Abstract
Municipal solid waste incineration (MSWI) fly ash has been classified as hazardous waste and needs treatment in an environmentally safe manner. Mechanochemical (MC) treatment is such a detoxification method, since it destroys dioxins and solidifies heavy metals. Milling, however, also introduces supplemental metals (Fe, Ni, Cr, Mn…), following wear of both steel balls and housing. Milling moreover reduces the particle size of fly ash and disperses catalytic metal, potentially rising the reactivity of fly ash to form and destroy ‘dioxins’, i.e. polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD + PCDF or PCDD/F). To test this issue, model fly ash (MFA) samples were composed by mixing of silica, sodium chloride, and activated carbon, and doped with CuCl2. Then, these samples were first finely milled without any additives for 0 h (original sample), 1 h and 8 h, and the effect of milling time (and hence particle size) was investigated on the formation of polycyclic aromatic hydrocarbons (PAHs), and of polychlorinated phenols (CP), benzenes (CBz), biphenyls (PCB) and dioxins (PCDD + PCDF) during de novo tests at 300 °C for 1 h, thus simulating the conditions prevailing in the post-combustion zone of an incinerator, where dioxins are formed and destroyed. These compounds are all characterized by their rate of generation (ng/g MFA) and their signature, i.e. internal distribution over congeners as a means of gathering mechanistic indications. PAH and CBz total yield did not decrease in MC treated MFA with milling time, while total pentachlorophenol (PeCP), PCB and PCDD/F yield decreased up to 86, 94 and 97%, respectively. International Toxic Equivalents (I-TEQ) concentration decreased more than 90%, while degree of chlorination varied inconsistently for PCB and PCDD/F, and average congener patterns of PCDD/F do not vary considerably with milling time for both gas and solid phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fly ash (FA) from municipal solid waste incineration (MSWI) has been classified as hazardous waste, because of the presence of toxic contaminants such as heavy metals, chloride salts, dioxins and other organic compounds (Ferreira et al. 2003). Currently, FA treatment becomes an urgent environmental issue in China, following fast growth of MSWI capacity. Extensive studies explain the formation of polychlorinated dibenzo-p-dioxins + dibenzofurans (PCDD + PCDF = PCDD/F) starting from FA (Addink and Olie 1995; Stieglitz 1998; Kuzuhara et al. 2003; Fujimori et al. 2009; Yang et al. 2015; Zhang et al. 2016). Such FA contains the necessary precursors or carbon, together with catalytic metals, to synthesize PCDD/F. Obvious precursors are polychlorinated phenols (CP), benzenes (CBz) and biphenyls (PCB), as well as polycyclic aromatic hydrocarbons (PAHs). Vogg and Stieglitz (1986) proposed de novo synthesis as a catalytic oxidative breakdown of a macromolecular chlorinated carbon matrix, yielding mainly carbon dioxide and monoxide, accompanied by Products of Incomplete Combustion: chlorobenzenes (CBz), chlorophenols (CP), polychlorinated naphthalenes (PCN), biphenyls (PCB), dibenzo-p-dioxins + dibenzofurans (PCDD/F), etc. Thus, de novo synthesis also generates precursors, linking various precursor routes with de novo synthesis (Addink and Olie 1995). CP condenses rapidly to form PCDD (Karasek and Dickson 1987; Peng et al. 2016), whereas CBz form PCB (Kim et al. 2004) and possibly some PCDF. Huang and Buekens (1995) proposed the ratio of PCDD to PCDF as a simplified discriminant between precursor routes and de novo activity.
Only part of the emerging PCDD is formed directly from the carbon matrix, while other compounds are also synthesized from single ring structures that are de novo created (Addink et al. 1995; Stieglitz et al. 1997; Hell et al. 2001) or present as Products of Incomplete Combustion (PIC). Also, the chlorination of basic structures (dibenzo-p-dioxins + dibenzofurans) and dechlorination of fully octachlorinated dibenzo-p-dioxin (OCDD) and dibenzofuran (OCDF) could play a substantial role, explaining part of the signature of PCDD/F (Iino et al. 1999, 2000; Wikström and Marklund 2000; Ryu et al. 2006). Chlorination on catalytic surfaces in MSWI fly ash has been found to occur largely at the 2,3,7,8 positions (Luijk et al. 1994; Addink et al. 1996; Ryu et al. 2003a, b, 2004). Conversely, surface-mediated dechlorination was also occurring at the 2,3,7,8 positions, resulting in PCDD/F isomer distributions very different from those produced by chlorination (Hagenmaier et al. 1987; Wiesmuller 1990; Iino et al. 2000, 2001).
According to the Stockholm Convention, persistent organic pollutants (POPs) are a threat to the environment and all POPs should be eliminated and decomposed in an environmentally safe manner. In particular, both thermal and mechanochemical (MC) detoxification and decomposition are considered as potential countermeasures for POPs contaminated materials.
Mechanochemical treatment has shown a remarkable potential to destroy safely any halogenated pollutant, as a solvent-free and non-thermal alternative for disposing of the existing PCB and other POPs stock piles, as well as PCDD/F in fly ash (Cagnetta et al. 2016b). Successfully treated were as follows: chlorophenol (Wei et al. 2009; Lu et al. 2012), 1,2,3-trichlorobenzene (Mio et al. 2002), hexachlorobenzene (Loiselle et al. 1997; Zhang et al. 2014), hexabromobenzene (Zhang et al. 2002), perfluorooctane sulphonates (PFOS) (Zhang et al. 2013), polychlorinated biphenyls (PCBs) (Nah et al. 2008) and polymers such as polyvinyl chloride (Shu et al. 2001) (PVC).
