quality assurance, blank and standards were run after five determinations to calibrate the instrument. Analytical variances of the data remained below 10% for all the measurements.
Significant effects were assessed using analysis of variance (ANOVA) following appropriate transformations whenever required. Standard error of means (SEM) and coefficients of variation (CV) were computed for expressing data variability. The statistical analysis were done using SPSS programme.
Results and Discussion
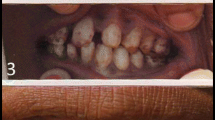
Fluoride concentration in drinking water samples ranged from 0.03 to 2.24 ppm (Table 1). For most of the water samples, the values remained below the permissible limit as established by Ministry of Health, Government of India. For Khemli village however, the concentrations of fluoride in both, well water (1.87–2.24 ppm) and borewell water (1.14–1.42) remained above the permissive limit. The area considered in this study receives high amount of air-borne fluoride from phosphate fertilizer factories (Table 1). Concentration of fluoride in air was found to be the highest at Khemli (1.95–7.61 μg m−3) and lowest at Devali village (0.36–2.46 μg m−3). About 93% of 2 h air samples at Khemli contained fluoride above 2.0 μg m−3. Fluoride concentration in agricultural crops and dietary vegetables were substantially high (Table 2). In grains, the concentration, on dry weight basis, ranged between 27.5 and 121.4 μg g−1 and in vegetables, between 37.6 and 143.4 μg g−1 (Table 2). Among the grain crops, fluoride accumulated maximally in maize (37.6–121.4 μg g−1 dry weight) and among vegetables, in spinach leaf (56.2–143.4 μg g−1 dry weight). In school going children (aged 5–15), the cases of dental fluorosis ranged from 14.0% to 43.0% (Table 3). The incidence of dental fluorosis in boys and girls did not differ significantly. Of the 10 villages selected in the study, Khemli was found to be the most severly affected (Table 4), where about 49.0% of the adult population has shown the sign of dental fluorosis.
Trends in fluorosis were found to be synchronous to those with fluoride concentrations in drinking water, air, crops and dietary vegetables. High concentration of fluoride in air and in drinking water and other sources could account for high incidences of fluorosis in Khemli village. According to WHO standards, permissive limit for fluoride in drinking water is 1.0 ppm (Meenakshi et al. 2004). In dry tropics, where water intake is very high, fluoride in water even at low level may induce harmful effects (Bassin et al. 2004).
Furthermore, high concentration of air-borne fluoride could result in high accumulation of fluoride in crops and dietary vegetables grown in the area (Jelenko and Pokomy 2010). Air-borne fluoride also contributes to raised fluoride levels in open water systems such as wells and ponds. These sources, in addition to their use for drinking water are often used for crops irrigation. Thus, enhanced fluoride intake through drinking water coupled with dietary intake could significantly substantiate total fluoride accumulation in body tissue. Inhalation exposure to air-borne fluoride could further substantiate total tissue level fluoride. According to an US Department of Agriculture report, air-borne fluoride has caused more worldwide damage to domestic animals than any other pollutant (USDA 1972). Devali was found to be the least affected village considered in this study, where fluoride in drinking water (0.01–0.14 ppm) and in ambient air (0.36–2.46 μg m−3) remained the lowest.
The incidence of fluorosis in villagers including school going children in SE Udaipur appeared alarming. In particular, children with fast growing tissues could get affected more severely and quickly. Children have also shown such symptoms as stomach pain, intermittent diarrhea, chronic constipation and gas formation which could be linked with fluoride intake (Ando et al. 2001). Excess intake of fluoride leads to the accumulation of dermaten sulphate, which demineralize the area around teeth and bones. Such demineralized area in teeth get perforated and chipped besides being discoloured. A high percentage of adults in these villages (aged 40–60) containing dental fluorosis also complained for joint pains. These symptoms may be indicative of skeletal fluorosis followed by dental fluorosis (Pandey 2005b). Furthermore, in the fluorosis areas, the residents including children showed high content of urinary fluoride and low concentration of inorganic phosphate in urine (Fig. 1). The latter is an indicative of altered glomerular filtration. These determinants are often used as biochemical marker of fluoride exposure (Choubisa 2001). Asynchrony in urinary fluoride and inorganic phosphate indicate the state of fluoride exposure which could be linked with the adverse health effects in residents of fluorosis areas (Ando et al. 2001). In the studied area, except for Khemli village, the concentrations of fluoride in most of the water samples were found to be below the permissible limit. It seemed that atmospheric emissions from phosphate fertilizer factories could substantiate fluoride intake through inhalation and dietary intake. The area considered in this study is exposed to emission from phosphate fertilizer factories where fluoride rich phosphate rocks are processed for production of phosphate fertilizer. The source add substantial amount of fluoride into the air environment (Pandey 2005b). Air-borne fluoride can easily accumulate in uncovered food stuffs. Since fluoride contamination in food stuffs is hard to remove by washing, consumption of contaminated food stuffs constitute an important source of total fluoride. Air-borne fluoride deposited onto the open waters such as wells and ponds could further contribute to human exposure through drinking water and irrigation-linked food chain contamination. These multiple routes of exposure could exacerbate the incidence of fluorosis even in those villages where water fluoride levels were below the permissible limit.
