Abstract
Freshwater mussels are at-risk taxa and may be exposed to high levels of carbon dioxide (CO2) because of the potential use of CO2 to control the movement of invasive aquatic fish species. One potential behavioral response to a change in the partial pressure of CO2 (pCO2) may be altered valve movement. In this study, three species of mussels were fitted with modified sensors and exposed to two regimes of pCO2 to define thresholds of impaired valve movement. The first experiment demonstrated that Pyganodon grandis were much more tolerant to rising pCO2 relative to Lampsilis siliquoidea (acute closure at ∼200,000 μatm in comparison to ∼80,000 μatm). The second experiment consisted of monitoring mussels for 6 days and exposing them to elevated pCO2 (∼70,000 μatm) over a 2-day period. During exposure to high pCO2, Lampsilis cardium were open for nearly the entire high pCO2 period. Conversely, P. grandis were closed for most of the period following exposure to high pCO2. For L. siliquoidea, the number of closures decreased nearly 40-fold during high pCO2. The valve movement responses observed suggest species differences, and exposure to elevated pCO2 requires a reactive response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mussels provide many vital ecosystem services and are integral components of freshwater food webs (Vaughn et al. 2008). Globally, freshwater mussel species are among the most at-risk taxa on the planet (Ricciardi and Rasmussen 1999) with 71% of freshwater mussels either extinct or under threat (Williams et al. 1993). Habitat degradation, invasive species, overexploitation, and climate change are among the main reasons for declines of mussel populations (e.g., Strayer and Dudgeon 2010; Strayer et al. 2004; Williams et al. 1993); therefore, monitoring the responses of mussels to environmental stressors is important for predicting population outcomes and to inform conservation practices.
Carbon dioxide (CO2) is a naturally occurring component of freshwater ecosystems, but one that also has the potential to be a stressor that can negatively impact freshwater organisms (Hasler et al. 2016). Many rivers (i.e., unionid mussel habitat) are currently supersaturated with CO2 (Butman and Raymond 2011), can reach partial pressures upwards of 35,000 μatm, and often have wide variation in CO2 across daily and seasonal time scales (Crawford et al. 2016). To date, it remains unclear how the partial pressure of CO2 (pCO2) will change in freshwater in the future (Hasler et al. 2016). However, as a result of climate change, pCO2 in the Great Lakes may increase concomitantly with the atmosphere (Phillips et al. 2015), which would expose freshwater biota to relatively marginal increases in pCO2. Perhaps, the most impending action that could change pCO2 in freshwater is the proposal to use zones of elevated pCO2 (e.g., 50–100 times current levels) to deter the movement of invasive fishes (Noatch and Suski 2012; Donaldson et al. 2016; Cupp et al. 2017). More importantly, elevated pCO2 similar to what might be used to deter fish movement in freshwater has been shown to induce ionic, molecular, and metabolic responses for mussels, inducing several physiological costs (Hannan et al. 2016a, 2016b, 2016c; Jeffrey et al. 2016). Taken together, the increased likelihood of mussels experiencing elevated pCO2 in the future makes it imperative that we quantify signs of disturbance to understand the impacts of potential environmental stressors on these threatened taxa.
Valve movement, the opening and closing of the two valves of a mussel, is a rhythmic behavior that may be one of the several behaviors or physiological processes altered by exposure to elevated pCO2. Valve movement is completed through contraction of the adductor muscles, and this cyclical opening and closing of the valves has been shown to be essential for feeding (Robson et al. 2009; Robson et al. 2010), respiration (Schick et al. 1986), and metabolism (Schick et al. 1988). In addition, valve closure is an important anti-predator behavior, but extended valve closure can induce energetic costs by limiting oxygen uptake, reducing feeding rates, and impairing acid-base regulation (Robson et al. 2007; Robson et al. 2010). In past studies, mussels have been found to alter valve movements (i.e., open or closed more frequently) in response to challenges such as exposure to conspecific cues present in water (e.g., masticated conspecifics; Robson et al. 2007), various substances, including serotonin applied to the cerebral ganglia (Salánki 1963), toxic environments (e.g., maganese and uranium, Markich et al. 2000; noxious dinoflagellates, Nagai et al. 2006), and elevated water temperatures (Dowd and Somero 2013). At present, the pCO2 levels that induce changes to valve movement, and how mussels alter valve movement in response to exposure to elevated pCO2, are not known; however, some evidence suggest that high pCO2 can be used as an anesthetic in molluscs (Salánki 1963; Cooper 2011). Defining both the levels of ambient pCO2 that result in closure, as well as the patterns of closure, may be critical for defining thresholds for mussel valve movement. Understanding how mussel behavior responds to high pCO2 may define the behavioral and physiological consequences of exposure, as well as how mussel communities may change in environments that undergo a rise in pCO2. Species differences with respect to valve movement and tolerance to rising pCO2 may also be possible given the range of pCO2 different species may have experience in the wild over both recent and evolutionary time.
