Abstract
The main objective of this study was to assess the possible effects of airborne particulate matter less than 10 μm in diameter (PM10) from the Middle Eastern Dust (MED) events on human health in Khorramabad (Iran) in terms of estimated hospital admissions (morbidity) for cardiovascular diseases (HACD) and for respiratory diseases (HARD) during the period of 2015 to 2016. The AirQ program developed by the World Health Organization (WHO) was used to estimate the potential health impacts to daily PM10 exposures. The numbers of excess cases for cardiovascular/respiratory morbidity were 20/51, 72/185, and 20/53 on normal, dusty, and MED event days, respectively. The highest number of hospital admissions was estimated for PM10 concentrations in the range of 40 to 49 μg/m3, i.e, lower than the daily (50 μg/m3) limit value established by WHO. The results also showed that 4.7% (95% CI 3.2–6.7%) and 4.2% (95% CI 2.6–5.8%) of HARD and HACD, respectively, were attributed to PM10 concentrations above 10 μg/m3. The study demonstrates a significant impact of air pollution on people, which is manifested primarily as respiratory and cardiovascular problems. To reduce these effects, several immediate actions should be taken by the local authorities to control the impacts of dust storms on residents’ health, e.g., developing a green beltway along the Iran-Iraq border and management of water such as irrigation of dry areas that would be effective as mitigation strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution includes particles and gaseous pollutants, but particles are of paramount of importance with respect to health effects (e.g., Sicard et al. 2010; Jakubiak-Lasocka et al. 2015; Khaefi et al. 2017). In the twentieth century, adverse effects of air pollution on human health were demonstrated. For instance, the air pollution episodes in Europe (Meuse Valley and London) and in the USA (Donora, Pa) caused observable excess mortality and morbidity (Nemery et al. 2001; Fattore et al. 2011; Yari et al. 2016). Among common air pollutants, particulate matter with an aerodynamic diameter of less than or equal to 10 μm (PM10) is particularly important for human health because PM10 represent the particles mass that penetrate into the respiratory tract (Schwartz et al. 1993; Wang et al. 2009; Weuve et al. 2012). The major sources of PM10 are anthropogenic, e.g., road traffic, combustion, power plant activities, and industrial processes or natural, e.g., sea salt and desert dust (Gharehchahi et al. 2013). Exposure to ambient PM10 can cause several adverse health outcomes such as lung irritation, asthma exacerbation, chronic bronchitis, cancer, increased hospital admissions, and mortality resulting from respiratory and cardiovascular diseases (e.g., Sicard et al. 2011; Jeong 2013; Neisi et al. 2016).
Dust storms occur when high wind speeds occur over low, dry vegetation and open soil areas (WMO 2013). These dust storms are associated with environmental and socioeconomic problems (Gerivani et al. 2011; Soleimani et al. 2016; Goudarzi et al. 2017). Over the past two decades, increasing frequency and intensities of dust storms transported from Iran’s western neighboring countries have influenced the western and central parts of the country with high PM10 levels for several days at a time (Ebrahimi et al. 2014). Middle Eastern Dust (MED) storms especially from the Arabian Peninsula, Jordan, Iraq, Syria, and Kuwait affected Iran and likely resulted in the observed increased rates of morbidity and mortality for cardiovascular and respiratory disease (Shahsavani et al. 2012; Ebrahimi et al. 2014; Khaniabadi et al. 2017a). Respiratory disease hospitalizations have increased during MED events in Saudi Arabia (Habeebullah 2013). During previous dust storms for instance in Australia (Brisbane, Barnett et al. 2012), China (Beijing, Xie et al. 2005), Iran (Ahvaz city, Shahsavani et al. 2012), Mauritania (Ozer 2006), and Spain (Cabello et al. 2012), the maximum hourly PM10 concentrations were 894, 798, 5338, 2998, and 378 μg/m3, respectively. In some areas, measured hourly PM10 concentrations were greater than 6000 μg/m3 during dust storms (Naddafi et al. 2012). Significant correlations (p < 0.05) were observed between dust storms and mortality for cardiovascular and respiratory diseases in South Korea (Kwon et al. 2002) and between dust events and daily hospital admissions for respiratory and cardiovascular diseases, pneumonia, and hypertensions in China (Meng and Lu 2007). In addition, significant correlations were found between the PM10 levels and the number of cardiovascular emergency admissions during dust events in Sanandaj (Iran) over the time period 2009–2010 (Ebrahimi et al. 2014). Major desert dust storms have occurred in Iran since 2004 (Khaniabadi et al. 2017a; Maleki et al. 2016). The present study estimated the effects of dust storms on hospital admissions due to cardiovascular diseases (HACD) and respiratory diseases (HARD) attributed to exposure to high PM10 concentrations in Khorramabad.