The study indicates that exposure to excess fluoride has caused dental fluorosis and altered glomerular filtration both, in adults and children in rural community of NE Udaipur. However, unlike many other parts of Rajasthan, fluorosis did not appear endemic to this area. Air-borne fluoride being added from phosphate fertilizer factories could substantiate the total fluoride intake in the residents through multiple routes including inhalation and dietary intake of contaminated food stuffs. Atmospheric sources further add fluoride to open waters such as wells and ponds leading to human exposure through drinking and irrigation linked contamination. Hence the approaches such as provision of defluoridated drinking water would be least productive unless the residents are getting relief from air-borne fluoride being added from phosphate fertilizer factories. Majority of the affected cases belong to poor socio-economic status and could hardly afford to take calcium and vitamin-C rich diet. It is therefore invitable to develop and implement suitable control measures to reduce air-emission linked health problems of this region.
References
Ando M, Tadano M, Yamamoto S, Tamura K, Asanuma S, Watanable T, Kondo T, Sakurai S, Ji R, Liang C, Chen X, Hong Z, Cao S (2001) Health effects of fluoride pollution caused by coal burning. Sci Total Environ 271:107–117
Bassin EB, Mittleman MA, Wypij D, Joshipura K, Douglass CW (2004) Problems in exposure assessment of fluoride in drinking water. J Public Health Dent 64:45–49
Bellack E (1972) Methods and materials for fluoride analysis. J Am Water Works Assoc 64:62–66
Choubisa SL (2001) Endemic fluorosis in southern Rajasthan, India. Fluoride 34:61–70
Desai VK, Solanki DM, Kantharia SL, Bhavsar BS (1993) Monitoring of neighborhood fluorosis through a dental fluorosis survey in schools. Fluoride 26:181–186
Jelenko I, Pokomy B (2010) Historical biomonitoring of fluoride pollution by determining fluoride content in roe deer (Capreolus capreolus L.) antlers and mandibles in the vicinity of the largest Slovene thermal power plant. Sci Total Environ 409:430–438
Lakdawala DR, Punchar BD (1973) Fluoride content of water and commonly consumed food in Bombay and a study of dietary intake. Indian J Med Res 16:1679–1687
Madhavan N, Subramanian V (2001) Fluoride concentrations in river water of a South Asia. Curr Sci 80:1312–1319
Meenakshi, Garg VK, Kavita, Renuka, Malik A (2004) Ground water quality in some village of Haryana, India: focus on fluoride and fluorosis. J Hazard Mater 106:85–97
Pandey J (2005a) Fluoride distribution and fluorosis in some rural areas of Udaipur. J Int Med Sci Assoc 18:133–135
Pandey J (2005b) Evaluation of air pollution phytotoxicity downwind of a phosphate fertilizer factory in India. Environ Monit Assess 100:249–266
Samal UN (1988) Dental fluorosis in school children in the vicinity of an aluminium factory in India. Fluoride 21:137–141
Susheela AK (1999) Fluorosis management program in India. Curr Sci 77:1250–1256
Susheela AK, Mondal NK, Gupta R, Ganesh K, Brahmankar S, Bhasin S, Gupta G (2010) Effective interventional approach to control anaemia in pregnant women. Curr Sci 98:1320–1330
United States Department of Agriculture (1972) Air pollutants affecting the performance of domestic animals. Agricultural handbook no. 380, p 109
World Health Organization (WHO) (1970) Fluorides and human health. Geneva, p 273
Acknowledgments
We are grateful to Prof. H. R. Tyagi, former Convener, Department of Environmental Science, MLS University for laboratory facility and other support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, J., Pandey, U. Fluoride Contamination and Fluorosis in Rural Community in the Vicinity of a Phosphate Fertilizer Factory in India. Bull Environ Contam Toxicol 87, 245–249 (2011). https://doi.org/10.1007/s00128-011-0344-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0344-6