Thus, the objectives of the present study were to (1) define an upper level of pCO2 that mussels would abruptly respond to by observing when changes in valve movement occurred during an exposure to incrementally rising pCO2 and (2) quantify valve movement of three freshwater mussel species in response to a 48-h exposure to elevated pCO2, as well as during a 48-h period before and after the exposure.
Materials and methods
Mussel collection and husbandry
Fatmucket (Lampsilis siliquoidea [Barnes, 1823], hereinafter “FM”) were reared from the glochidial stage and delivered overnight from an aquaculture research facility at Missouri State University, Springfield, MO, USA, to the Aquatic Research Facility, Urbana, IL, in June 2015. Giant floater (Pyganodon grandis [Say, 1829], hereinafter “GF”) and plain pocketbook (Lampsilis cardium [Rafinesque, 1820], hereinafter “PB”) were collected by benthic grab from a barrow pit near Champaign, IL, USA, and the Mississippi River near Cordova, IL, USA, respectively, between July and August 2015, and transported to the Aquatic Research Facility at the University of Illinois, Urbana-Champaign, IL, USA, in coolers. Following transport, all three species were placed into separate 128.7 L recirculating tank systems supplied with 64.4 L of fresh water that was drawn from a 0.04 ha naturalized, earthen-bottom pond. The recirculating systems consisted of three plastic holding containers (one of which was only used to treat water and house the recirculating pump), a UV sterilizer (TMC Vecton 8 W, 11 L min−1 flow rate, Pentair, Apopka, FL, USA), an aquarium heater/chiller (Teco 500, TECO-US, Aquarium Specialty, Columbia, SC, USA), and a low-pressure air blower (SL24H, Sweetwater/Pentair, Apopka, FL, USA). Each holding tank also had 5 cm of sand (Old Castle all-purpose sand, Atlanta GA) as mussel habitat. Mussels were provided a commercial shellfish diet (a mix of algae species ranging in particle sizes; ration = 1 L; Instant Algae, Reed Mariculture Inc., Campbell, CA, USA) every other day. Within holding containers, each sensor-equipped mussel was placed in a small, plastic, open container (15.6 cm × 15.6 cm × 6.35 cm, Ziploc, S. C. Johnson & Son, Racine, WI) to prevent movement towards other mussels and tangling of sensors across individuals, but mussels were still exposed to the recirculating water. Experiments commenced 1–3 months after mussels were brought to the facility, and food was withheld during the experiments. Length of time spent in laboratory conditions does not seem to alter species-specific physiological responses to elevated pCO2 (Hannan et al. 2016a, 2016b).
Valve gape measurements
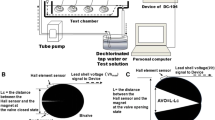
In the experiments described below, the valve gape of each mussel (i.e., the distance between two valves of the shell) was monitored every 10 s (Robson et al. 2009) using linear Hall effect circuits modified from Wilson et al. (2005). The sensor (transducer) component of the circuit (1.5 mm × 4 mm × 3 mm; 0.1 g; A1324, Allegro Microsystems, Inc., Worcester, MA, USA) was sealed using a plastic sealant and affixed near the siphon (posterior end) of the right valve of each mussel, while a neodymium iron boron nickel-plated magnet (4 mm diameter; 3 mm height; 0.3 g; M1219-3; Comos Group of Companies, Clifton, NJ, USA) was attached on the other valve directly opposite to the sensor. Both the sensors and magnets were affixed using a cyanoacrylate adhesive. Sensors were connected to a microcontroller board (Arduino Uno; Somerville, MA, USA), which was connected to a computer for data logging and power supply purposes. The sensor, when near a magnetic field, produces a voltage change that decreases non-linearly as the distance between the sensor and a magnet increases. Thus, closure of the valves can be inferred from monitoring the voltage output of the sensor for each mussel. Because voltage output varies based on relative placement of the sensor and magnet, output voltages cannot be grouped across individuals.