Materials and methods
Study area
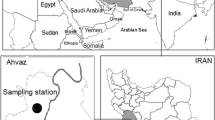
Khorramabad (33° 29′ 16″ N; 48° 12′ 21″ E) is the capital of the Iranian province of Lorestan (Fig. 1) and is located in southwestern Iran. The population of Khorramabad was estimated as 540,000 inhabitants in 2014 (Iranian statistical center). Khorramabad is exposed to MED storms and is one of the most polluted cities in the world in terms of PM10 (Goudie 2014). In recent years, in addition to the MED storms, the number of vehicles and new heavy industries, such as a petrochemical complex, has strongly increased local emissions and produced poor air quality. The city is enclosed by the Zagros Mountains (1170 m a.s.l.) trapping the air pollutants in the boundary layer and producing high air pollutant levels exceeding the air quality standards (Mirhosseini et al. 2013).
Particulate matter sampling
PM10 concentrations and air quality data were obtained from the air quality monitoring agency (Lorestan Environmental Protection Agency (LEPA)). An air pollution-monitoring site is located at the Daneshkade Behdasht station and the LEPA is responsible for its maintenance and operation. The monitoring station is fully automated and provides hourly PM10 concentrations using a β-ray absorption monitor (MetOne Model BAM-1020-Continuous Beta, USA). The hourly PM10 concentrations, from 1 January 2015 to 1 January 2016, were obtained from the LEPA and 24-h concentrations were computed for this study. For the aggregation of hourly data to longer averaging periods (i.e., 24-h) a minimum data capture rate of 75% was imposed to calculate a valid aggregated value. The number of dust event days was determined by using data from Iranian Environmental Protection Agency. Dust event days were detected based on visibility, wind speed, and PM10 hourly concentrations (Hoffmann et al. 2008).
Air quality health impact assessment: AirQ software
The WHO software tool AirQ (Air Quality Health Impact Assessment, AirQ2.2.3) performs calculations that allow quantification of the health effects of exposure to air pollution, including estimates of the reduction in life expectancy (Fattore et al. 2011; Shakour et al. 2011; Khaniabadi et al. 2017b). The AirQ model estimates the effects of short-term changes in air pollution (based on risk estimates from time-series studies) and the effects of long-term exposures. The AirQ model requires relative risk (RR) and baseline incidence (BI) values based on existing exposure-response relationships developed from prior epidemiological studies (Ghozikali et al. 2016; Conti et al. 2017). In epidemiology, the RR is the risk (probability) of developing a disease relative to exposure, per 10 μg/m3 increase of the air pollutant (Sicard et al. 2011; Omidi et al. 2016; Khaniabadi et al. 2017a). A relative risk of 1 indicates that there is no increase in risk. In fact, under certain circumstances, it might be possible to have a RR value of less than 1, which would suggest that instead of being a risk factor the exposure of interest might actually be protective.
The counts of daily respiratory and cardiovascular hospitalizations due to accidents were excluded from the analysis. The values of RR and BI for HACD and HARD attributed to PM10 exposure (Table 1) were obtained from published WHO (2004) data based on epidemiological studies and meta-analysis of time-series and panel studies such as APHEA-2 providing quantitative estimates of the short-term health effects of air pollution (e.g., Atkinson and Anderson 1997; Burret and Doles 1997; Katsouyanni et al. 1997; Touloumi 1997).
The attributable proportion (AP) is defined as the fraction of health consequences in a population exposed to a specific air pollutant (Fattore et al. 2011; Khaniabadi et al. 2017a). The AP can be related to the RR values by:
where AP is the attributable proportion of health outcomes and RR(c) is the relative risk for a given health outcome in category c of exposure (e.g., residential or industrial), taken from prior exposure-response functions based on epidemiological studies. P(c) is the population proportion in category c. The rate of attributable proportion related to the exposure can be estimated by:
where IE is the incidence of exposure which is the rate of the health outcomes attributable to the exposure, for a given concentration level, and I is the baseline incidence which is the baseline frequency of the given outcome in the studied population. Knowing the population size, the number of estimated excess cases associated with the exposure can be calculated by:
where NE is the number of cases attributed to the exposure and N is the size of the population investigated.