MC degradation of pollutants proceeds possibly without any additives during milling, thus avoiding an increase of the treated waste weight. In numerous studies, however, fly ash is mixed with suitable additives, such as calcium oxide CaO (Nomura et al. 2005), magnesium and amine (Birke et al. 2006), CaO and SiO2 as a mixture (Wei et al. 2009; Cagnetta et al. 2016a) and Fe/SiO2 (Zhang et al. 2014) for rapid destruction. In another, recent study, fly ash is washed free from chlorides, thus avoiding the MC formation of PCDD/F (Chen et al. 2016). Conversely, Yan et al. (2007) milled fly ash from medical waste incinerator without any additive, yet achieved a good degradation efficiency of PCDD/F. Milling treatment can be performed at atmospheric temperature, while very high, up to several thousand degrees Celsius temperature at points of action in the sub-microscopic deformation zone, where a grain collides at high velocity with a solid surface, may occur (quasi-adiabatic energy accumulation, so-called triboplasma), which can bring chemical and physico-chemical changes of solids due the influence of mechanical energy (Heinicke et al. 1984). Kuzuhara et al. (2003) showed that the method and level of mixing might markedly influence upon the results of consecutive de novo tests.
In the present study, milling tests of model fly ash were conducted from a different perspective: since milling affects the reactivity of fly ash, it is verified in how far such milling may influence the results of a consecutive de novo test. Following a series of preliminary tests, a model fly ash was composed and samples of fresh and milled fly ash (1 and 8 h of milling) were subjected to de novo tests (300 °C, 1 h), and the formation was monitored not only of PCDD/F but also of a range of potential precursor compounds. Thus, the amount of the potential precursors PAH, CP, CBz and PCB was established. Moreover, the PCDD/F was analysed in terms of not only PCDD and of PCDF (ng/g) but also their homologue profiles as well as the identity of (theoretically) 136 individual PCDD and PCDF congeners was established.
Materials and methods
Chemicals
CuCl2·2H2O (purity >99.0%), n-hexane (purity 95.0%), dichloromethane, ethyl acetate, iso-octane (ACS, ≥99%), anhydrous Na2SO4, CH3OH, NaCl (purity >99.5%), NaOH and HCl of analytical grade reagents (AR) were procured from Aladdin Chemistry Co., Ltd. SiO2 (AR) was obtained from Sinopharm Chemical Reagent Co., Ltd., China.
Preparation of MFA
Model fly ash (MFA) samples were prepared from silica (SiO2) as matrix, sodium chloride as source of chloride and activated carbon (AC) as source of carbon. A selected amount of CuCl2 was added to this MFA as catalyst and chlorinating agent. Activated carbon powder (AC) was first washed with distilled water and acetone, respectively, and then completely dried for about 4 h at 100 °C. Silica powder (100–120 mesh) was rinsed with distilled water and then dried for about 4 h at 100 °C. The MFA was mixed with CuCl2 by grinding these together in a mortar for about 10 min in the following weight proportions: AC (2.5 wt%), sodium chloride (NaCl; 10 wt% Cl), 0.2 wt% copper chloride CuCl2 was added and silica (SiO2) formed the balance.
Ball milling of fly ash
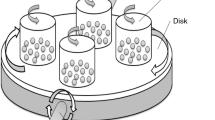
An all-dimensional planetary ball mill (QXQM-2, Changsha Tencan Powder Technology Co., Ltd., China) was used for the MC treatment (Chen et al. 2016). The samples were milled for 1 and 8 h. Compared with the conventional planetary ball mill, this unit adds a dimension of rotation. The disk and all pots slowly rotate (1 rpm) around main spindle to avoid sedimentation of materials caused by gravity, making powders to be ground more completely. The milling pots, with volume of 500 mL, and balls with either 8 (2.1 g) or 12 (7.1 g) mm diameter were made of special stainless steel (1Gr18Ni9Ti). About 40 g fly ash samples were charged into each pot, together with equal weight big (10) and small balls (43) under atmospheric conditions, with a ball to powder ratio of 4 wt/wt The mill was operated for 16 h, with a 30-min driving belt cooling interval every 30 min (Kuzuhara et al. 2003). The rotation speed of the disk was set at 300 rpm and the rotational direction changed automatically every 30 min. After MC treatment, MFA was collected for further analysis.
Analytical methods
The chemical composition of fly ash was analysed by X-ray fluorescence (XRF), and also coupled plasma atomic emission spectrometry (ICP-AES, iCAP6300) was used. The crystalline structure of the fly ash particles was identified by X-ray diffraction analysis (XRD, X’Pert PRO PAN alytical B. V.). The microcosmic surface of fly ash with 0, 1 and 8 h milling was characterized by SEM (scanning electron microscopy) using scanning electron microscopy (Hitachi SU-70). The BET (Brunauer-Emmett-Teller) surface area and average particle size was measured by means of a Micromeritics Tristar 3020 instrument.
Experimental procedure
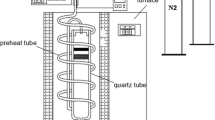
Figure 1 shows a schematic diagram of the experimental apparatus used for de novo tests. A sample of 2 g of model fly ash (MFA) was placed into a vertical quartz tube (53 cm in length and 5 cm in diameter) and fixed by means of glass wool. Then, the tube was placed for 1 h in a preheated electric furnace at 300 °C in a flow of 10% oxygen/90% nitrogen (300 mL/min) to simulate the conditions in the post-combustion zone of a MSWI. The glass wool used in our experiments was first rinsed by acetone and then dried at 100 °C. A blank test was run without sample at similar experimental conditions, and after pre-treatment, the sample was analysed for all selected compounds and no compounds were detected in the blank test.