Acute incremental exposure
Using FM (N = 6) and GF (N = 5) mussels, an experiment was carried out to define the CO2 level when abrupt valve closure occurred and to observe the duration of time that the mussels remained closed (PB mussels were not used due to sensor malfunction). Carbon dioxide was added to the central basin of the recirculating system using the common technique of bubbling compressed CO2 gas (commercial grade, 99.9% purity) through an air stone (Summerfelt and Sharrer 2004; Clingerman et al. 2007). Bubbling lasted for a 5-min period at a rate of 0.95 m3 s−1 (17,000 L), followed by a 5-min wait period. After this 5-min wait period, additional CO2 was added, followed by another wait period. This wait period was necessary to allow the pCO2 level to stabilize and be accurately determined. The process of incrementally adding CO2 continued until all the mussels in the experiment were closed and pCO2 increased approximately 14,000 μatm every 5 min. Upon cessation of CO2 addition, and the subsequent decline in pCO2 (pCO2 decreased approximately 5000 μatm every 5 min), the pCO2 at which mussels opened and the duration of the closure were recorded. The CO2 level was measured using a modified infrared probe (GMT221, 0–20%, Vaisala, Vantaa, Finland) (Johnson et al. 2009), and both the time of each mussel’s closure and re-opening, as well as the duration of the closure, were recorded.
Monitoring of valve movement across differing pCO2
An impact assessment approach was used to determine if elevated pCO2 changed valve movement. The monitoring period to define valve movement before, during, and after an exposure to elevated pCO2 lasted a total of 144 h. For the first 48 h, beginning at noon on the first day of the experiment, animals were held at ambient CO2 (Table 1). Exposure to elevated CO2 began at noon on the third day and ended at noon on the fifth day of the experiment. Mussels (N = 7 FM, 6 PB, 4 GF) were subsequently monitored for 48 h after the exposure to CO2 (from noon on day 5 to noon on day 7 of the experiment).
Elevated pCO2 was achieved by bubbling into the central basin of the recirculating system through an air stone until the target pCO2 was reached (Table 1). The target pCO2 used, ∼70,000 μatm, in the current study was chosen based on levels known to induce sub-lethal physiological disturbances in freshwater mussels (Hannan et al. 2016c) as well as a level that would not result in an immediate valve movement response. Target pCO2 was maintained using a pH controller (PINPOINT®, American Marine Inc., Ridgefield, CT, USA) that would automatically add CO2 to the basin should the pH rise above the target level during the exposure period (Reynaud et al. 2003). Dissolved CO2 and total alkalinity (TA) levels were measured using digital titration kits (Model CA-23 (dissolved CO2) and Model AL-AP (TA), Hach Company, Loveland, CO, USA) before, during, and after exposure to CO2, and pCO2 was monitored using a modified infrared probe. Other water quality parameters monitored included pH (WTW pH 3310 meter, Cole Palmer, Vernon Hills, IL, USA), dissolved oxygen, and temperature (YSI 550A, Yellow Springs Instruments, Irvine, CA, USA) (Table 1). All measurements were taken every 6 h over the course of the experiment (n = 8 for each period).
Data analyses
Sensor data were assessed to determine when each mussel was closed by comparing voltage output values during the experiment to outputs of the mussel during the sensor attachment process when mussels were out of water and closed, and by visually inspecting the data (Fig. 1). Three response variables were measured for each mussel during the 6-day CO2 exposure experiment; however, only total time spent closed and number of closures were analyzed due to average duration of closure per individual correlating positively with total time spent closed. To compare these two response variables across the different exposure periods (levels: before, during, and after CO2 exposure) and across species (levels: FM, PB, and GF), generalized linear mixed models were conducted using the ‘glmmPQL’ function in R (R Development Core Team 2010), which uses a penalized quasi-likelihood to estimate model parameters (Breslow and Clayton 1993; Bolker et al. 2009). Fixed factors in each model included exposure period and species, as well as the interaction between exposure period and species. Mussel ID was included in each model as a random factor to account for repeated measures across the same individual within a treatment. A quasipoisson error distribution was fitted to the count data (i.e., number of closures), while the model for total time spent closed was fitted using quasi error distributions (quasipoisson and quasi error distributions model over-dispersion because the dispersion parameter is not fixed) (Bolker et al. 2009). Visual inspection of model residuals was used to assess normality and heteroscedasity, and models were graphically validated following Zuur et al. (2009). Significance of fixed factors, including interactions, was assessed at the 95% significance level. The ‘multcomp’ package (Torsten et al. 2008) was used to complete Tukey’s post hoc analyses to define pairwise differences between factor levels. Pairwise comparisons were only considered significant at the 99% significant level.