Exposure assessment
The PM10 concentrations were pre-processed in Excel to convert the data to the inputs to run the AirQ program. For that, annual and seasonal averages, annual and seasonal maxima values, and 98th percentile were calculated. The PM10 concentrations were parsed into 10 μg/m3 intervals, corresponding to exposure categories. The model assumes that PM10 concentrations are representative of the mean exposure of the population. In agreement with the dust event categories (Carsten et al. 2008), the number of excess cases for HACD and HARD was estimated for the three ranges of PM10 levels (<50, 50–200, and >200 μg/m3) and three RR values (low, central, and high 95% confidence interval) using AirQ2.2.3 software.
Results
PM10 concentrations
The US 24-h National Ambient Air Quality Standards (NAAQS) for PM10 is 150 μg/m3 (US-EPA 2006). The PM10 statistics such as annual average, annual maximum, summer and winter averages, summer, and winter maxima, and 98th percentile concentrations are presented in Table 2. In Khorramabad, the annual average PM10 concentration was 67.3 μg/m3 in 2015 with a summer average of 68.3 μg/m3 and a slightly lower average in winter (65.9 μg/m3). The maximum 24-h concentration (621 μg/m3) was observed in summer compared to a winter maximum of 535 μg/m3. The annual 98th percentile in 2015 was 287 μg/m3. In 2015 in Khorramabad, 22 days had PM10 concentrations exceeding the NAAQS criterion value (i.e., 150 μg/m3). In 2014, a previous study reported 90 days with daily PM10 concentrations exceeding 150 μg/m3 in Khorramabad, with an annual average of 80.6 μg/m3 and an annual maximum of 422 μg/m3 (Nourmoradi et al. 2016).
According to the Hoffmann classification for dust storms (Table 3), the number of days for the normal, dusty, light dust storm (DS1), dust storm (DS2), strong dust storm (DS3), and serious strong dust storm (DS4) categories were 181, 175, 7, 2, 0 and 0, respectively, in Khorramabad in 2015. For the dust event categories (Carsten et al. 2008), the number of DS1 and DS2 days were 9. The number of days for dusty category (PM10 > 50 μg/m3) was higher than the days with normal values (PM10 < 50 μg/m3).
Person-days
Figure 2 depicts the percentage of days in which people living in Khorramabad were exposed to different ranges of PM10 concentrations related to normal, dusty, and MED storm days. In 2015, the exposure time to PM10 for normal (<50 μg/m3), dusty (50–200 μg/m3), and MED (>200 μg/m3) conditions were 49.8, 47.1, and 3.0% in a year, respectively. In 2015, the highest morbidity rate (i.e., 14% of the total number) was related to the PM10 concentrations in the range 40–49 μg/m3.
Short-term health effects
The cardiovascular and respiratory hospitalizations during normal, dusty, and MED storm days, produced by PM10 exposure, in terms of attributable proportions (AP) are presented in Table 4 for low, high, and central RR values. The number of excess of morbidity for cardiovascular diseases on normal, dusty, and MED event days for the central RR was 19.8, 71.6, and 20.2 individuals, respectively. The estimated numbers of excess respiratory diseases morbidity were 51.2, 184.8, and 53.0 persons during normal, dusty, and MED event days, respectively. The sum of excess HACD and HARD cases associated with a short-term PM10 exposure were 112 and 289 people based on the central RR value. The ratios of number of excess cases in dusty air to normal air are similar for both HACD and HARD (ratio = 4.6). The estimated AP was 4.7% (95% CI 3.2–6.7%) for HACD and 4.2% (95% CI 2.6–5.8%) for HARD, respectively.
Figure 3 shows the cumulative number of each health outcome (number of excess cases) including the lower (lower curve), central (middle curve), and higher (upper curve) relative risks, corresponding to 5% (underestimated risk), 50% (central risk) and 95% (overestimated risk) confidence interval, respectively. For concentrations exceeding 150 μg/m3, 31.8 and 82.3 HACD and HARD cases can be attributed to the PM10, respectively. For each increase of 10 μg/m3 in PM10 concentration, the risk of HACD and HARD rises by 0.60 and 0.48%, respectively. In addition, about 97% of hospitalizations for cardiovascular and respiratory diseases was associated to PM10 concentrations lower than 200 μg/m3 and 3% was related to MED events in 2015.