Sampling and analysis of PCDD/F
During the de novo experiment in the continuous flow reactor, all semi-volatile organics were absorbed on XAD-2 cartridges and in dichloromethane (DCM). These represent the gas-phase compounds while those remaining in the solid residue were collected as solid-phase compounds. Gas-phase (XAD-2 resin, DCM and adsorbed condensate) and solid-phase (fly ash residue) samples were Soxhlet-extracted with DCM for 24 h. The extracts and washes were replaced by 100 mL n-hexane before further treatment. This extract was divided into three parts. One was used for CP and CBz analysis, one for additional pre-treatment to analyse PCDD/F and coplanar PCB and one for PAH.
For PAH formation in de novo tests, the raw extract was evaporated and passed through a silica gel column and, after nitrogen dry, was kept for analysis at 4 °C. The fraction for CP and CBz analysis was made double in amount by adding double deionized water and was pre-treated according to a method described by Huang et al. (2017), and few drops of 5 mol/L NaOH were used to increase its pH >12; it was shaken well and kept for a while, until two separate layers appeared. This step was repeated three times. Organic layer was used for CBz sample preparation and aqueous layer for CP samples. For chlorophenols, about five drops of 3 mol/L HCl were added to the water phase to adjust pH < 2 and 20 mL dichloromethane and ethyl acetate mixed solvent (4:1) was added and well mixed; then, organic layer was passed through anhydrous Na2SO4 to remove water. After rotary evaporation and nitrogen dry, 1 mL CH3OH was added and kept at 4 °C for analysis. For CBz, organic phase was passed through anhydrous Na2SO4 for water removal, then through multi-silica gel column. After nitrogen blow dry, 1 mL iso-octane was added in samples and kept at 4 °C for analysis. Before Soxhlet extraction, the raw extract for PCDD/F was spiked with 10 μL EPA1613-LCS standards. Then, the extracted solution was concentrated to 1–2 mL with a rotary evaporator and diluted with hexane to 10 mL. After adding 25 μL 1613-ISS clean-up internal standard, half of the concentrated solution went to sample clean-up including a multi-silica gel column and a basic-alumina column, while the other half was kept at 4 °C for possible reworking of the pre-treatment. The eluate was blown by nitrogen and kept at 4 °C after adding 1613-ISS standards and waiting for analyses by EPA method 1613 (US EPA 1994). The fraction including PCB was conducted in the same way as PCDD/F by using EPA method 1668B (US EPA 2008).
The 16 PAHs were analysed by means of gas chromatography with a HP-5 MS (30 m × 0.25 mm × 0.25 μm) coupled with mass spectrometry (Agilent 6890N GC/5975B MSD). The temperature program for GC oven was as follows: initial temperature 50 °C, held for 2 min; 50–200 °C at 15 °C/min; and 200–300 °C at 10 °C/min then held for 5 min. Carrier gas was helium (99.999%). The isotope standards were purchased from Accu Standard, Inc., USA. The quantification of PAH was performed by using selective ion monitoring (SIM) mode. The 12 chlorobenzene congeners were analysed by Agilent GC-ECD gas chromatograph (HP6890N GC-ECD), with a DB-5 column (30 m × 0.25 mm × 0.25 μm). The temperature program for GC oven was as follows: initial temperature 40 °C held for 4 min; then increased to 220 °C at the 10 °C/min; and held for 5 min. The quantification of CBz was performed by using electron capture detector (ECD). LC/MS/MS analyses for chlorophenols was performed using an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, USA) equipped with an electrospray ionization (ESI) source, operated in the negative ion multiple-reaction monitoring (MRM) mode. Agilent Mass Hunter Workstation was used for data acquisition and processing. Nitrogen was used as the sheath gas and drying gas. The nebulizer pressure was set to 45 psi and the flow rate of drying gas was 5 L/min. The flow rate and temperature of the sheath gas were 11 L/min and 350 °C, respectively. Chromatographic separation was carried out on a Zorbax SB C8 column (150 × 2.1 mm, 3.5 μm). The HPLC mobile phases consisted of (A) 25 mM ammonium acetate in distilled water (pH 4.0) and (B) methanol. The gradient program was as follows: 0–10 min, 45–55% of B; 10–15 min, 55–65% of B; 15–20 min, 65–75% of B; and 20–30 min, 75–95% of B. The flow rate was set at 0.2 mL/min. Mass spectrometric detection was completed by use of an electrospray ionization (ESI) source in negative ion MRM mode. Analyses for PCDD/F and PCB were performed by means of high-resolution chromatography with a DB-5MS column (60 m × 0.25 mm × 0.25 μm) coupled with high-resolution mass spectrometry (JMS-800D, JEOL, Japan). The temperature program for GC oven was as follows: initial temperature 75 °C, held for 2 min; 75–150 °C at15°C/min; and 150–290 °C at 2.5 °C/min then held for 1 min, with carrier gas, helium (99.999%), 1.2 mL/min. The isotope standards were purchased from Cambridge Isotope Laboratories. The target compounds were all tetra- to octa-CDD/F as well as the 17 2,3,7,8-substituted PCDD/F and 209 PCB congeners.