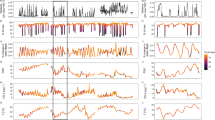
Examples of the raw sensor output (no unit) before (red), during (green), and after (blue) the CO2 exposure for a fatmucket, Lampsilis siliquoidea (a), pocketbook, Lampsilis cardium (b), and giant floater, Pyganodon grandis (c). Time is expressed as Unix time, which is the number of seconds elapsed since January 1, 1970. High sensor output values represent values typical of closed mussels and low sensor output values represent values typical of mussels that are open (i.e., magnet is not close to sensor)
For the acute incremental exposure experiment, a similar statistical test as described above was used to compare the pCO2 at which mussels of the two species (FM and GF) closed and subsequently re-opened. For this model, species and valve action (levels = closed and re-open) were included as fixed effects, mussel ID was included as a random factor, and a quasipoisson error distribution was used. Duration of the closure during the acute experiment was compared across species using a Cox proportional hazard survival analysis using the ‘survival’ package in R (Therneau 2015) because the response was a time to an event (i.e., valve closure).
Results
The pCO2 at which mussels closed following incremental increases in pCO2 and subsequently re-opened following cessation of CO2 additions differed across species (Table 2). GF mussels closed at pCO2 more than twice as high as FM mussels (170,263 ± 56,141 μatm vs 81,925 ± 44,160 μatm, respectively) and re-opened at a significantly lower pCO2 compared to FM mussels (21,210 ± 26,070 μatm vs 88,411 ± 31,661 μatm, respectively; Fig. 2). Within species, FM mussels closed and then re-opened at a similar pCO2 level, while GF mussels re-opened at a pCO2 over two-thirds lower than the pCO2 value at the time of their closure (Fig. 2). The duration of the closure between the times when the mussels initially closed and then re-opened was significantly longer for GF mussels compared to FM mussels (333 ± 329 min vs 109 ± 15 min, respectively; Cox proportional hazard, z = −1.79, df = 1, P = 0.07).
Carbon dioxide (CO2) partial pressures (μatm) that fatmucket (FM, N = 6) and giant floater (GF, N = 5) mussels “closed at” or “opened at” during the acute exposure to incremental rising of CO2 levels. Valve closure occurred during the step-wise increase in pCO2 that occurred every 5 min until all mussels were closed. Valve opening occurred after CO2 addition had ended and the system began to return to starting conditions. Box plots that do not share a letter represent within-species differences determined using a generalized linear mixed model. An asterisk or dagger represents across-species differences within valve actions. Horizontal bars in the box plot represent the median response value, and the 75 and 25% quartiles. Whiskers represent ±1.5 times the interquartile range, and outliers are indicated as dots
Before, during, and after the 2-day exposure to elevated pCO2, differences in valve movement, time spent closed and number of closures, were observed both within and across the three species of mussels examined (Table 2). During the elevated pCO2 exposure, PB mussels were closed for nearly 0% of the time, which was significantly different than the periods before and after exposure (Fig. 3a). Conversely, the duration that GF mussels were closed was not affected until after the period of CO2 exposure when closure duration decreased to approximately a third of the time spent closed during the CO2 exposure, which was significant relative to during the CO2 exposure period, but not compared to the pre-exposure period (Fig. 3a). In addition, CO2 exposure did not result in a significant change in the duration that FM mussels remained closed (Fig. 3a), but the number of closures decreased over 40-fold during the CO2 exposure period compared to before the CO2 exposure period (Fig. 3b). Among the three species of mussels, FM mussels were closed for a third of the duration that PB and GF mussels were closed before the pCO2 exposure period (Fig. 3a); however, FM had on average 10 times more closures than PB and GF during the same period (Fig. 3b). Finally, PB and GF mussels did not vary the number of closures across treatments (Fig. 3b).