Discussion
In this study, a WHO estimation tool was used to investigate the health effects of particulate matter (PM10) on the health of people living in Khorramabad (Iran). The impact of PM10 was estimated as the increase in cardiovascular and respiratory morbidity for short-term PM10 exposure. The AirQ2.2.3 program was used in epidemiological studies worldwide to assess the short-term health impacts of PM10 on mortality and morbidity cases (e.g., Tominz et al. 2005; Fattore et al. 2011; Shakour et al. 2011; Habeebullah 2013; Jeong 2013; Khaniabadi et al. 2017c).
In Khorramabad, the annual average, summer average, annual maximum, and 98th percentile of PM10 concentrations were 67.3, 68.2, 621, and 287 μg/m3, respectively, in 2015. Previous Iranian studies reported that, e.g., in Ilam city (180Km west from Khorramabad), the annual PM10 mean concentration was 78 μg/m3 in 2015 (Khaniabadi et al. 2017a). The PM10 average in summer (87 μg/m3) was higher than the winter (69 μg/m3). The annual maximum PM10 was observed in summer with 769 μg/m3 and the 98th percentile was 273 μg/m3. An annual mean of 116 μg/m3 was observed in Kermanshah (150Km North) in 2012 (Marzouni et al. 2016) as well as a summer mean, annual maximum and 98th percentile of PM10 concentrations were 126, 624, and 376 μg/m3, respectively. A mean PM10 concentration during stormy days of 187 μg/m3 was found in 2010 in Sanandaj (250 km North) as well as an annual maximum 24-h concentrations of PM10 equal to 600 μg/m3 (Ebrahimi et al. 2014). Higher annual mean (195.5 μg/m3) and annual maximum (782.1 μg/m3) of PM10 was observed in Makkah (Saudi Arabia) over 1-year period (March 2012 to February 2013). In this study, the number of days (184 days) assigned as dusty (i.e., PM10 > 50 μg/m3) was lower than the number of dusty days in Kermanshah in 2012 (322 days). Higher PM10 concentrations during summer are caused by higher temperatures and wind speeds leading to increased atmospheric turbulent and resuspension of dusts in Middle Eastern desert areas (Habeebullah 2013).
In Khorramabad and Ilam, 3% of estimated excess cases occurred during days with PM10 levels exceeding 200 μg/m3, i.e., MED storms in 2015. Similar to Kermanshah in 2012, Ilam in 2015, and in Northern Italy in 2006, the highest number of hospital admissions was observed for PM10 concentrations range of 40–49 μg/m3 in Khorramabad, i.e., lower than the daily limit value (50 μg/m3 established by the WHO guideline while the annual limit value (20 μg/m3) was largely exceeded (WHO 2006; Fattore et al. 2011; Marzouni et al. 2016; Khaniabadi et al. 2017a, c). In another study, the maximum number of hospital admissions was determined for the PM10 concentration range 200–249 μg/m3 in Saudi Arabia (Habeebullah 2013).
The results of this study revealed that 87% of HACD and HARD occurred when PM10 concentrations were higher than 20 μg/m3, and 97% of these impacts was attributed to PM10 concentrations less than 200 μg/m3. In a study in Trieste, Italy, the results showed that 2.5% of respiratory deaths were related to PM10 concentrations greater than 20 μg/m3 (Tominz et al. 2005). The greater number of people admitted to hospital, for concentrations exceeding 200 μg/m3, can be attributed to the Middle Eastern Dust events.
In this study, an excess of total morbidity (HARD + HACD) of 112 and 289 people was associated with a short-term PM10 exposure. In Tallinn (Estonia), the number of excess cases of HARD and HACD due to exposure to PM10 were estimated at 71 and 204 persons in 2006–2008 (Orru et al. 2011). The study of short-term health effects of PM10 in Suwon (South Korea) has estimated the number of excess cases for the HARD and the HACD at 462 and 179 people, respectively, in 2011 (Jeong 2013). In this study, for each 10 μg/m3 increase in PM10 level, HARD and HACD increased by 0.60 and 0.48%, respectively. In another study, in northern China, there was a 0.04% increase in HARD and HACD for each 10 μg/m3 increase in the PM10 level (Chen et al. 2010). In another study in Egypt, an increase of 4.1% in the HARD was associated with an increase of 10 μg/m3 in PM10 level (Shakour et al. 2011). A cohort study in 25 cities of China indicated that 1.8% (0.8–2.9%) and 1.7% (0.3–3.2%) increases (mean and 95% CI) in mortality risk was related to 10 μg/m3 increments of PM10 for cardiovascular mortality and respiratory mortality, respectively (Zhou et al. 2014). Older references showed that, e.g., in the USA, each 10 μg/m3 increase of PM10 concentration up to 150 μg/m3 caused 0.12% increase in the risk rate of mortality among inhabitants of San Jose during 1980–1986 (Fairley 1990). For each 100 μg/m3 increase in the PM10 concentration, 1.35 and 0.021% increase in the incidence of cardiovascular and respiratory diseases was observed respectively in Washington (Hefflin et al. 1994). For PM10 lower than 100 μg/m3, each 10 μg/m3 increase of PM10 level led to 1.1% increase in mortality risk in Los Angeles, USA (Shumway et al. 1988).