Results and discussion
Characteristics of fly ash after 0, 1 and 8 h of milling
Composition
The cations, anions and heavy metals of fly ash samples, milled at 0, 1 and 8 h, were analysed by both XRF and ICP techniques. As expected, the matrix material’s silicon showed the highest concentration (Table 1), followed by chlorine and sodium. Surprisingly, the concentration of Fe, Cu and Cr increased particularly after 1 and 8 h of milling: results show that the milling may increase some heavy metals due to wear of equipment and balls, while Ca disappears after 8 h and 0.4% in 0 h sample is below the Ca content in typical MSWI fly ash as MFA was prepared by mixing 2.5 wt% AC and Ca content in AC was 1.1% (not shown here), similarly 0.1, 0.01 and 0.1% content in 0 h milled samples (Table 1) observed for Al, Zn and Cu, respectively, was also lower than the MSWI fly ashes. When a ball hits another ball or the pot wall, compressing a small amount of powder, on the contact surface the temperature rises up to thousands of K in a microscopic area (~1 μm2) for a very short time (~10−9 s) (Urakaev and Boldyrev 2000), this brings deformation and disruption of solids, various energy-rich intermediates arise in the first place and if high mechanical energy is applied to it, then it can be converted to heat and it is possible to get various unexpected chemical reactions. If a metal exhibits stronger strength of microcrystal then it needs greater degree of disordering (degradation limit) its lattice structure, apparently in the current study, applied mechanical energy for long 8 h reached to degradation limit of Ca lattice and it totally disappeared in the last sample (Butyagin 1994).
Particle size and surface
The particle surface was observed by SEM. Figure 2a shows the fly ash, before milling: it consists of many large particles; 1 h of milling reduces these to smaller sizes (Fig. 2b); Fig. 2c–d shows particle aggregation, small particles sticking to larger ones. Further milling only brings slight change in size, but the particles stick together to form aggregates.
Specific surface and size
The BET specific surface area was 2.54, 5.62 and 6.29 m2 g−1 after 0, 1 and 8 h milling, respectively; their average particle size d P was 23.6, 10.7and 9.54 nm, respectively.
Crystallinity
The evolution of silica and sodium chloride crystals was monitored by X-ray diffraction (XRD) using Cu Kα radiation (from a copper tube running at 40 kV and 200 mA) and a diffractometer operating at 2θ of 0.02° (D-max-2550TC, Rigaku, Japan). Crystalline phases were identified by comparing the intensity and position of Bragg peaks with those listed by the joint committee on powder diffraction standards (JCPCS) data files. The two major components of model fly ash, SiO2 and NaCl, show weaker peak intensities after 1 and 8 h of milling (Fig. 3a–c); their wave shapes remain similar, yet with some peak heights declining, and suggesting that no clear reactions took place between these fly ash compounds during their mechanical treatment. The decrease in intensity is ascribed to the partial conversion of crystalline phases to amorphous states, an effect typically induced by ball milling (Suryanarayana 1995, 2001).
De novo synthesis of organic pollutants
After milling for 0, 1 and 8 h, the particle size becomes smaller, the specific surface larger and the reactive catalyst (CuCl2) better dispersed. The purpose of this study was to check how these various changes would affect the de novo activity, exhibited by the model fly ash tested. For this purpose, a de novo test was conducted under the constant conditions: a temperature of 300 °C and a reaction time of 1 h.
PAH Figure 4 shows the effect of 1 or 8 h of milling time on the formation of PAH during these de novo tests. The PAHs surviving the test include sum of gas and solid phases. Apparently, milling did not much affect the amount of PAH. This may be attributed to the test temperature of 300 °C, too low for producing much PAH (Kim et al. 2004). Among the 16 EPA PAH scrutinized, only the concentration of naphthalene (Nap) augmented with time (0.64, 0.73 and 1.18 ng/g at 0, 1 and 8 h, respectively) and distributed more in gas phase. As milling under goes for several hours, deformation and disruption of MFA particles bring out unexpected changes, macromolecular aromatic structures of carbon are also fractured and could yield only lower molecular PAH, like naphthalene. Under oxygen deficient conditions Nap could condense and produce higher molecular PAH. It has been suggested that the transformation of PAH to PCDD/F or PCB occurs via three main reaction steps including degradation, oxygen insertion and chlorination (Weber et al. 2001; Fullana et al. 2004). In the three cases, two and three ringed PAHs (naphthalene, phenanthrene and anthracene) dominated the PAHs analysed. Furthermore, their internal distribution after the three de novo tests stayed very similar and their total amount of PAH remained very low for the three tests, with preliminary milling for 0 h (at 2.95 ng/g), 1 h (2.56 ng/g) and at 8 h (3.26 ng/g). The result in so far is disappointing that the milling is clearly accompanied by the formation of amorphous carbon and graphite and that PAHs were regarded as likely precursors. Possibly the activated carbon used as raw material was already too much carbonized to generate PAH under the test conditions employed.
CP
In the fresh sample (0 h), all phenol congeners (mono- to pentachlorophenol) were observed after a de novo test, while only pentachlorophenol remained in the samples de novo tested after 1 and 8 h of milling. Already in the first sample, PeCP was dominant, with 324 ng/g (0 h), and later 95 ng/g (1 h) and 104 ng/g (8 h). In fresh MFA samples (0 h), 44 and 9 ng/g was observed for 2- and 3-MCP, respectively; 2,6-DCP amounted to 218 ng/g MFA; while 2,4,5-TCP, 2,3,4,5-TeCP and 2,3,4,6-TeCP showed 30, 165 and 15 ng/g MFA, respectively. In most de novo studies, chlorophenols are either not analysed or else the analytical results appear rather erratic, e.g. Schwarz and Stieglitz (1992), due to an easy transition from CP to PCDD at about 300 °C.
CBz
Figure 5 plots the de novo formation of CBz vs. sample milling time. The total amount of CBz: 8.24 ng/g (0 h), 4.57 ng/g (1 h) and 6.01 ng/g (8 h) generated remains very low, yet markedly decreases after 1 h then rises with preliminary milling time. Among CBz, the lower chlorinated congeners 1,3-DiCBz and 1,4-DiCBz were the dominant congeners; in contrast Hue et al. (2016) observed PeCBz and HxCBz as dominant congeners in waste incinerator fly ash in Vietnam.