Total time spent closed (min; a) and number of closures (b) before, during, and after the 48 h of elevated CO2 exposure for fatmucket (FM, N = 7), giant floater (GF, N = 4), and pocketbook (PB, N = 6). Box plots that do not share a letter represent within-species differences and box plots that do not share an asterisk or dagger represent across-species differences within periods of exposure determined using a generalized linear mixed model. Horizontal bars in the box plot represent the median response value, and the 75 and 25% quartiles. Whiskers represent ±1.5 times the interquartile range, and outliers are indicated as dots
Discussion
All three freshwater mussel species responded to a 48-h exposure to elevated pCO2 by changing valve movement, likely responding to elevated pCO2 as a stressor. When mussels are exposed to elevated pCO2, they experience changes such as an increase in heat shock protein 70 production (Jeffrey et al. 2016), increased Na2+ and glucose (Hannan et al. 2016b), and decreased Mg2+ (Hannan et al. 2016c) in hemolymph, likely from reduced internal or external pH, which can act as a stressor and cause a departure from homeostasis. Interestingly, however, the different species of mussels appeared to have utilized different strategies to deal with the stress of high pCO2. More specifically, during the exposure to elevated pCO2, PB and FM mussels decreased either the time they spent closed or the number of closures, respectively, resulting in longer periods of time spent with their shell open. It is possible that spending more time open during exposure to high pCO2 may assist with mediating acid/base disturbances, which have been shown to occur in several species of mussels exposed to high levels of CO2 (Hannan et al. 2016a, 2016b, 2016c). Furthermore, should mussels remain closed during exposure to high pCO2, mussels may be forced to rely on anaerobic metabolism and incur additional energetic costs (Byrne et al. 1990); thus, remaining open may be a behavioral adaptation to prevent the accumulation of harmful end products of anaerobic respiration. In addition to potential respiratory acidosis, the tendency for PB and FM mussels to remain open during pCO2 exposure suggests that the increase in pCO2 caused the posterior adductor muscle to relax, which could indicate anesthesia (Salánki 1963). PB and FM also showed similar valve movement after the elevated pCO2 exposure period ended. In fact, based on the individual measurements of sensor output, valve movement observed before the elevated pCO2 exposure period returned almost immediately once the stressor was removed. Similarly, when Dowd and Somero (2013) exposed Mytilus congeners to elevated temperature, a pattern that was prevalent during recovery periods was a tendency for valve movement to quickly return to control levels, even after subsequent bouts of exposure to high temperatures. Return to normal valve movement activity after exposure to a stressor is likely important for maintaining homeostasis (Romero et al. 2009) and may have ecological consequences, as prolonged periods of being open can lead to increased predation (Norton-Griffiths 1967; Robson et al. 2010).
Unlike FM and PB mussels, GF mussels utilize a different strategy during exposure to elevated pCO2. During elevated pCO2 exposure, GF mussels remained closed for a longer duration compared to FM and PB mussels. Furthermore, during the acute experiment, GF mussels did not close until CO2 levels reached nearly 200,000 μatm suggesting that they may be more tolerant to acute exposure to elevated pCO2 compared to FM, which closed at approximately 80,000 μatm. This greater tolerance to an acute elevation in pCO2 somewhat contradicts the finding that GF remained closed longer during elevated pCO2 exposure when compared to the other species. However, it may suggest that GF responds to elevated pCO2 based on exposure time following a threshold value of pCO2 less than 200,000 μatm, rather than absolute pCO2. Being closed longer during exposure, elevated pCO2 was likely an attempt to limit exposure to the external environment, thus providing protection against the potential for respiratory acidosis induced by high pCO2 (see above). However, in intertidal mussels, there is a clear trade-off, as remaining closed for an extended duration also increases anaerobic respiration (Zandee et al. 1985), reduces food intake (Robson et al. 2010), and causes a rise in metabolic wastes due to impaired excretion (Nagai et al. 2006). A higher tolerance to elevated pCO2 may have been an attempt to limit these trade-offs, though because we used freshwater mussels, it may be that these trade-offs are more or less severe. Furthermore, if GF mussels in this study experienced these trade-offs while being closed for extended durations, it is likely that the significant increased time spent open by GF mussels during the recovery period following the elevated pCO2 exposure allowed mussels to release harmful by-products and to increase feeding (McCorkle et al. 1979; Famme 1980; Schick et al. 1986). Similar responses in valve movement have been observed in freshwater mussels exposed to emersion, where mussels decrease their time spent closed following emersion (Byrne et al. 1990).