A significant correlation between PM10 levels and HARD with a central relative risk of 1.14 (1.01–1.29) was observed, with a number of cases higher during the cold season than the warm season (Chen et al. 2010; Guo et al. 2010). A recent study, carried out in Greece for a 13-year period 2001–2013, assessed the annual number of HARD due to the exposure to inhalable PM10 in Athens (Moustris et al. 2017). The annual mean PM10 concentrations ranged from 30 to 65 μg/m3 over time. The AirQ2.2.3 software was used to evaluate adverse health effects by PM10 and the results show that the annual mean of HARD cases per 100,000 inhabitants ranged between 20 (suburban area) and 40 (city center area). Moreover, a strong relation between the annual number of HARD cases and the annual number of days exceeding the European Union daily PM10 threshold value (40 μg/m3) was found (Moustris et al. 2017). When the mean annual PM10 concentration exceeds the threshold value, the number of HARD associated with PM10 increases by 25% on average (Moustris et al. 2016).
Different studies reported the number of HARD cases per 100,000 inhabitants (with the associated mean annual PM10 concentration): 32 people in Volos, Greece (41 μg/m3) over the time period 2007–2011 (Moustris et al. 2016), 39 in Suwon, South Korea (52 μg/m3) in 2011 (Jeong 2013), 77 in Tehran, Iran (91 μg/m3) in 2010 (Naddafi et al. 2012), 2504 in Makkah, Saudi Arabia (196 μg/m3) in 2012–2013 (Habeebullah et al. 2013), and 4919–5002 in Cairo, Egypt (306–441 μg/m3) in 2008–2009 (Shakour et al. 2011).
A study in Sydney (Australia) found a significant relationship between respiratory diseases and dust events with a relative risk value of 1.2 (95% CI 1.15–1.26) for respiratory diseases (Merrifield et al. 2013). Another investigation was conducted to determine the influence of Asian Dust Storms (ADS) on the hospitalizations due to asthma and chronic obstructive pulmonary disease (COPD) over the tie period 2006–2012. The PM10 concentrations during ADS events reach 147 μg/m3 whereas the concentrations are around 62 μg/m3 during normal days. Hospital visits were significantly associated (p < 0.05) with the occurrence of Asian dust and increased significantly in the days with ADS for asthma (RR = 1.21; 95% CI 1.01–1.19) and COPD (RR = 1.29; 95% CI 1.05–1.59) compared with control days (Park et al. 2015). The numbers of excess cases for COPD and respiratory mortality were 336 and 26 persons, respectively, in 2015 (Khaniabadi et al. 2017a).
Conclusions
The study demonstrates a likely significant impact of air pollution on people living in Khorramabad, which is manifested primarily as a range of respiratory and cardiovascular problems. The results have strongly suggests the importance of the Middle Eastern Dust (MED) events in Khorramabad on increases of health outcomes attributable to PM10. Although the findings are consistent with previous studies conducted worldwide, further investigation is required to refine the specific relative risk and baseline incidence values specific to the Iranian territory and related to variations in climate, geography, and demographic characteristics. Additional investigation is required to estimate the adverse health effects due to other pollutants, such as nitrogen dioxide, sulfur dioxide, ozone, carbon monoxide, and volatile hydrocarbons. In order to reduce the adverse health effects of particulate matter, health advisories provided by health authorities should be given to the public with particular emphasis on vulnerable people (e.g., children, elderly) with chronic lung and heart pathologies (e.g., asthmatic) to reduce their exposures during the dusty days. Furthermore, mitigation measures and strategies, as preventive risk, should be initiated by the appropriate government agencies to control air pollution and dust events in Iran. Activities such as spreading mulch, washing streets, management of water bodies, and planting some new species of plants to intercept airborne dust could reduce the dust concentrations in the ambient air.