PCB
Table 2 shows the PCB profile at different milling times. From the isomers analysed, International Union of Pure and Applied Chemistry (IUPAC) congener #189 is showing the highest concentration at 0 h milling. Like for PeCP, the formation of PCB is highest for the 0 h sample. The total amount of PCB and their I-TEF value respectively decreased by 37 and 70% after 1 h of milling and after 8 h, a 41% decrease in concentration was observed, while the I-TEF value slightly increased by 12%. Deca-CB is predominantly formed after 1 h of milling. Milling brings amorphization of catalysts like Cu and Fe and also modification of graphitic structure of activated carbon (Cagnetta et al. 2016a) that reduced reactivity of fly ash to support dioxins and dl-PCB formation. However, still there are predecessors (unburned carbon, chlorine, catalysts) of de novo formation present in fly ash and sample is well mixed by milling even after long 8 h. Moreover, to the best of our knowledge, such problem has never been investigated; why few isomers are formed in high concentration in mechanically active fly ash than in samples without treatment. Penta-CB (#123) is such a case in present study which needs further assessment in respect to de novo formation, as it showed quite unusual behaviour and a high concentration (185.88 pg/g) was observed in 8 h milled sample as compared to value (113.13 pg/g) obtained in 0 h milled sample only in gas phase. In solid phase, concentration of Penta-CB (#123) decreased gradually with milling time.
The PCDD/F homologue profiles (present signatures of isomer groups from TCDD through OCDF) in fly ash were analysed (Fig. 6). After de novo tests on raw MFA-samples, PCDD/F formation (especially PCDF) was substantial. The presence of PAH, CBz, PeCP and PCB indicated de novo formation of these precursor compounds (Dickson et al. 1989; Hell et al. 2001). The dominance of OCDD, HpCDF and OCDF is typical for waste incineration (Everaert and Baeyens 2002), as there was a catalyst and chlorine source (CuCl2.2H2O and NaCl) in model fly ash.
Many studies suggested that there should be good correlation between precursor molecules, like PCB, CP and CBz, and PCDD/F formation (Pandelova et al. 2006 and Pandelova et al. 2009). A high correlation (R 2 = 0.95 not shown here) between total PCDD/F and total PCB amount was found in all samples with different milling times, studied here. This result is supported by many studies focusing on PCB and PCCD/F formation during combustion within incinerators, where PCB either by oxidation or by chlorination led to dioxins formation (Lemieux et al. 2001). The total conc. of pentachlorophenol (dominant and only congener found in samples with 1 and 8 h milling) showed a fairly high correlation (R 2 = 0.99) with total dioxins. Almost all CP isomers correlate strongly with PCDD/F in the gas phase, but only certain isomers, in particular TeCP and PeCP, are of importance in the particle phase (Tuppurainen et al. 2000).
Relationship between I-TEQ reductions of all organics with milling time
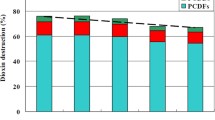
In order to test the milling effect on de novo formation of selected organic compounds in model fly ash, it was milled for 1 and 8 h without any additive. Previous studies, focusing on degradation of organic compounds, reported that mechanochemical treatment after 2 h achieved a high removal efficiency of OCDD and OCDF of up to 99.3 and 99.9% with CaO additive (Nomura et al. 2005). Cagnetta et al. (2016a) attained after 4 h a degradation efficiency of 76% of dioxins by milling secondary copper smelting fly ash together with calcium oxide and silica. Results of the pg-TEQ/g values and their corresponding percent reduction in gas and solid phases, during milling, are given in Table 3. Total reduction efficiency (gas + solid) for PCDD/F and PCB was more than 90% except 2,3,7,8 PCDD showed slightly lower reduction value of 88%. Interestingly total Penta CB #123 increased 21%, while PeCP showed 68% reduction after 8 h treatment. Percent reduction efficiencies are calculated by the following Eq. (1)
After 8 h of milling, PCDD/F and PCB showed very low amount both in gas and solid phase in de novo tests, some of PCB toxic congeners were not detected even after 1 h. This could be attributed the fact that MC treatment induces mass transport process with a significant impact on the physical and chemical behaviour of solids at different scales (Baláž et al. 2013), and MFA has lost its dioxin generation potential to some extent. Only PentaCB (#123) showed 64% increase in 8 h milled sample; this could be attributed to the fact that, although milling reduced reactivity of fly ash to support dioxins and dl-PCB formation, but still there are predecessors (unburned carbon, chlorine, catalysts) of de novo formation present in fly ash and sample is well mixed by milling, so there is a possibility that PentaCB (#123) formation increased, which needs further investigation. Present study showed better results in terms of total PCDD/F and PCB formation reduction without any additive and at rotational speed of 300 rpm after 8 h, when compared with the study of Nomura et al. (2005) at a rotational speed of 700 rpm. Pentachlorophenol showed 60 and 75% reduction in gas and solid phases, respectively; the possible reason might be that mechanical treatment time and milling intensity were not enough in the present study to inactivate FA for de novo potential of precursor formation. Current value of PeCP is less than the residual PeCP concentration (1.6%) after 8 h milling in another study, reason might be that they used 400 rpm rotational speed and CaO as an additive (Wei et al. 2009). PAH and CBz in present study showed increasing trend with grinding time, although total conc. Remains very low for both categories. This issue needs further assessment of mechanical treated FA’s for toxic compounds, under influence of different milling conditions in detail (milling time, milling materials, intensity, etc.). As very low rotational speeds lead to increased periods of milling, this thereby induces a large inhomogeneity in the powder due to inadequate kinetic energy input and insufficient localized heat input. Conversely, very high speeds can lead to excessive heating of the vessel, high wear of the balls causing increased contamination and lower powder yields (Chen et al. 2007).