Differences in how species responded to changes in pCO2 were not unexpected, as species differences in response to stressors are known, including elevated pCO2 and acidification (Widdicombe and Spicer 2008; Kroeker et al. 2010). Specifically for unionid mussels in response to elevated pCO2, Hannan et al. (2016b) found that FM and threeridge (Amblema plicata; [Say 1817]) mussels demonstrated species-specific difference in hemolymph Ca2+ and Cl− following exposure to pCO2 of 55,000 μatm for 28 days and suggested that shell thickness and access to internal bicarbonate play a role in physiological responses to high pCO2. PB and FM are closely related phylogenetically (i.e., both Lampsilis genus; Campbell et al. 2005), tend to have thick shells, and showed similar responses to elevated pCO2 (i.e., closed less often or less frequently), while GF mussels have thinner shells (Pennak 1989) and may have a higher tolerance to elevated pCO2. Taken together, observed species differences in valve movement may have been due to shell thickness and access to internal bicarbonate, which has been suggested by physiological-based studies on the mussels monitored in this study (Hannan et al. 2016a, 2016b). It is also possible that species differences with respect to preferred habitat may be an important factor in why the species differed in their responses to elevated pCO2; however, because FM were hatchery-reared, it is difficult to link species differences in all three mussels monitored. For the wild mussels used in this study (PB and GF), PB are found in small creeks to large rivers, and GF are found in sluggish waters, lakes, and ponds (Cummings and Mayer 1992). It is possible that these systems would differ widely with respect to pCO2 with GF habitat potentially having higher pCO2 for longer periods of the year (Cole et al. 1994; Butman and Raymond 2011; Hasler et al. 2016), and this might suggest that GF would be more tolerant to elevated pCO2 and why their response differed from PB.
Valve movement is a behavior that is critical to maintain homeostasis in mussels. Modifications in valve movement as a result of external challenges can be an effective measure of demonstrating thresholds for disturbance, as well as potential outcomes. Overall, valve movement was shown to be a useful measurement for understanding the behavioral response of mussels to elevated pCO2. Mussel species reacted to elevated pCO2 by altering valve movement, presumably in response to acid-base disturbances as a result of reduced internal or external pH. Furthermore, the three species of mussels demonstrated differences in tolerance to elevated pCO2 as well as the duration of valve closures and number of closures. The consequences of these species-specific differences in valve movement are not well understood; however, as specific strategies of valve movement during exposure to elevated pCO2 maximize survival, community-level changes could arise if environments become elevated in pCO2. Specifically, should CO2 barriers be used to control the movement of invasive fishes, it is likely that one response by mussels will be to alter valve movement. Future work should aim to understand how physiological stress experienced during exposure to elevated pCO2 may be modulated by valve movement and to monitor both physiological and behavioral responses to elevated pCO2 in individuals. Other intrinsic factors such as sex and size of individuals may also yield differences in how individuals respond to elevated pCO2.