References
Atkinson R, Anderson H (1997) Daily time-series for cardiovascular hospital admissions and previous day s air pollution in London, UK. Occup Environ Med 54:535–540
Barnett A, Fraser J, Munck L (2012) The effects of the 2009 dust storm on emergency admissions to a hospital in Brisbane, Australia. Int J Meteorol 56:719–726
Burret R, Doles R (1997) Association between ambient carbon monoxide levels and hospitalization for congestive heart failure in the elderly in 10 Canadian cities. Epidemiology 8:162–167
Cabello M, Orza J, Barrero M, Gordo E, Berasaluce A, Canton L, Duenas C, Fernandez M, Perez M (2012) Spatial and temporal variation of the impact of an extreme Saharan dust event. J Geophys Res 117. doi:10.1029/2012JD017513
Carsten H, Roger F, Ralf W, Yong L, Michael S (2008) Effects of grazing and topography on dust flux and deposition in the Xilingele grassland, Inner Mongolia. Journal Arid Environment 5:792–807
Chen R, Pan G, Kan H, Tan J, Song W, Wu Z (2010) Ambient air pollution and daily mortality in Anshan, China: a time-stratified case-crossover analysis. Sci Total Environ 408:6086–6091
Conti GO, Heibati B, Kloog I, Fiore M, Ferrante M (2017) A review of AirQ models and their applications for forecasting the air pollution health outcomes. Environ Sci Pollut Res. doi:10.1007/s11356-016-8180-1
Ebrahimi SJA, Ebrahimzadeh L, Eslami A, Bidarpoor F (2014) Effects of dust storm events on emergency admissions for cardiovascular and respiratory diseases in Sanandaj, Iran. J Environ Health Sci Eng 12:110
Fairley D (1990) The relationship of daily mortality to suspended particulates in Santa Clara County, 1980–1986. Environ Health Pers 89:159–168
Fattore E, Paiano V, Borgini A, Tittarelli A, Bertoldi M, Crosignani P, Fanelli R (2011) Human health risk in relation to air quality in two municipalities in an industrialized area of Northern Italy. Environ Res 111:1321–1327
Gerivani H, Lashkaripour GR, Ghafoori M (2011) The source of dust storm in Iran: a case study based on geological information and rainfall data. Carpathian J Earth Environ Sci 6:297–308
Gharehchahi E, Mahvi AH, Amini H, Nabizadeh R, Akhlaghi AA, Shamsipour M, Yunesian M (2013) Health impact assessment of air pollution in Shiraz, Iran: a two-part study. Iran J Environ Health Sci Eng 11:2–8
Ghozikali MG, Heibati B, Naddafi K, Kloog I, Conti GO, Polos R, Ferrante M (2016) Evaluation of chronic obstructive pulmonary disease attributed to atmospheric O3, NO2 and SO2 using AirQ model. Environ Res 144:99–105
Goudarzi G, Daryanoosh SM, Godini H, Hopke PK, Sicard P, De Marco A, Dehdari-Rad H, Harbizadeh A, Jahedi F, Mohammadi MJ, Savari J, Sadeghi S, Kaabi Z, Omidi-Khaniabadi Y (2017) Health risk assessment of exposure to the Middle-Eastern Dust storms in Iranian megacity of Kermanshah. Public Health. doi:10.1016/j.puhe.2017.03.009
Goudie AS (2014) Desert dust and human health disorders. Environ Int 63:101–113
Guo Y, Tong S, Zhang Y, Barnett A, Jia Y, Pan X (2010) The relationship between particulate air pollution and emergency hospital visits for hypertension in Beijing, China. Sci Total Environ 201:4446–4450
Habeebullah T (2013) Health impacts of PM10 using AirQ2.2.3 model in Makkah. J Basic Appl Sci 9:259–268
Hefflin BJ, Jalaludin B, McClure E, Cobb N, Johnson CA, Jecha L, Etzel RA (1994) Surveillance for dust storms and respiratory diseases in Washington State, 1991. Arch Environ Health: Int J 49:170–174
Hoffmann C, Funk R, Sommer M, Li Y (2008) Temporal variations in PM10 and particle size distribution during Asian dust storms in Inner Mongolia. Journal of Atmospheric Environment 42:8422–8431
Jakubiak-Lasocka J, Lasocki J, Badyda AJ (2015) The influence of particulate matter on respiratory morbidity and mortality in children and infants. Adv Exp Med Biol 849:39–48
Jeong S (2013) The impact of air pollution on human health in Suwon City. Asian J Atmos Environ 7:227–233