Degrees of chlorination
The degree of chlorination (d c , average number of chlorine substituent) was one of way to judge mechanochemical treatment effect for de novo formation of chlorinated compounds, and can be calculated as follows: To see how far the chlorination of dioxins has proceeded, we calculated the degree of chlorination of PCDD/F and PCB, d c by Eq. (2), which is defined as the sum of the products of the mole fraction f j and the number of chlorine atoms n j for each homologue:
The results in Table 3 show that the degrees of chlorination slightly vary from 7.14 for G0 to 7.07 for G1, while 7.16 in G8 sample was observed for dioxins in gas phase, and in solid phase, a small decrease in Cl content was observed. Dioxin like PCB showed a gradual decrease in value with milling time in solid, while in gas-phase dechlorination did not occur, indicating that milling treatment apparently has no obvious effect on chlorination/dechlorination of organic compounds.
Resulting isomer patterns of PCDD/F in samples with 0, 1 and 8 h milling
The PCDD/F isomer patterns are given in Figs. 7 and 8. For dioxins and furans, a maximum is found within the hepta- and octa-chlorinated isomers (0, 1 and 8 h) for both gas and solid phases and isomers exhibited same distribution in all samples. Comparing both phases, it was observed that O8CDD and O8CDF isomers generally decreased as the milling time increased. For other chlorinated isomers with same chlorination number, most of them decrease with varied extent, but some of the isomers did not change in their concentration after milling, yet few isomers showed higher conc. after 8 h than samples with 0 h milling. The most of the toxic congeners of PCDD/F were reduced after 8 h milling suggesting that isomers with alpha (1,4,6,9-position) and beta positions (2,3,7,8-position) showed same behaviour, and similar results were found by Peng et al. (2010).
Conclusion
The effect of preliminary milling on the consecutive de novo synthesis of polycyclic aromatic hydrocarbons (PAH), chlorophenols (CP), chlorobenzenes (CBz), polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDD/F) and polychlorinated biphenyls (PCB) was tested at a single reaction time, temperature and gas flow rate. Milling affect samples in several ways: it reduces particle size, enhances sample homogeneity and dispersion of the active agent (CuCl2) and destroys chloroaromatics compounds by mechanochemical forces. The output of PAH and CBz were not too much affected by this treatment, whereas PeCP, PCB and PCDD/F showed variable responses, though the total concentration of PCDD/F and PCB was greatly reduced during milling. The total concentration of pentachlorophenol decreased after 1 and 8 h of milling (324 initially to 95 and 104 ng/g).
Milling time significantly affected the I-TEQ concentration and showed a good reduction percentage over the time period of 8 h. Most toxic congeners were almost completely degraded of milling, while few PCB toxic congeners were detected even after 1 h. Milling time did not change of degree of chlorination for PCB and PCDD/F.
Investigation of detailed isomer patterns of PCDD/F showed no change with milling treatment time, although few congeners exceptionally increased in concentration after 8 h treatment. Same degradation of isomers with the alpha (1,4,6,9-position) and beta positions (2,3,7,8-position) was associated with milling treatment time. Detailed investigation of the 209 PCB congeners was not made in the present study.
Future work will also consider the other parameters of milling treatment (such as milling speed, milling ball materials during mechanical treatment) and de novo testing (temperature and time, gaseous atmosphere, and type of catalysts). Results obtained in the present study may provide a basis of comparison for future work, focusing on all organic formation in mechanically treated samples.
References
Addink R, Olie K (1995) Mechanisms of formation and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans in heterogeneous systems. Environ Sci Technol 29:1425–1435
Addink R, Cnubben P, Olie K (1995) Formation of polychlorinated dibenzo-p-dioxins/dibenzofurans on fly ash from precursors and carbon model compounds. Carbon 33:1463–1471
Addink R, Antonioli M, Olie K, Govers HAJ (1996) Reactions of dibenzofuran and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin on municipal waste incinerator fly ash. Environ Sci Technol 30:833
Baláž P, Achimovičová M, Baláž M, Billik P et al (2013) Hallmarks of mechanochemistry: from nanoparticles to technology. Chem Soc Rev 42:7571–7637
Birke V, Mattik J, Runne D, Benning H, Zlatovic D (2006) Dechlorination of Recalcitrant Polychlorinated Contaminants Using Ball Milling. NATO Security through Science Series C: Environmental Security. 111–127
Butyagin PY (1994) Problems in mechanochemistry and prospects for its development. Russ Chem Rev 63(12):965–976
Cagnetta G, Hassan MM, Huang J, Yu G, Weber R (2016a) Dioxins reformation and destruction in secondary copper smelting fly ash under ball milling. Sci Rep. doi:10.1038/srep22925