References
Bolker BM, Brooks ME, Clark CJ et al (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. doi:10.1016/j.tree.2008.10.008
Breslow NE, Clayton DG (1993) Approximate inference in generalized linear mixed models. J Am Stat Assoc 88:9–25
Butman D, Raymond PA (2011) Significant efflux of carbon dioxide from streams and rivers in the United States. Nat Geosci 4:839–842. doi:10.1038/ngeo1294
Byrne RA, Gnaiger E, McMahon RF, Dietz TH (1990) Behavioral and metabolic responses to emersion and subsequent reimmersion in the freshwater bivalve, Corbicula fluminea. Biol Bull 178:215–259
Campbell DC, Serb JM, Buhay JE et al (2005) Phylogeny of North American amblemines (Bivalvia, Unionoida): prodigious polyphyly proves pervasive across genera. Invertebr Biol 124:131–164. doi:10.1111/j.1744-7410.2005.00015.x
Clingerman J, Bebak J, Mazik PM, Summerfelt ST (2007) Use of avoidance response by rainbow trout to carbon dioxide for fish self-transfer between tanks. Aquac Eng 37:234–251. doi:10.1016/j.aquaeng.2007.07.001
Cole JJ, Caraco NF, Kling GW, Kratz TK (1994) Carbon dioxide supersaturation in the surface waters of lakes. Science 265:1568–1570
Cooper JE (2011) Anesthesia, analgesia, and euthanasia of invertebrates. ILAR J 52:196–204
Crawford JT, Stanley EH, Dornblaser MM, Striegl RG (2016) CO2 time series patterns in contrasting headwater streams of North America. Aquat Sci. doi:10.1007/s00027-016-0511-2
Cummings KS, Mayer CA (1992) Field guide to freshwater mussels of the Midwest. Illinois Natural History Survey Manual 5
Cupp AR, Erickson RA, Fredricks KT, et al. (2017) Responses of invasive silver and bighead carp to a carbon dioxide barrier in outdoor ponds. 305:297–305.
Donaldson MR, Amberg JJ, Adhikari S et al (2016) Carbon dioxide as a tool to deter the movement of invasive bigheaded carps. Trans Am Fish Soc 145:657–670. doi:10.1080/00028487.2016.1143397
Dowd WW, Somero GN (2013) Behavior and survival of Mytilus congeners following episodes of elevated body temperature in air and seawater. J Exp Biol 216:502–514. doi:10.1242/jeb.076620
Famme P (1980) Effect of shell valve closure by the mussel Mytilus edulis L. on the rate of oxygen consumption in declining oxygen tension. Comp Biochem Physiol 67A:167–170
Hannan KD, Jeffrey JD, Hasler CT, Suski CD (2016a) Physiological responses of three species of unionid mussels to intermittent exposure to elevated carbon dioxide. Conservation Physiology 4:1–13. doi:10.1093/conphys/cow066
Hannan KD, Jeffrey JD, Hasler CT, Suski CD (2016b) The response of two species of unionid mussels to extended exposure to elevated carbon dioxide. Comp Biochem Physiol -Part A Mol Integr Physiol 201:173–181. doi:10.1016/j.cbpa.2016.07.009
Hannan KD, Jeffrey JD, Hasler CT, Suski CD (2016c) Physiological effects of short- and long-term exposure to elevated carbon dioxide on a freshwater mussel, Fusconaia flava. Can J Fish Aquat Sci 73:1538–1546. doi:10.1139/cjfas-2016-0083
Hasler CT, Butman D, Jeffrey JD, Suski CD (2016) Freshwater biota and rising pCO2? Ecol Lett 19:98–108. doi:10.1111/ele.12549
Jeffrey JD, Hannan KD, Hasler CT, Suski CD (2016) Molecular and whole-animal responses to elevated CO2 exposure in a freshwater mussel. J Comp Physiol B 187:87–101. doi:10.1007/s00360-016-1023-z
Johnson MS, Billett MF, Dinsmore KJ et al (2009) Direct and continuous measurement of dissolved carbon dioxide in freshwater aquatic systems—method and applications. Ecohydrology 3:68–78. doi:10.1002/eco.95
Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13:1419–1434. doi:10.1111/j.1461-0248.2010.01518.x
Markich SJ, Brown PL, Jeffree RA, Lim RP (2000) Valve movement responses of Velesunio angasi (Bivalvia: Hyrdiidae) to manganese and uranium: an exception to the free ion activity model. Aquat Toxicol 51:155–175
McCorkle S, Shirley TC, Dietz TH (1979) Rhythms of activity and oxygen consumption in the common pond clam, Ligumia subrostrata (Say). Can J Zool 57:1960–1964
Nagai K, Honjo T, Go J et al (2006) Detecting the shellfish killer Heterocapsa circularisquama (Dinophyceae) by measuring bivalve valve activity with a Hall element sensor. Aquaculture 255:395–401. doi:10.1016/j.aquaculture.2005.12.018
Noatch MR, Suski CD (2012) Non-physical barriers to deter fish movements. Environ Rev 20:1–12. doi:10.1139/A2012-001