Katsouyanni K, Touloumi G, Spix C (1997) Short-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from times series data from the APHEA project. BMJ 314:1658–1663
Khaefi M, Hassani G, Yari AR, Soltani F, Dobaradaran S, Moogahi S, Mohammadi MJ, Mahboubi M, Alavi N, Farhadi M, Khaniabadi YO (2017) Association of particulate matter impact on prevalence of chronic obstructive pulmonary disease in Ahvaz, Southwest Iran during 2009–2013. Aerosol Air Qual Res 17:203–207
Khaniabadi YO, Daryanoosh SM, Amrane A, Polosa R, Hopke PK, Goudarzi G, Mohammadi MJ, Sicard P, Armin H (2017a) Impacts of middle eastern-dust storms on human health. Atmos Pollut Res. doi:10.1016/j.apr.2016.11.005
Khaniabadi YO, Hopke PK, Goudarzi G, Daryanoosh SM, Jourvand M, Basiri H (2017b) Cardiopulmonary mortality and COPD attributed to ambient ozone. Environ Res 152:336–341. doi:10.1016/j.envres.2016.10.008
Khaniabadi YO, Goudarzi G, Daryanoosh SM, Borgini B, Tittarelli A, De Marco A (2017c) Exposure to PM10, NO2, and O3 and impacts on human health. Environ Sci Pollut Res 24:2781–2789. doi:10.1007/s11356-016-8038-6
Kwon HJ, Cho SH, Chun Y, Lagarde F, Pershagen G (2002) Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ Res 90:1–5
Maleki H, Sorooshian A, Goudarzi G, Nikfal A, Baneshi MM (2016) Temporal profile of PM10and associated health effects in one of the most polluted cities of the world (Ahvaz, Iran) between 2009 and 2014. Aeolian Res 22:135–140
Marzouni MB, Alizadeh T, Banafsheh MR, Khorshiddoust AM, Ghozikali MG, Akbaripoor S, Sharifi R, Goudarzi G (2016) A comparison of health impacts assessment for PM10 during two successive years in the ambient air of Kermanshah, Iran. Atmos Pollut Res 7:768–774
Meng Z, Lu B (2007) Dust events as a risk factor for daily hospitalization for respiratory and cardiovascular diseases in Minqin, China. Atmos Environ 41:7048–7058
Merrifield A, Schindeler S, Jalaludin B, Smith W (2013) Health effects of the September 2009 dust storm in Sydney, Australia: did emergency department visits and hospital admissions increase? Environ Health 12. doi:10.1186/1476-069X-12-32
Mirhosseini S, Birjandi M, Zare M, Fatehizadeh A (2013) Analysis of particulate matter (PM10 and PM2.5) concentration in Khorramabad city. Int J Env Health Eng 3:1–4
Moustris KP, Proias GT, Larissi JK, Nastos PT, Koukouletsos KV, Paliatsos AG (2016) Health impacts due to particulate air pollution in Volos City, Greece. J Environ Sci Health A 51:15–20
Moustris KP, Ntourou K, Nastos PT (2017) Estimation of particulate matter impact on human health within the urban environment of Athens City, Greece. Urban Sci 1. doi:10.3390/urbansci1010006
Naddafi K, Hassanvand MS, Yunesian M, Momeniha F, Nabizadeh R, Faridi S, Gholampour A (2012) Health impact assessment of air pollution in megacity of Tehran, Iran. Iran J Environ Health Sci Eng 9:28
Neisi A, Goudarzi G, Babaei A, Vosoughi M, Hashemzadeh H, Naimabadi A, Mohammadi MJ, Hashemzadeh B (2016) Study of heavy metal levels in indoor dust and their health risk assessment in children of Ahvaz City, Iran. Toxin Rev 35:16–23
Nemery B, Hoet P, Nemmar A (2001) The Meuse Valley fog of 1930: an air pollution disaster. Lancet 357:704–708
Nourmoradi H, Khaniabadi YO, Goudarzi G, Daryanoosh SM, Khoshgoftar M, Omidi F, Armin H (2016) Air quality and health risks associated with exposure to particulate matter: a cross-sectional study in Khorramabad, Iran. Health Scope 5:e31766
Omidi Y, Goudarzi G, Heidari AM, Daryanoosh SM (2016) Health impact assessment of short-term exposure to NO2 in Kermanshah, Iran using AirQ model. Environ Eng and Manag J 3:91–97
Orru H, Maasikmets M, Lai T, Tamm T, Kaasik M, Kimmel V, Orru K, Merisalu E, Forsberg B (2011) Health impacts of particulate matter in five major Estonian towns: main sources of exposure and local differences. Air Qual Atmos Health 4:247–258
Ozer P (2006) Dust in the wind and public health: example from Mauritania. International Conference on Desertification. Migration, Health, remediation and Local Governance: Brussels, 22 September, 2006