Cagnetta G, Huang J, Wang B, Deng S, Yu G (2016b) A comprehensive kinetic model for mechanochemical destruction of persistent organic pollutants. Chem Eng J 291:30–38
Chen CN, Chen YL, Tseng WJ (2007) Surfactant-assisted de-agglomeration of graphite nanoparticles by wet ball mixing. J Mater Process Technol 190:61–64. doi:10.1016/j.jmatprotec.2007.03.109
Chen Z, Lu S, Mao Q, Buekens A, Chang W, Wang X, Yan J (2016) Suppressing heavy metal leaching through ball milling of fly ash. Energies. doi:10.3390/en9070524
Dickson LC, Lenoir D, Hutzinger O (1989) Surface- catalyzed formation of chlorinated dibenzodioxins and dibenzofurans during incineration. Chemosphere 19:277
Everaert K, Baeyens J (2002) The formation and emission of dioxins in large scale thermal processes. Chemosphere 46:439–448
Ferreira C, Ribeiro A, Ottosen L (2003) Possible applications for municipal solid waste fly ash. J Hazard Mater 96:201–216
Fujimori T, Takaoka M, Takeda N (2009) Influence of Cu, Fe, Pb, and Zn chlorides and oxides on formation of chlorinated aromatic compounds in MSWI fly ash. Environ Sci Technol 43:8053–8059
Fullana A, Nakka H, Sidhu S (2004) PCDF formation from PAH reactions. Organohalogen Cpds 66:1226
Hagenmaier H, Kraft M, Brunner M, Haag R (1987) Catalytic effect of fly ash from waste incinerator facilities on the formation and decomposition of PCDD/Fs. Environ Sci Technol 19:1080–1084
Heinicke G, Hennig HP, Linke E, Steinike U, Thiessen K, Meyer PK (1984) Tribochemistry, Akademie-Verlag Berlin 1984, 495 S., 329 Abb., 106 Tab. Preis: 98, –M, Cryst Res Technol 19:1424
Hell K, Stieglitz L, Dinjus E (2001) Mechanistic aspects of the de-novo synthesis of PCDD/PCDF on model mixtures and MSWI fly ashes using amorphous 12C- and 13C-labeled carbon. Environ Sci Technol 35:3892–3898
Huang H, Buekens A (1995) On the mechanisms of dioxin formation in combustion processes. Chemosphere 31:4099–4117
Huang J-X, Chen J-H, Lu S-Y (2017) Measurement of chlorobenzenes and chlorophenols in fly ashes from MSWI using GC-ECD and LC-MS/MS. Chin J Environ Eng 11(2):1293–1299 (in Chinese)
Hue NT, Thuy NTT, Tung NH (2016) Polychlorobenzenes and polychlorinated biphenyls in ash and soil from several industrial areas in North Vietnam: residue concentrations, profiles and risk assessment. Environ Geochem Health 38:399
Iino F, Imagawa T, Takeuchi M, Sadakata M (1999) De novo synthesis mechanism of polychlorinated dibenzofurans from polycyclic aromatic hydrocarbons and the characteristic isomers of polychlorinated naphthalenes. Environ Sci Technol 33(7):1038–1043
Iino F, Imagawa T, Gullett B (2000) Dechlorination-controlled polychlorinated dibenzofuran isomer patterns from municipal waste incinerators. Environ Sci Technol 34:3143–3147
Iino F, Tsuchiya K, Imagawa T, Gullett B (2001) An isomer prediction model based on dechlorination kinetics for polychlorinated naphthalenes, polychlorinated dibenzo-p-dioxins, and polychlorinated biphenyls from municipal waste incinerators. Environ Sci Technol 35:3175–3181
Karasek FW, Dickson LC (1987) Model studies of polychlorinated dibenzo-p-dioxin formation during municipal refuse incineration. Science 237(4816):754–756. doi:10.1126/science.3616606
Kim K-S, Hong K-H, Ko Y-H, Kim M-G (2004) Emission characteristics of PCDD/Fs, PCBs, chlorobenzenes, chlorophenols, and PAHs from polyvinylchloride combustion at various temperatures. J Air Waste Manage Assoc 54:555–562
Kuzuhara S, Sato H, Kasai E, Nakamura T (2003) Influence of metallic chlorides on the formation of PCDD/Fs during low-temperature oxidation of carbon. Environ Sci Technol 37:2431–2435
Lemieux PM, Lee CW, Ryan JV, Lutes CC (2001) Bench-scale studies on the simultaneous formation of PCBs and PCDD/Fs from combustion systems. Waste Manag 21:419–425
Loiselle S, Branca M, Mulas G, Cocco G (1997) Selective mechanochemical dehalogenation of chlorobenzenes over calcium hydride. Environ Sci Technol 31(1):261–265
Lu S, Huang J, Peng Z, Li X, Yan J (2012) Ball milling 2,4,6-trichlorophenol with calcium oxide: dechlorination experiment and mechanism considerations. Chem Eng J 195–196:62–68
Luijk R, Dorland C, Smit P, Jansen J, Govers HAJ (1994) The role of bromine in the de novo synthesis in a model fly ash system. Chemosphere 28:1299–1309
Mio H, Saeki S, Kano J, Saito F (2002) Estimation of mechanochemical dechlorination rate of poly vinyl chloride. Environ Sci Technol 36:1344–1348
Nah IW, Hwang KY, Shul YG (2008) Effect of metal and glycol on mechanochemical dechlorination of polychlorinated biphenyls (PCBs). Chemosphere 73(1):138–141
Nomura Y, Nakai S, Hosomi M (2005) Elucidation of degradation mechanism of dioxins during mechanochemical treatment. Environ Sci Technol 39:3799–3804
Pandelova M, Lenoir D, Schramm KW (2006) Correlation between PCDD/F, PCB and CBz in coal/waste combustion. Influence of various inhibitors. Chemosphere 62(7:1196–1205
Pandelova M, Stanev I, Henkelmanna B, Lenoira D, Schramm KW (2009) Correlation of PCDD/F and PCB at combustion experiments using wood and hospital waste. Influence of (NH4)2SO4 as additive on PCDD/F and PCB emissions. Chemosphere 75( 5:685–691