Norton-Griffiths M (1967) Some ecological aspects of the feeding behaviour of the oystercatcher Haematopus ostralegus on the edible mussel Mytilus edulis. Ibis 109:412–424
Pennak RW (1989) Fresh-water invertebrates of the United States: protozoa to mollusca, 3rd edn. Wiley, New York
Phillips J, McKinley G, Bennington V et al (2015) The potential for CO2-induced acidification in freshwater: a Great Lakes case study. Oceanography 25:136–145. doi:10.5670/oceanog.2015.37
R Development Core Team (2010) R: a language and environment for statistical computing, Vienna, Austria. URL http://www.R-project.org/
Reynaud S, Leclercq N, Romaine-Lioud S et al (2003) Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob Chang Biol 9:1660–1668
Ricciardi A, Rasmussen JB (1999) Extinction rates of North American freshwater fauna. Conserv Biol 13:1220–1222
Robson AA, Garcia De Leaniz C, Wilson RP, Halsey LG (2010) Behavioural adaptations of mussels to varying levels of food availability and predation risk. J Molluscan Stud 76:348–353. doi:10.1093/mollus/eyq025
Robson AA, Thomas GR, Garcia De Leaniz C, Wilson RP (2009) Valve gape and exhalant pumping in bivalves: optimization of measurement. Aquat Biol 6:191–200. doi:10.3354/ab00128
Robson AA, Wilson RP, Garcia de Leaniz C (2007) Mussels flexing their muscles: a new method for quantifying bivalve behaviour. Mar Biol 151:1195–1204. doi:10.1007/s00227-006-0566-z
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389. doi:10.1016/j.yhbeh.2008.12.009
Salánki J (1963) The effect of serotonin and catecholamines on the nervous control of periodic activity in fresh-water mussel (Anodonta cygnea). Comp Biochem Physiol 8:163–171
Schick JM, Gnaiger E, Widdows J et al (1986) Activity and metabolism in the mussel Mytilus edulis L. during intertidal hypoxia and aerobic recovery. Physiol Biochem Zool 59:627–642
Schick JM, Widdows J, Gnaiger E (1988) Calorimetric studies of behavior, metabolism and energetics of sessile intertidal animals. Am Zool 28:161–181
Strayer D, Downing J, Haag W et al (2004) Changing perspectives on pearly mussels, North America’s most imperiled animals. Bioscience 54:429–439
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J North Am Benthol Soc 29:344–358. doi:10.1899/08-171.1
Summerfelt ST, Sharrer MJ (2004) Design implication of carbon dioxide production within biofilters contained in recirculating salmonid culture systems. Aquac Eng 32:171–182
Therneau T (2015) A package for survival analysis in S. R package version 2.38, https://CRAN.R-project.org/package=survival
Torsten H, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Vaughn CC, Nichols SJ, Spooner DE (2008) Community and foodweb ecology of freshwater mussels. J North Am Benthol Soc 27:409–423. doi:10.1899/07-058.1
Widdicombe S, Spicer JI (2008) Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J Exp Mar Bio Ecol 366:187–197. doi:10.1016/j.jembe.2008.07.024
Williams JD, Warren ML, Cummings KS et al (1993) Conservation status of freshwater mussels of the United States and Canada. Fisheries 18:6–22. doi:10.1577/1548-8446(1993)018<0006:csofmo>2.0.co;2
Wilson R, Reuter P, Wahl M (2005) Muscling in on mussels: new insights into bivalve behaviour using vertebrate remote-sensing technology. Mar Biol 147:1165–1172. doi:10.1007/s00227-005-0021-6
Zandee DI, Holwerda DA, Kluytmans JH, De Zwaan A (1985) Metabolic adaptations to environmental anoxia in the intertidal bivalve mollusc Mytilus edulis L. Netherlands J Zool 36:322–343
Zuur AF, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This work was supported by the Illinois Department of Natural Resources, and the United States Geological Survey, through funds provided by the United States Environmental Protection Agency’s Great Lakes Restoration Initiative. J. Tiemann, K. Cummings, J. Tix, C. Sullivan, and J. Sherwood provided valuable help collecting mussels. We would also like to thank A. Wright for providing valuable help with mussel husbandry and laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Hasler, C.T., Hannan, K.D., Jeffrey, J.D. et al. Valve movement of three species of North American freshwater mussels exposed to elevated carbon dioxide. Environ Sci Pollut Res 24, 15567–15575 (2017). https://doi.org/10.1007/s11356-017-9160-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9160-9