Park J, Nam L, Myoung H, Yoonki K, Woo J (2015) The influence of asian dust, haze, mist, and fog on hospital visits for airway diseases. Tuberc Respir Dis 78:326–335
Schwartz J, Slater D, Larson T, Pierson W, Koenig J (1993) Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 174:826–831
Shahsavani A, Naddafi K, Jafarzade HN, Mesdaghinia A, Yunesian M, Nabizadeh R, Arahami M et al (2012) The evaluation of PM10, PM2.5, and PM1 concentrations during the Middle Eastern Dust (MED) events in Ahvaz, Iran, from April through September 2010. J Arid Environ 77:72–83
Shakour A, El-Shahat M, El-Taieb N, Hassanein M, Mohamed A (2011) Health impacts of particulate matter in greater Cairo, Egypt. J Am Sci 7:840–848
Shumway R, Azari A, Pawitan Y (1988) Modeling mortality fluctuations in Los Angeles as functions of pollution and weather effects. Environ Res 45:224–242
Sicard P, Mangin A, Hebel P, Malléa P (2010) Detection and estimation trends linked to air quality and mortality on French Riviera over the 1990–2005 period. Sci Total Environ 408:1943–1950
Sicard P, Lesne O, Alexandre N, Mangin A, Collomp R (2011) Air quality trends and potential health effects—development of an aggregate risk index. Atmos Environ 45:1145–1153
Soleimani Z, Goudarzi G, Sorooshian A, Marzouni MB, Maleki H (2016) Impact of middle eastern dust storms on indoor and outdoor composition of bioaerosol. Atmos Environ 138:135–143. doi:10.1016/j.atmosenv.2016.05.023
Tominz R, Mazzoleni B, Daris F (2005) Estimate of potential health benefits of the reduction of air pollution with PM10 in Trieste, Italy. Epidemiol Prev 29:149–155
Touloumi G (1997) Short term effect of ambient oxidant exposure on mortality: a combined analysis within the APHEA project. Am J Epidemiol 146:177–185
US-EPA, United States Environmental Protection Agency (2006) National Ambient Air Quality Standards (NAAQS)
Wang S, Feng X, Zeng X, Ma Y, Shang K (2009) A study on variations of concentrations of particulate matter with different sizes in Lanzhou, China. Atmos Environ 43:2823–2828
Weuve J, Puett R, Schwartz J, Yanosky J, Laden F, Grodstein F (2012) Exposure to particulate air pollution and cognitive decline in olderwomen. Arch Intern Med 72:219–227
WHO (2004) Meta-analysis of time-series studies and panel studies of particulate matter (PM) and ozone (O3). WHO task group. WHO/EURO 04/5042688
WHO, World Health Organization (2006) WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and ulfurdioxide. Summary of risk assessment. World Health Organization Regional Office for Europe
WMO, World Meteorological Organization (2013) Establishing a WMO Sand and Dust Storm Warning Advisory and Assessment System Regional Node for West Asia: current capabilities and needs
Xie S, Yu T, Zhang Y, Zeng L, Qi L, Tang X (2005) Characteristics of PM10, SO2, NOx and O3 in ambient air during the dust storm period in Beijing. Sci Total Environ 345:153–164
Yari AR, Goudarzi G, Geravandi S, Dobaradaran S, Yousefi F, Idani E, Jamshidi F, Shirali S, Khishdost M, Mohammadi MJ (2016) Study of ground-level ozone and its health risk assessment in residents in Ahvaz City, Iran during 2013. Toxin Rev 35:201–206. doi:10.1080/15569543.2016.1225769
Zhou M, Liu Y, Wang L, Kuang X, Xu X, Kan H (2014) Particulate air pollution and mortality in a cohort of Chinese men. Environ Pollut 186:1–6
Acknowledgments
The authors wish to thanks to the Lorestan Environmental Protection Agency (LEPA) for providing the PM10 data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Khaniabadi, Y.O., Fanelli, R., De Marco, A. et al. Hospital admissions in Iran for cardiovascular and respiratory diseases attributed to the Middle Eastern Dust storms. Environ Sci Pollut Res 24, 16860–16868 (2017). https://doi.org/10.1007/s11356-017-9298-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9298-5