Peng Z, Ding Q, Sun Y, Jiang C, Gao X, Yan J (2010) Characterization of mechanochemical treated fly ash from a medical waste incinerator. J Environ Sci 22(10):1643–1648
Peng Y, Chen J, Lu S, Huang J, Zhang M, Buekens A, Li X, Yan J (2016) Chlorophenols in municipal solid waste incineration: a review. Chem Eng J 292:398–414
Ryu J-Y, Mulholland JA, Chu B (2003a) Chlorination of dibenzofuran and dibenzo-p-dioxin vapor by copper(II) chloride. Chemosphere 51:1031–1039
Ryu J-Y, Mulholland JA, Dunn JE (2003b) Polychlorinated dibenzofuran (PCDF) prediction from dibenzofuran chlorination. Organohalogen Compd 63:49–52
Ryu J-Y, Mulholland JA, Dunn JE, Iino F, Gullett BK (2004) Potential role of chlorination pathways in PCDD/F formation in a municipal waste incinerator. Environ Sci Technol 38:5112–5119
Ryu J–Y, Choi K, Mulholland JA (2006) Polychlorinated dibenzo-p-dioxin (PCDD) and dibenzofurna (PCDF) isomer patterns from municipal waste combustion: formation mechanism fingerprints. Chemosphere 65:1526–1536
Schwarz G, Stieglitz L (1992) Formation of organohalogen compounds in fly ash by metal-catalyzed oxidation of residual carbon. Chemosphere 25:277–282
Shu S, Junya K, Fumio S, Kaoru S, Seiichi M, Tsuyoshi I (2001) Effect of additives on dechlorination of PVC by mechanochemical treatment. J Mater Cycles Waste Manage 3(1):20–23
Stieglitz L (1998) Selected topics on the de novo synthesis of PCDD/PCDF on fly ash. Environ. Eng Sci 15:5–18
Stieglitz L, Bautz H, Roth W, Zwick G (1997) Investigation of precursor reactions in the de-novo-synthesis of PCDD/PCDF on fly ash. Chemosphere 34:1083–1090
Suryanarayana C (1995) Bibliography on mechanical alloying and milling. Cambridge International Science Publishing, Cambridge
Suryanarayana C (2001) Mechanical alloying and milling. Prog Mater Sci 46:1–184
Tuppurainen KA, Ruokojärvi PH, Asikainen AH, Aatamila M, Ruuskanen J (2000) Chlorophenols as precursors of PCDD/Fs in incineration processes: correlations, PLS modeling, and reaction mechanisms. Environ Sci Technol 34:4958–4962
Urakaev FK, Boldyrev VV (2000) Mechanism and kinetics of mechanochemical processes in comminuting devices: 1. Theory. Powder Technol 107:93–107
USEPA (1994) Method 1613: tetra- through octa-chlorinated dioxins and furans by isotope dilution HRGC/HRMS, USEPA. USEPA Press, Washington
USEPA (2008) Method 1668B: chlorinated biphenyl congeners in water, soil, sediment, biosolids, and tissue by HRGC/HRMS, USEPA. USEPA Press, Washington
Vogg H, Stieglitz L (1986) Thermal behavior of PCDD/PCDF in fly ash from municipal incinerator. Chemosphere 15:1373–1378
Weber R, Iino F, Imagawa T, Takeuchi M, Sakurai T, Sadakata M (2001) Formation of PCDF, PCDD, PCB, and PCN in de novo synthesis from PAH: mechanistic aspects and correlation to fluidized bed incinerators. Chemosphere 44:1429–1438
Wei YL, Yan JH, Lu SY, Li XD (2009) Mechanochemical decomposition of pentachlorophenol by ball milling. J Environ Sci-China 21(12):1761–1768
Wiesmuller T (1990) Examinations of the catalytic dechlorination of octachlorodibenzo-p-dioxin and octachlorodibenzofuran and application of the obtained mixtures in toxicological studies. Dissertation, University of Tubingen, Federal Republic of Germany
Wikström E, Marklund S (2000) Secondary formation of chlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls, benzenes and phenols during MSW combustion. Environ Sci Technol 34:604–609
Yan JH, Peng Z, Lu SY, Li XD, Ni MJ, Cen KF, Dai HF (2007) Degradation of PCDD/Fs by mechanochemical treatment of fly ash from medical waste incineration. J Hazard Mater 147:652–657
Yang J, Yan M, Li X, Lu S, Chen T, Yan J, Olie K, Buekens A (2015) Formation of dioxins on NiO and NiCl2 at different oxygen concentrations. Chemosphere 133:97–102
Zhang Q, Matsumoto W, Saito HF, Baron M (2002) Debromination of hexabromobenzene by its co-grinding with CaO. Chemosphere 48(7):787–793
Zhang KL, Huang J, Yu G, Zhang QW, Deng SB, Wang B (2013) Destruction of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) by ball milling. Environ Sci Technol 47(12):6471–6477
Zhang W, Wang H, Jun H, Yu M, Wang F, Zhou L (2014) Acceleration and mechanistic studies of the mechanochemical dechlorination of HCB with iron powder and quartz sand. Chem Eng J 239:185–191
Zhang M, Yang J, Buekens A, Olie K, Li X (2016) PCDD/F catalysis by metal chlorides and oxides. Chemosphere 159:536–544
Acknowledgements
This Project was Supported by the General Program of National Natural Science Foundation of China (No. 51676172), the Zhejiang Provincial Natural Science Foundation of China (R14E060001), National Basic Research Program (973 Program) of China (No. 2011CB201500), the Science and Technology Project of Zhejiang Province (No. 2009C13004), and the Program of Introducing Talents of Discipline to University (B08026) and the Zhejiang University’s Pao Yu-Kong International Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
Rights and permissions
About this article
Cite this article
Mubeen, I., Buekens, A., Chen, Z. et al. De novo formation of dioxins from milled model fly ash. Environ Sci Pollut Res 24, 19031–19043 (2017). https://doi.org/10.1007/s11356-017-9528-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9528-x